Physiological and Transcriptome Responses of Pinus massoniana Seedlings Inoculated by Various Ecotypes of the Ectomycorrhizal Fungus Cenococcum geophilum during the Early Stage of Drought Stress
Abstract
1. Introduction
2. Materials and Methods
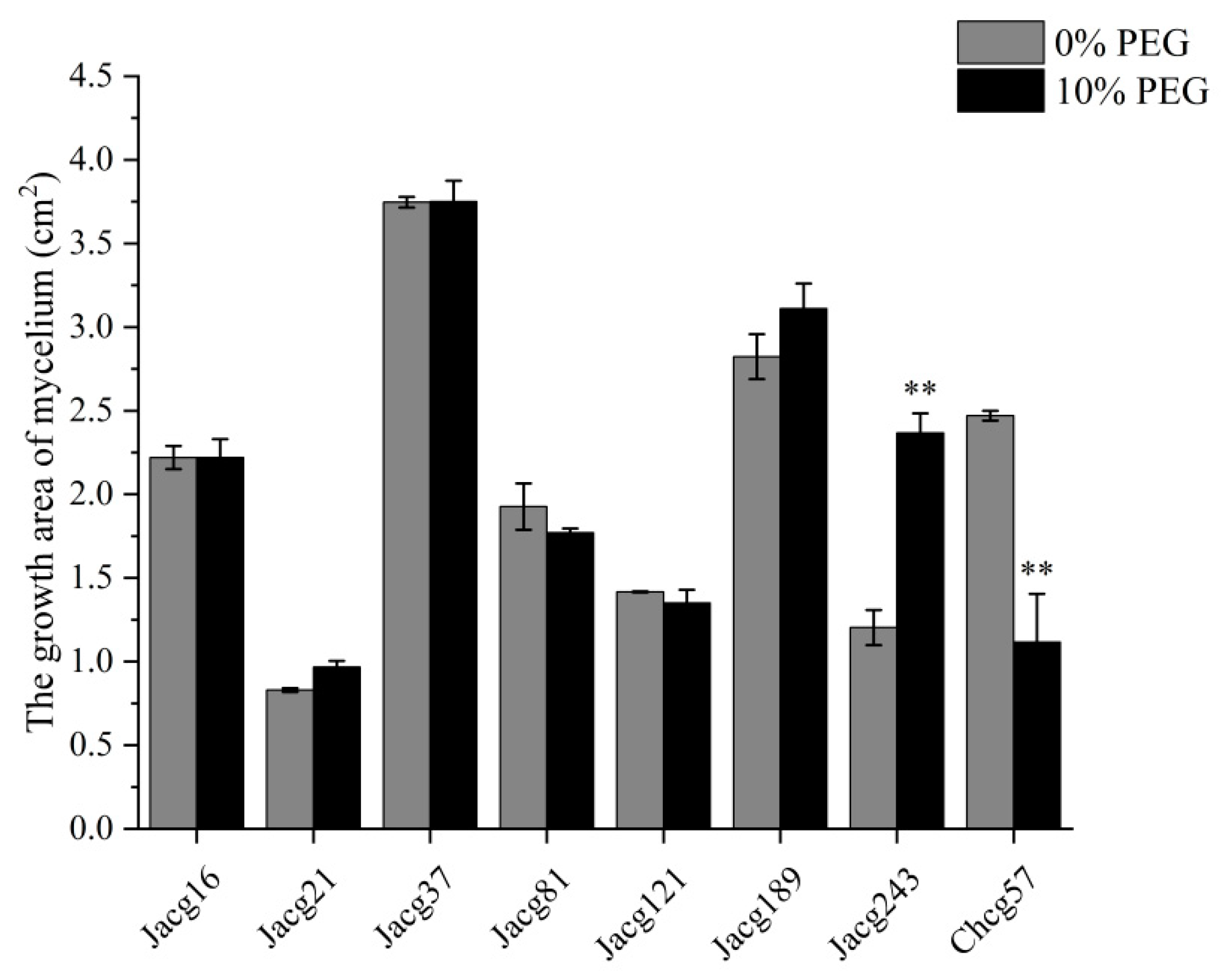
2.1. The Mycelial Growth of Different Ecotypes of C. geophilum under Drought Treatment
2.2. Preparation of Mycorrhizal Seedlings of P. massoniana
2.3. Experimental Design for Drought Stress of P. massoniana Seedlings
2.4. Determination of Photosynthetic Index
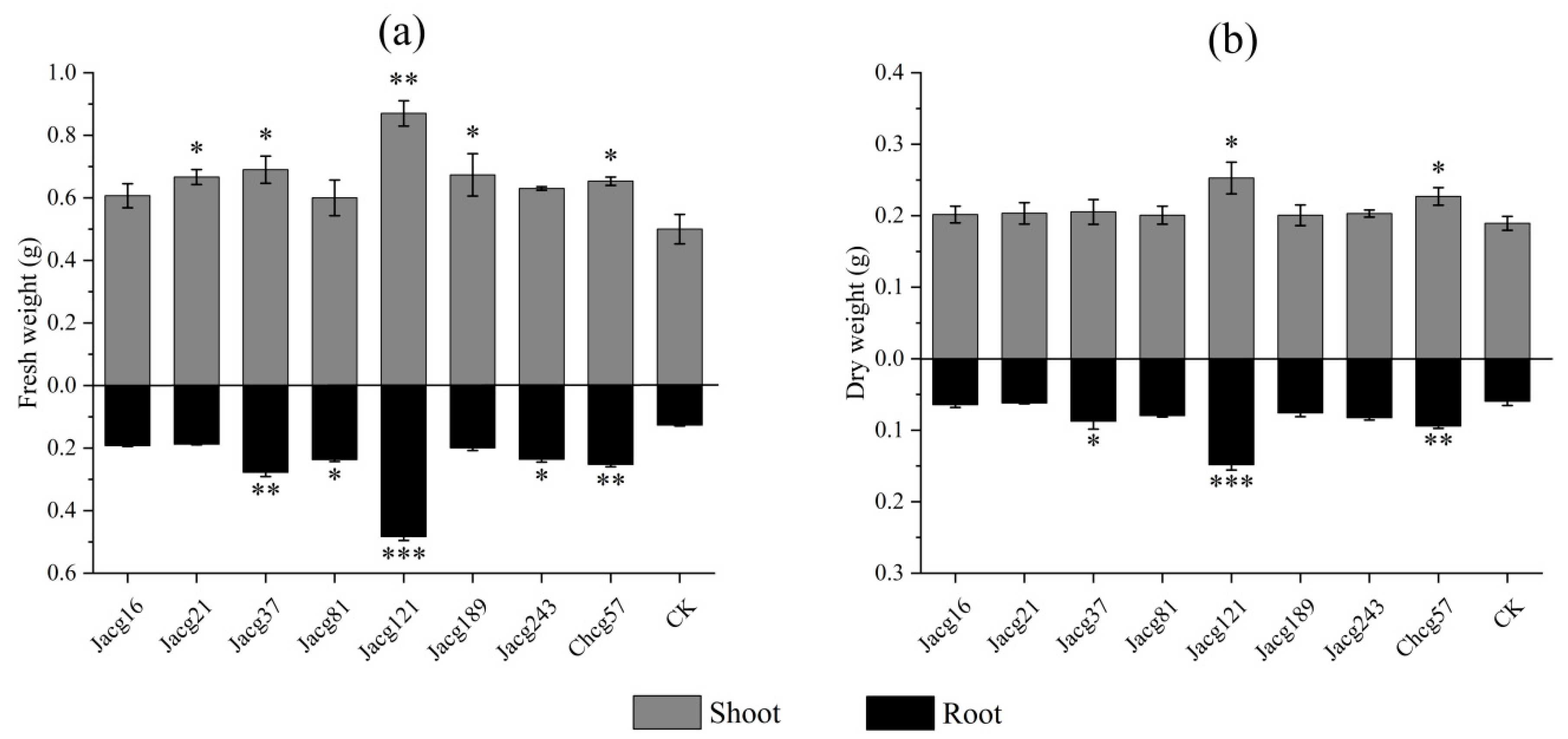
2.5. Determination of Morphological and Physiological Indicators
2.6. RNA-Seq and qRT-PCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Assessing the Drought Tolerance of Various C. geophilum Ecotypic Strains and Their Impact on the Growth of P. massoniana Seedlings through Inoculation
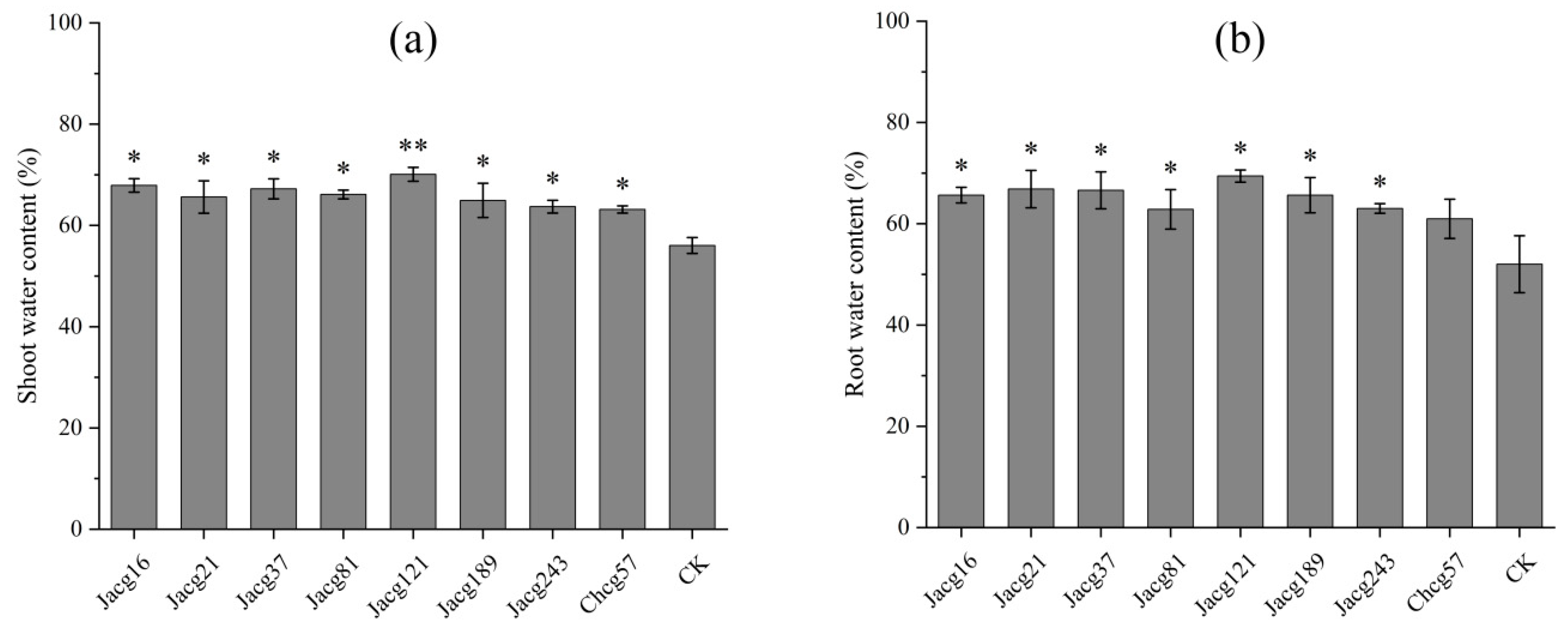
3.2. Effects of Inoculation with Different Ecotypic Strains of C. geophilum on Water Content and Photosynthetic Parameters of P. massoniana Seedlings during the Early Stage of Drought Stress
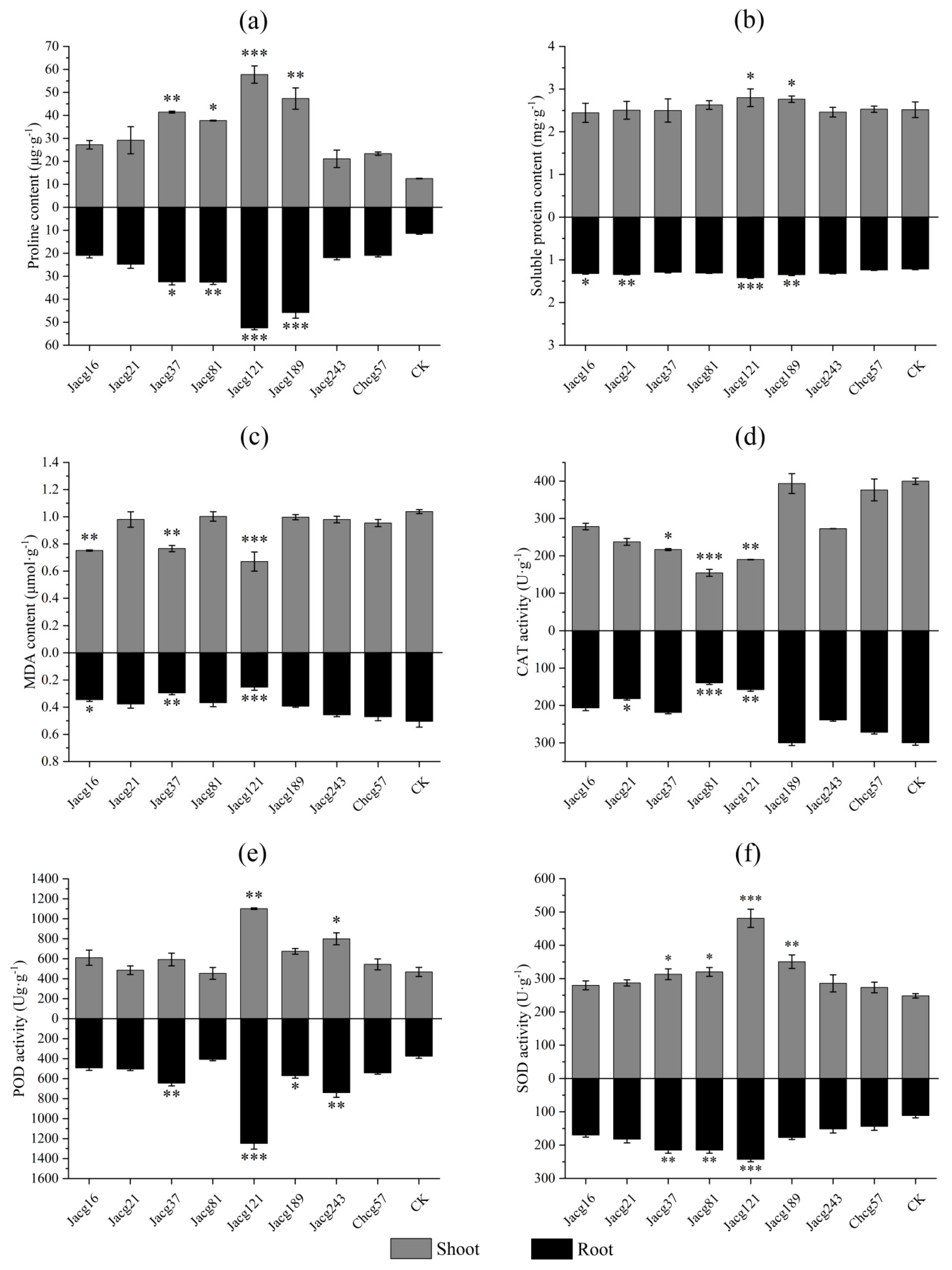
3.3. Effects of Inoculation with Different Ecotypic Strains of C. geophilum on Physiological Indexes of P. massoniana Seedlings during the Early Stage of Drought Stress
3.4. Comprehensive Evaluation of Drought Tolerance in P. massoniana Mycorrhizal Seedlings Inoculated by Various Ecotypes of C. geophilum
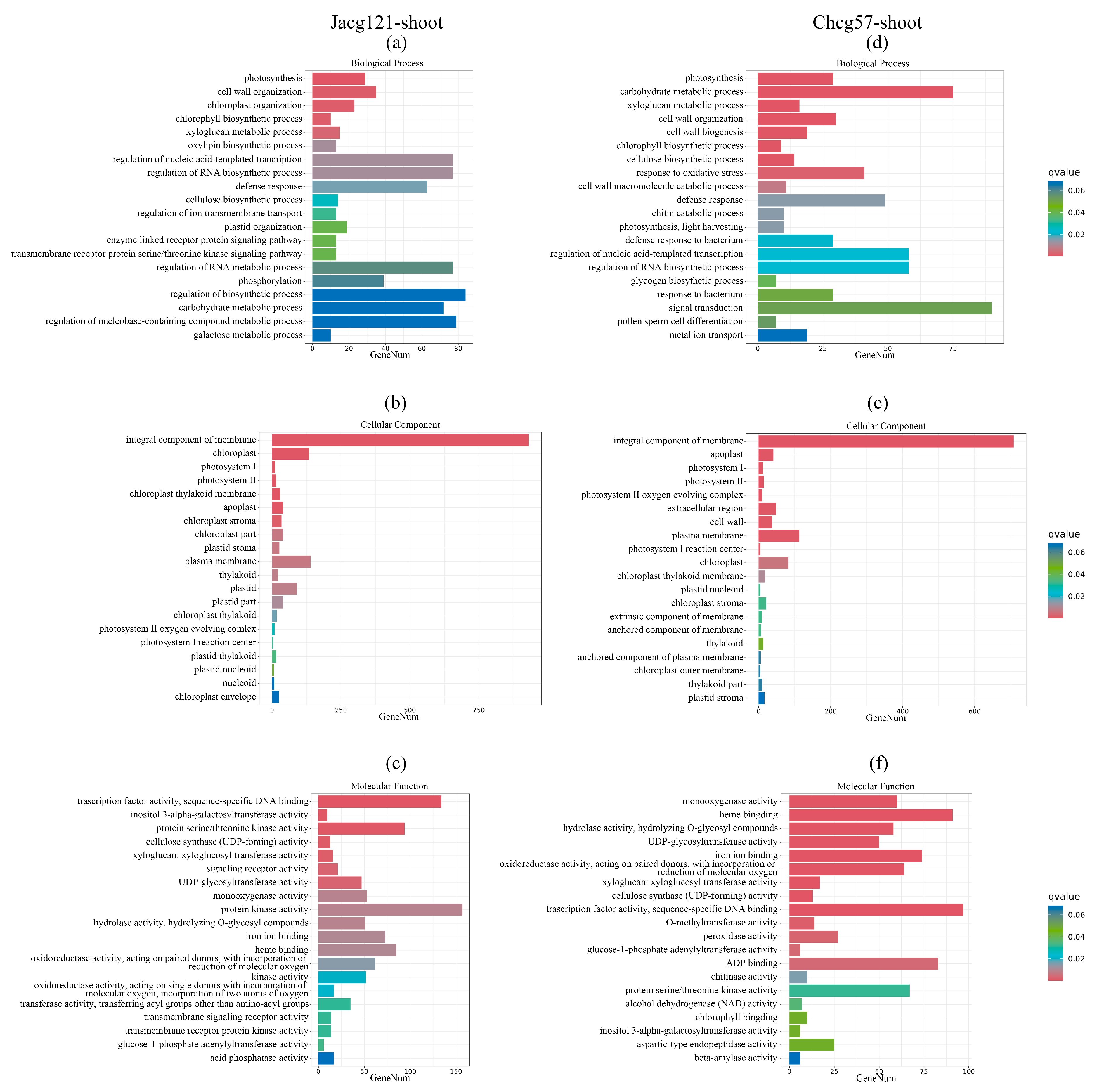
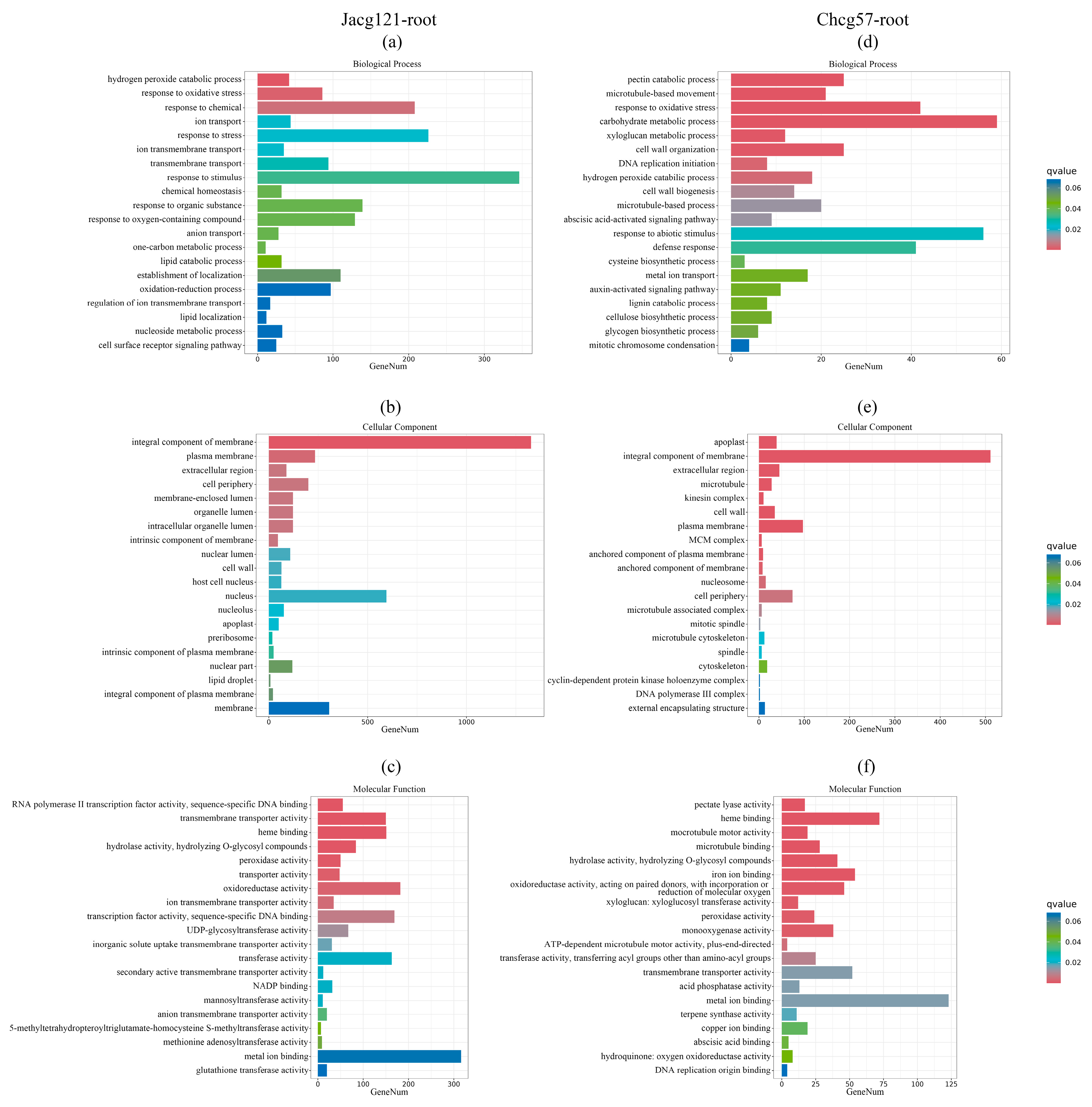
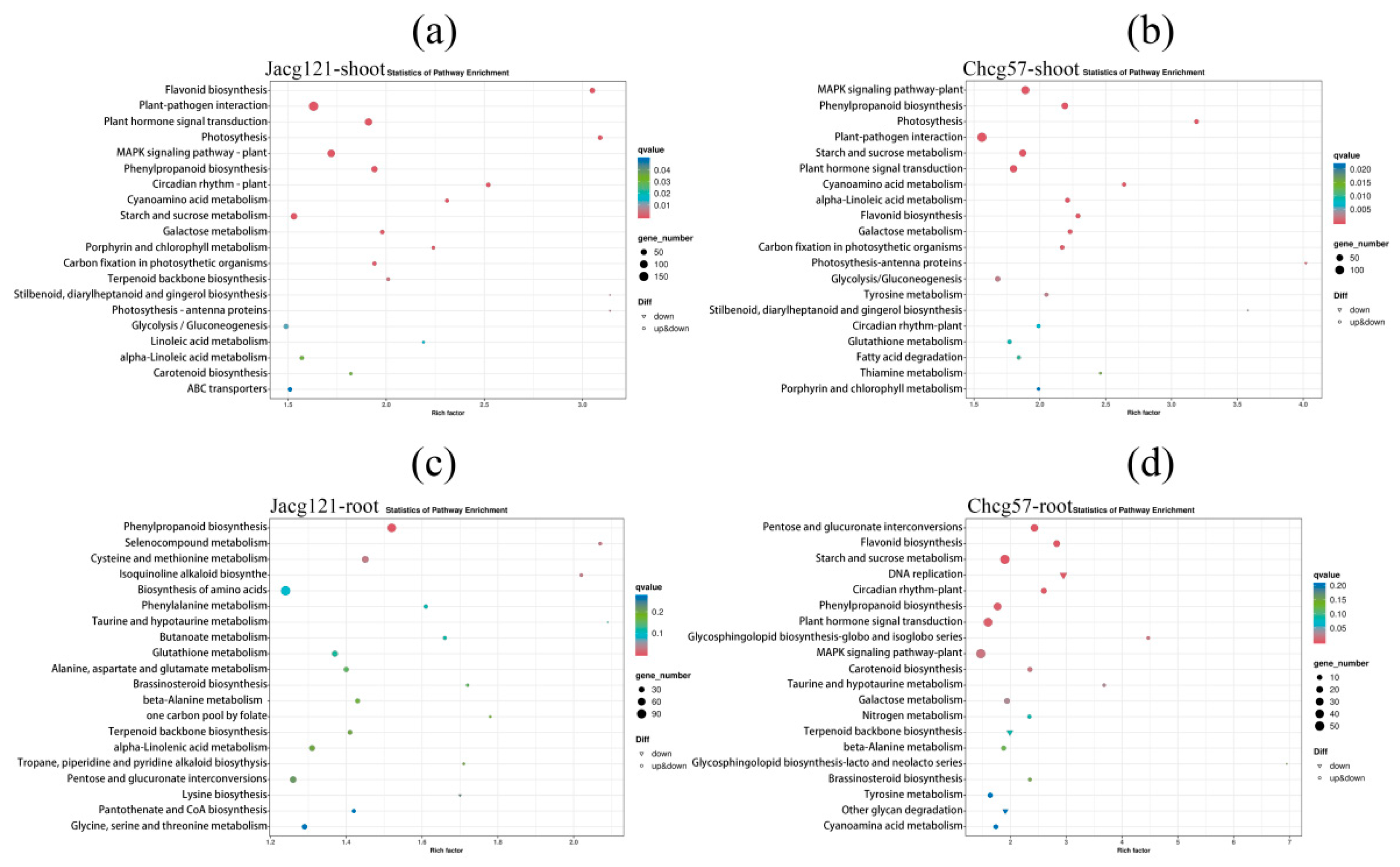
3.5. Transcriptional Responses in C. geophilum-Inoculated P. massoniana Seedlings with Various Drought Tolerance Levels under the Early Stage of Drought Stress
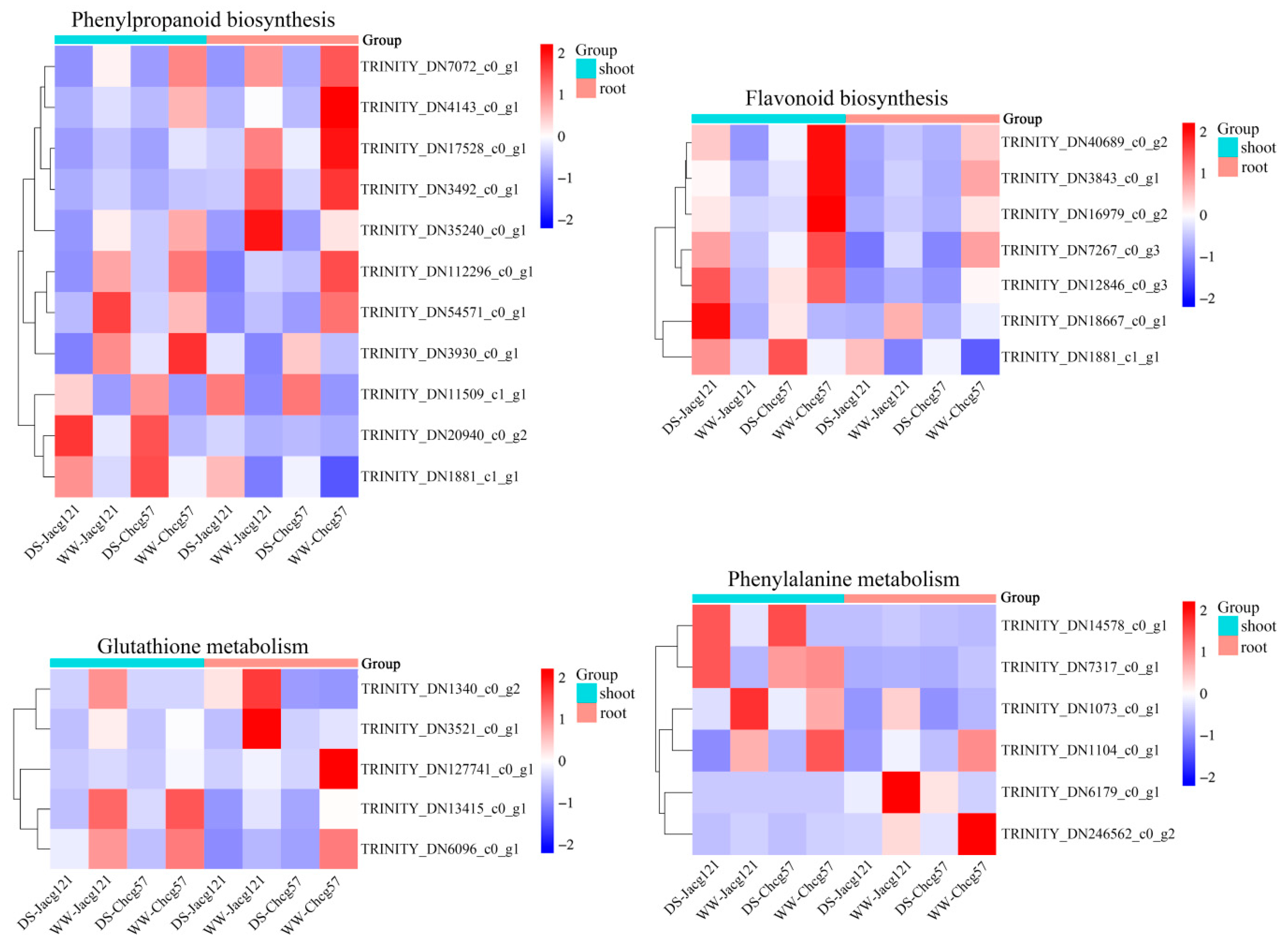
3.6. Analysis of Key Genes for Drought Tolerance of P. massoniana Seedlings Inoculated by C. geophilum under the Early Stage of Drought Stress
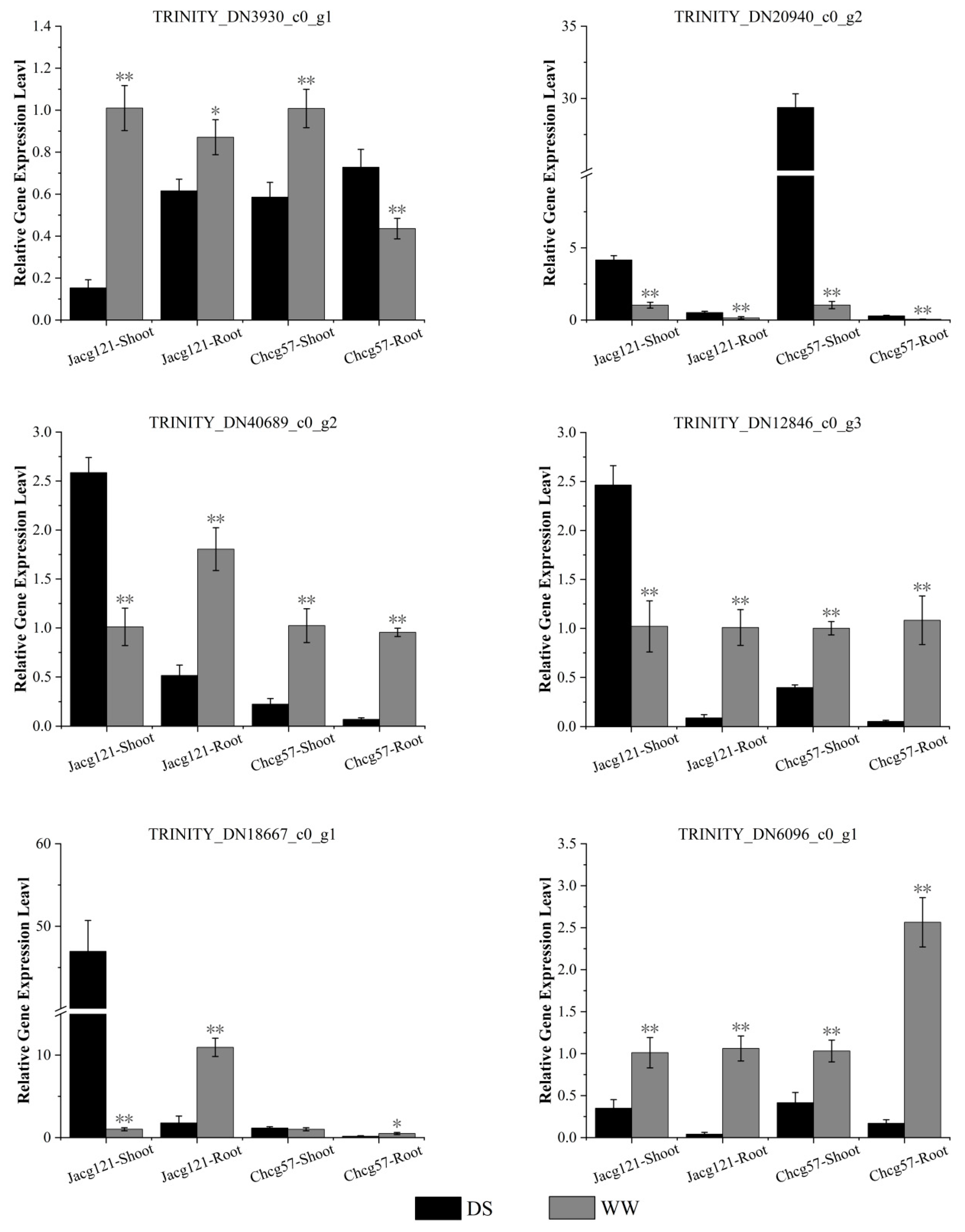
3.7. RT-qPCR Verification of DEGs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Praba, M.L.; Cairns, J.E.; Babu, R.C.; Lafitte, H.R. Identification of physiological traits underlying cultivar differences in drought tolerance in rice and wheat. J. Agron. Crop Sci. 2010, 195, 30–46. [Google Scholar] [CrossRef]
- Avery, W.; Easterling, D.R.; Kunkel, K.E.; Lewis, K.L.M.; Crimmins, A. Impacts, Risks, and Adaptation in the United States: Fourth National Climate Assessment, Volume II; U.S. Global Change Research Program: Washington, DC, USA, 2018; pp. 572–603.
- Wang, H.; Zhang, Z.; Kong, X.; Lui, S.; Shen, Z. Preliminary deduction of potential distribution and alternative hosts of invasive pest, Dendroctonus valens (Coleoptera: Scolytidae). Sci. Silvae Sin. 2007, 43, 71–76. [Google Scholar]
- Choat, B. Predicting thresholds of drought-induced mortality in woody plant species. Tree Physiol. 2013, 33, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.N.; Ribeiro, R.V.; Ferreira-Silva, S.L.; Viégas, R.A.; Silveira, J.A.G. Comparative effects of salinity and water stress on photosynthesis, water relations and growth of Jatropha curcas plants. J. Arid Environ. 2010, 74, 1130–1137. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, Y.; Wang, X.; Liu, Q.; Ran, J. Transcriptome analysis of needle and root of Pinus massoniana in response to continuous drought stress. Plants 2021, 10, 769. [Google Scholar] [CrossRef]
- Tan, J.; Tang, S.; Chen, H. Advances of drought resistance in Pinus massoniana. Guangxi For. Sci. 2017, 46, 7. [Google Scholar]
- Liu, Y.; Li, X.; Kou, Y. Ectomycorrhizal Fungi: Participation in nutrient turnover and community assembly pattern in forest ecosystems. Forests 2020, 11, 453. [Google Scholar] [CrossRef]
- Vaario, L.; Xing, S.; Xie, Z.; Lun, Z.; Sun, Y. In situ and in vitro colonization of Cathaya argyrophylla (Pinaceae) by ectomycorrhizal fungi. Mycorrhiza 2006, 16, 137–142. [Google Scholar] [CrossRef]
- Mrnka, L.; Kuchár, M.; Cieslarová, Z.; Matějka, P.; Száková, J.; Tlustoš, P.; Vosátka, M. Effects of endo- and ectomycorrhizal fungi on physiological parameters and heavy metals accumulation of two species from the family salicaceae. Water Air Soil Pollut. 2012, 223, 399–410. [Google Scholar] [CrossRef]
- Wu, B.; Gao, C.; Chen, L.; Buscot, F.; Goldmann, K.; Purahong, W.; Ji, N.; Wang, Y.; Lü, P.; Li, X. Host phylogeny is a major determinant of Fagaceae-associated ectomycorrhizal fungal community assembly at a regional scale. Front. Microbiol. 2018, 9, 2409. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, C.; Chen, L.; Ji, N.; Wu, B.; Li, X.; Lü, P.; Zheng, Y.; Guo, L. Host plant phylogeny and geographic distance strongly structure Betulaceae-associated ectomycorrhizal fungal communities in Chinese secondary forest ecosystems. FEMS Microbiol. Ecol. 2019, 95, fiz037. [Google Scholar] [CrossRef] [PubMed]
- Plassard, C.; Louche, J.; Ali, M.A.; Duchemin, M.; Legname, E.; Cloutier-Hurteau, B. Diversity in phosphorus mobilisation and uptake in ectomycorrhizal fungi. Ann. For. Sci. 2011, 68, 33–43. [Google Scholar] [CrossRef]
- Zou, Y.; Srivastava, A.K.; Ni, Q.D.; Wu, Q. Disruption of mycorrhizal extraradical mycelium and changes in leaf water status and soil aggregate stability in rootbox-grown trifoliate orange. Front. Microbiol. 2015, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Zhang, H.; Yao, X. Prospects of exploitation and utilization of ecto-mycorrhiza. Anhui Agric. Sci. Bull. 2008, 14, 4. [Google Scholar]
- Ahmed, C.B.; Rouina, B.B.; Sensoy, S.; Boukhriss, M.; Abdullah, F.B. Exogenous proline effects on photosynthetic performance and antioxidant defense system of young olive tree. J. Agric. Food Chem. 2010, 58, 4216–4222. [Google Scholar] [CrossRef]
- Zhao, M.; Hao, W.; Ning, X.; Hao, L.; Yan, H.; Mu, Y.; Bai, S. Screening of Excellent Ectomycorrhizal Fungi-tree for Drought Resistant with Pinus sylvestris var. mongolica. Bull. Bot. Res. 2020, 40, 133–140. [Google Scholar]
- Park, J.Y. Effects of field inoculation with mycorrhizae of Cenococcum graniforme on Basswood growth. Bi-Mon. Res. Notes 1970, 26, 27–28. [Google Scholar]
- Guo, Z.; Wang, Y.; Wu, B.; Xu, Y.; Yao, H.; Li, Z.; Yu, Q.; Li, X.; Guo, L. Population genetic diversity and structure of ectomycorrhizal fungus Cenococcum geophilum. Mycosystema 2021, 40, 920–935. [Google Scholar]
- Coleman, M.D.; Bledsoe, C.S.; Lopushinsky, W. Pure culture response of ectomycorrhizal fungi to imposed water stress. Can. J. Bot. 1989, 67, 29–39. [Google Scholar] [CrossRef]
- Li, M.; Yuan, C.; Zhang, X.; Pang, W.; Zhang, P.; Xie, R.; Lian, C.; Zhang, T. The transcriptional responses of ectomycorrhizal fungus, Cenococcum geophilum, to drought stress. J. Fungi. 2022, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Marx, D.H. The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. II. Production, identification, and biological activity of antibiotics produced by Leucopaxillus cerealis var. piceina. Phytopathology 1969, 59, 153–163. [Google Scholar]
- Zhang, Z. Plant physiology Experiment Instruction, 2nd ed.; Higher Education Press: Beijing, China, 1990. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Minoru, K.; Susumu, G.; Shuichi, K.; Yasushi, O.; Masahiro, H. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Yi, X.; Du, Z.; Su, Z. PlantGSEA: A gene set enrichment analysis toolkit for plant community. Nucleic Acids Res. 2013, 41, W98–W103. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Hu, W.; Li, Y.; He, J.; Zhu, H.; Zhou, Z. Screening of drought resistance indices and evaluation of drought resistance in cotton (Gossypium hirsutum L.). J. Integr. Agric. 2020, 19, 495–508. [Google Scholar] [CrossRef]
- Peng, S.; Wang, X.; Li, J.; Xia, D.; Ge, Z.; Xue, J. Effects of ectomycorrhizal fungi inoculation on growth and photosynthetic characteristics of Broussonetia papyrifera seedlings under drought stress. Chin. J. Ecol. 2021, 40, 2719–2726. [Google Scholar]
- Wang, Y.; Tu, G. Effects of drought and rewatering on physiological properties of five species of Pinus massoniana seedlings. J. Cent. South Univ. For. Technol. 2021, 41, 12. [Google Scholar]
- Yan, T.; Zhang, P.; Pang, W.; Zhang, X.; Lian, C.; Zhang, T. Effects of high temperature-triggered transcriptomics on the physiological adaptability of Cenococcum geophilum, an ectomycorrhizal fungus. Microorganisms 2022, 10, 2039. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, T.; Yuan, C.; Li, C.; Rensing, C.; Chen, Y.; Xie, R.; Zhang, T.; Lian, C. Comparative physiological and transcriptome analysis provide insights into the response of Cenococcum geophilum, an ectomycorrhizal fungus to cadmium stress. J. Fungi. 2022, 8, 724. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Tsuruta, M.; Matsushita, N.; Goto, S.; Shen, Z.; Tsugama, D.; Zhang, S.; Lian, C. Physiological and transcriptional responses of the ectomycorrhizal fungus Cenococcum geophilum to salt stress. Mycorrhiza 2022, 32, 327–340. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Allen, M.F. Mycorrhizal fungi: High way for water and nutrients in arid soils. Vadose Zone J. 2007, 6, 291–297. [Google Scholar] [CrossRef]
- Rasouli, F.; Amini, T.; Skrovankova, S.; Asadi, M.; Hassanpouraghdam, M.B.; Ercisli, S.; Buckova, M.; Mrazkova, M.; Mlcek, J. Influence of drought stress and mycorrhizal (Funneliformis mosseae) symbiosis on growth parameters, chlorophyll fluorescence, antioxidant activity, and essential oil composition of summer savory (Satureja hortensis L.) plants. Front. Plant Sci. 2023, 14, 1151467. [Google Scholar] [CrossRef]
- Casieri, L.; Lahmidi, N.A.; Doidy, J.; Veneault-Fourrey, C.; Migeon, A.; Bonneau, L.; Courty, P.; Garcia, K.; Charbonnier, M.; Delteil, A.; et al. Biotrophic transportome in mutualistic plant-fungal interactions. Mycorrhiza 2013, 23, 597–625. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y. Effects of shading and drought on light-induced stomatal dynamics in Betula platyphylla seedling. Chin. J. Appl. Ecol. 2022, 33, 2331–2338. [Google Scholar]
- Firdos, K.; Aisha, A.N.; Muhammad, S.; Fahad, A.-Q.; Muhammad, A. Trehalose: A key organic osmolyte effectively involved in plant abiotic stress tolerance. J. Plant Growth Regul. 2019, 38, 608–618. [Google Scholar]
- Wang, J.; Zhang, H.; Gao, J.; Zhang, Y.; Tang, M. Effects of ectomycorrhizal fungi (Suillus variegatus) on the growth, hydraulic function, and non-structural carbohydrates of Pinus tabulaeformis under drought stress. BMC Plant Biol. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Mckiernan, A.B.; Hovenden, M.J.; Brodribb, T.J.; Potts, B.M.; Davies, N.W.; O’Reilly-Wapstra, J.M. Effect of limited water availability on foliar plant secondary metabolites of two Eucalyptus species. Environ. Exp. Bot. 2014, 105, 55–64. [Google Scholar] [CrossRef]
- Feng, X.; Yang, Z.; Wang, X. Tissue-specific transcriptome analysis of drought stress and rehydration in Trachycarpus fortunei at seedling. PeerJ 2021, 9, e10933. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Suzuki, N.; Miller, G.; Tognetti, V.B.; Vandepoele, K.; Gollery, M.; Shulaev, V.; Breusegem, F.V. ROS signaling: The new wave? Trends Plant Sci. 2011, 6, 300–309. [Google Scholar] [CrossRef]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Alvarez, M.; Huygens, D.; Fernandez, C.; Yessy, G.; Olivares, E.; Saavedra, I.; Alberdi, M.; Valenzuela, E. Effect of ectomycorrhizal colonization and drought on reactive oxygen species metabolism of Nothofagus dombeyi roots. Tree Physiol. 2009, 29, 1047–1057. [Google Scholar] [CrossRef]
- Pan, X.; Li, J.; Wang, J.; He, Q.; Su, Y.; Ma, J.; Du, K. The impact of drought stress on physiological indicators of four shrub species on the Qinghai-Tibet Plateau. For. Res. 2013, 26, 352–358. [Google Scholar]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, M.; Rahimmalek, M.; Arzani, A.; Talebi, M. The effect of water stress on phytochemical accumulation, bioactive compounds and expression of key genes involved in flavonoid biosynthesis in Chrysanthemum morifolium L. Ind. Crop. Prod. 2018, 120, 295–304. [Google Scholar] [CrossRef]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive oxygen species in plants: From source to sink. Antioxidants. 2022, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhu, M.; Hao, L.; Chen, X.; Gao, Y.; Guo, X.; Li, H. GhMAPKKK49, a novel cotton (Gossypium hirsutum L.) MAPKKK gene, is involved in diverse stress responses. Acta Physiol. Plant. 2016, 13, 28. [Google Scholar]
- Zhu, X.; Zhang, N.; Liu, X.; Li, S.; Yang, J.; Hong, X.; Wang, F.; Si, H. Mitogen-activated protein kinase 11 (MAPK11) maintains growth and photosynthesis of potato plant under drought condition. Plant Cell Rep. 2021, 40, 491–506. [Google Scholar] [CrossRef]
- Hou, H.; Liu, L.; Li, Q.; Wang, J.; Du, B. A cascade enzyme system integrating peroxidase mimic with catalase for linear range expansion of H2O2 assay: A mechanism and application study. Small 2023, 19, e2300444. [Google Scholar] [CrossRef]
- Bengough, A.G.; Mckenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Zheng, Y.; Yuan, C.; Matsushita, N.; Lian, C.; Geng, Q. Analysis of the distribution pattern of the ectomycorrhizal fungus Cenococcum geophilum under climate change using the optimized MaxEnt model. Ecol. Evol. 2023, 13, e10565. [Google Scholar] [CrossRef]
- Zhang, T.; Pang, W.; Yan, T.; Zhang, P.; He, J.; Rensing, C.; Yang, W.; Lian, C. Metal-non-tolerant ecotypes of ectomycorrhizal fungi can protect plants from cadmium pollution. Front. Plant Sci. 2023, 14, 1301791. [Google Scholar] [CrossRef]

| ID | Pn (μmol·m−2·s−1) | Gs (mol·m−2·s−1) | Ci (μmol·m−2·s−1) | Tr (mmol·m−2·s−1) |
|---|---|---|---|---|
| Jacg16 | 1.57 ± 0.06 | 0.023 ± 0.001 | 322.17 ± 4.37 *** | 0.95 ± 0.01 |
| Jacg21 | 2.45 ± 0.32 * | 0.019 ± 0.004 | 214.01 ± 3.12 | 1.00 ± 0.02 |
| Jacg37 | 3.22 ± 0.03 ** | 0.036 ± 0.001 *** | 280.97 ± 1.73 * | 1.72 ± 0.01 *** |
| Jacg81 | 3.53 ± 0.08 *** | 0.026 ± 0.007 * | 207.72 ± 8.04 | 1.16 ± 0.03 * |
| Jacg121 | 3.58 ± 0.12 *** | 0.046 ± 0.003 *** | 303.57 ± 4.77 ** | 2.25 ± 0.01 *** |
| Jacg189 | 1.93 ± 0.02 | 0.035 ± 0.003 ** | 327.84 ± 0.94 *** | 1.59 ± 0.01 ** |
| Jacg243 | 1.47 ± 0.04 | 0.020 ± 0.001 | 261.70 ± 3.00 | 0.98 ± 0.01 |
| Chcg57 | 2.30 ± 0.08 | 0.024 ± 0.007 | 280.65 ± 1.61 * | 1.20 ± 0.03 * |
| CK | 1.08 ± 0.01 | 0.008 ± 0.001 | 161.93 ± 3.55 | 0.93 ± 0.01 |
| Code | CI1 | CI2 | CI3 | μ(X1) | μ(X2) | μ(X3) | D | Comprehensive Evaluation * |
|---|---|---|---|---|---|---|---|---|
| Jacg16 | −0.083 | −0.382 | −1.990 | 0.437 | 0.354 | 0.000 | 0.386 | 6 |
| Jacg21 | −0.118 | −1.057 | −0.437 | 0.427 | 0.154 | 0.450 | 0.391 | 5 |
| Jacg37 | 0.555 | −0.492 | −0.107 | 0.610 | 0.321 | 0.545 | 0.564 | 3 |
| Jacg81 | 0.188 | −1.574 | 1.465 | 0.510 | 0.000 | 1.000 | 0.483 | 4 |
| Jacg121 | 1.991 | 0.511 | 0.417 | 1.000 | 0.619 | 0.697 | 0.919 | 1 |
| Jacg189 | 0.319 | 1.792 | 0.337 | 0.546 | 1.000 | 0.673 | 0.621 | 2 |
| Jacg243 | −0.454 | 0.230 | −0.627 | 0.336 | 0.536 | 0.394 | 0.369 | 7 |
| Chcg57 | −0.704 | 0.580 | −0.060 | 0.268 | 0.640 | 0.559 | 0.347 | 8 |
| CK | −1.693 | 0.393 | 1.002 | 0.000 | 0.584 | 0.866 | 0.160 | 9 |
| Index weight (wj) | 0.770 | 0.140 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, J.; He, J.; Li, M.; Matsushita, N.; Geng, Q.; Lian, C.; Zhang, S. Physiological and Transcriptome Responses of Pinus massoniana Seedlings Inoculated by Various Ecotypes of the Ectomycorrhizal Fungus Cenococcum geophilum during the Early Stage of Drought Stress. J. Fungi 2024, 10, 71. https://doi.org/10.3390/jof10010071
Zhang X, Zhang J, He J, Li M, Matsushita N, Geng Q, Lian C, Zhang S. Physiological and Transcriptome Responses of Pinus massoniana Seedlings Inoculated by Various Ecotypes of the Ectomycorrhizal Fungus Cenococcum geophilum during the Early Stage of Drought Stress. Journal of Fungi. 2024; 10(1):71. https://doi.org/10.3390/jof10010071
Chicago/Turabian StyleZhang, Xiaohui, Jinyan Zhang, Juan He, Mingtao Li, Norihisa Matsushita, Qifang Geng, Chunlan Lian, and Shijie Zhang. 2024. "Physiological and Transcriptome Responses of Pinus massoniana Seedlings Inoculated by Various Ecotypes of the Ectomycorrhizal Fungus Cenococcum geophilum during the Early Stage of Drought Stress" Journal of Fungi 10, no. 1: 71. https://doi.org/10.3390/jof10010071
APA StyleZhang, X., Zhang, J., He, J., Li, M., Matsushita, N., Geng, Q., Lian, C., & Zhang, S. (2024). Physiological and Transcriptome Responses of Pinus massoniana Seedlings Inoculated by Various Ecotypes of the Ectomycorrhizal Fungus Cenococcum geophilum during the Early Stage of Drought Stress. Journal of Fungi, 10(1), 71. https://doi.org/10.3390/jof10010071





