Abstract
The two etiologic agents of cryptococcal meningoencephalitis, Cryptococcus neoformans and C. gattii, have been commonly designated as either an opportunistic pathogen for the first species or as a primary pathogen for the second species. Such a distinction has been based on epidemiological findings that the majority of patients presenting meningoencephalitis caused by C. neoformans are immunocompromised while C. gattii infection has been reported more often in immunocompetent patients. A recent report, however, showed that GM-CSF (granulocyte-macrophage colony-stimulating factor) neutralizing antibodies were prevalent in the plasma of “apparently immunocompetent” C. gattii patients with meningoencephalitis. Because GM-CSF is essential for differentiation of monocytes to macrophages and modulating the immune response, it is not surprising that the lack of GM-CSF function predisposes otherwise healthy individuals to infection via inhalation of environmental pathogens such as C. gattii. Since the test for anti-GM-CSF autoantibodies is not included in routine immunological profiling at most hospitals, healthy patients with GM-CSF neutralizing antibodies are usually categorized as immunocompetent. It is likely that a comprehensive immunological evaluation of patients with C. gattii meningoencephalitis, who had been diagnosed as immunocompetent, would reveal a majority of them had hidden immune dysfunction. This paper reviews the relationship between GM-CSF neutralizing antibodies and the risk for C. gattii infection with CNS involvement.
1. Introduction
The etiologic agents of cryptococcosis, Cryptococcus neoformans and C. gattii are sibling species (85%–90% genomic identity) [1] that cause cryptococcosis in humans and a wide range of mammals. The disease in humans proceeds by inhalation of airborne cryptococci, which disseminate to the central nervous system (CNS) and cause menigoencephalitis. Unless treated, cryptococcal CNS infection is fatal and the fatality rate is high in spite of the most advanced therapeutic measures [2,3] Epidemiological studies have clearly shown that cryptococcosis due to C. neoformans is worldwide in distribution and though it can cause disease in otherwise healthy individuals, a majority of the reported cases have been from patients whose immune system have been compromised by various causes such as HIV infection, corticosteroid treatment and other immunosuppressive underlying conditions [3,4]. This is why C. neoformans has been widely known as an opportunistic pathogen. Epidemiological deviancy in C. neoformans infection, however, has been reported among patients in the Far East countries: China, Korea and Japan. In these countries, most of the cryptococcosis patients were caused by C. neoformans and were HIV sero-negative with or without any apparent predisposing underlying condition [5,6,7]. Cryptococcosis caused by C. gattii in HIV+ patients have been reported mostly in tropical and subtropical regions of the world such as Africa [8], South America [9] and Southern California [10].
Several studies have confirmed that C. gattii infects apparently healthy hosts with no known predisposing risk factor more frequently than immunocompromised individuals regardless of geographic regions and is therefore widely regarded as a primary pathogen [9,11,12,13,14,15,16]. While individuals with depleted CD4+ T cells resulting from HIV infection are highly susceptible to cryptococcal CNS infection due to C. neoformans [17,18], C. gattii infrequently infects such patients compared to C. neoformans even in the C. gattii endemic regions of the world [8,9,13,16]. Difference in host selection is not surprising considering their biological differences. The two species are readily distinguishable by the antigenic specificity of their polysaccharide capsule and their ability to utilize certain amino acids as carbon or nitrogen sources. C. neoformans includes strains of serotype A, D and AD which are generally unable to assimilate D-proline as a nitrogen source and glycine as a carbon source. C. gattii on the other hand can utilize D-proline as a nitrogen source and glycine as a source for both carbon and nitrogen [19,20]. These biochemical differences allow separation of the two species by using CGB agar media [19], which is commercially available (Hardy Diagnostics, Santa Maria, CA, USA; Thermo Fisher Scientific Remel Products, Lenexa, KS, USA). Recent studies have revealed further differences between the two species with regards to the utilization of D-amino acids as a nitrogen source in general [21] and the major target organs in mice [22].
It has been speculated that C. gattii infection in apparently healthy individuals is the result of increased environmental exposure to the fungus. Such speculation has been based on the over-representation of C. gattii infection among the Australian Aboriginal people living in rural areas [15,23] where daily exposure to the natural reservoir of C. gattii such as Eucalyptus and other trees, organic debris and soil is likely higher than the population living in urban areas. Analysis of the risk factors for C. gattii infection among patients in British Columbia, Canada showed that, in addition to environmental exposure, certain underlying medical conditions to be of significant risk [16]. These underlying medical conditions which carry a significant risk for C. gattii infection include preexisting oral steroid use, emphysema, chronic bronchitis, chronic obstructive pulmonary disease, sarcoidosis and pneumonia [16]. Environmental exposure risks included cutting/chopping wood, pruning and cleaning up branches [16].
The host specific mechanisms explaining the increased incidence of C. gattii infection in individuals with these risk factors have not been evaluated. Countless numbers of tourists and inhabitants are exposed to the environment of C. gattii endemic regions such as Vancouver, Canada, Southeast Asia and South America each year but the number of patients afflicted by C. gattii has only been a fraction of the exposed populations: less than one case per million per year in Australia [13] and Colombia [9] to 5.8 cases/million/year even in the highest case clusters reported in Vancouver Island, British Columbia during 1999–2007 [24]. This suggests that although the onset of C. gattii disease may be triggered by exposure, inhaled C. gattii cells in healthy individuals are readily eliminated by the host defense systems unless certain predisposing risk factors are present in these patients. Such endogenous risk factors may also contribute to increased persistence of dormant C. gattii or be required to trigger reactivation of dormant C. gattii to cause infection. GM-CSF neutralizing antibody appears to be one such host-dependent risk factor. Defining C. gattii as a primary pathogen may become obsolete as more subtle immune dysfunctions are recognized in otherwise healthy patients with C. gattii meningoencephalitis.
2. How Were GM-CSF Neutralizing Antibodies Identified as a Risk Factor for C. gattii Infection?
In 2013, Rosen et al., detected anti-GM-CSF autoantibodies which inhibited GM-CSF signaling in the plasma of four previously healthy HIV-negative patients suffering from cryptococcal meningoencephalitis [25]. This finding prompted them to screen archived plasma samples of 103 patients with culture proven cryptococcosis who had been treated at the National Institutes of Health between 1955 and 1984. Of the 103 archived plasma samples, 67 were from patients without any evidence of immunodeficiency and 36 were from patients with either a history of iatrogenic immunosuppression or an underlying medical condition that is known to predispose patients to cryptococcosis such as hematological malignancy or diabetes prior to their diagnosis of cryptococcosis. Of the 67 archived samples from patients without any recognized immunodeficiency, three were positive for the presence of anti-GM-CSF antibodies compared with none of the healthy controls (n = 64), diseased controls (n = 43, adult patients with undiagnosed immunodeficiency) or cryptococcosis patients with recognized immunodeficiency (n = 36). These results suggested that GM-CSF neutralizing antibodies could be a previously hidden risk factor for cryptococcal meningoencephalitis in HIV negative and otherwise healthy individuals. Of the seven cryptococcal strains (four from current and three from the archived cases) isolated from GM-CSF autoantibody positive patients, three had been reported as C. neoformans, one as C. gattii and three as Cryptococcus without identification at species level. The three Cryptococcus unspeciated strains had been isolated from the immunocompetent patients whose plasma samples had been archived and the strains had been maintained as lyophilized cultures.
Since the majority of cryptococcosis cases reported from Far East Asia are from HIV sero-negative patients with or without any apparent risk factors, we hypothesized that anti-GM-CSF autoantibodies could explain many of the cryptococcosis cases in otherwise healthy patients in Far East Asia [5,6,7]. As reported in 2014 [26], we screened 20 plasma samples from normal healthy volunteers and 21 otherwise healthy cryptococcosis patients from China with meningoencephalitis for the presence of functional anti-GM-CSF autoantibodies. To our surprise, GM-CSF neutralizing antibodies were detected in the plasma of only one patient who had been infected by C. gattii and one healthy volunteer, but none of the 20 patients infected by C. neoformans (Table 1) [26].

Table 1.
Detection of anti-GM-CSF autoantibodies in plasma from Chinese immunocompetent, otherwise healthy cryptococcosis patients with CNS infection [26].
| No. Patient Samples | Etiologic Agent | Anti-GM-CSF AB |
|---|---|---|
| 20 | C. neoformans, VNl, VNlll | 0 |
| 1 | C. gattii, VGl | 1 |
| 20 (Normal volunteers) | None | 1 |
The C. gattii strain infected the GM-CSF autoantibody positive patients was of the VGI molecular type, which is the major C. gattii molecular type found in Southeast Asia [27]. The plasma from the C. gattii infected patient with GM-CSF autoantibodies inhibited phosphorylation of STAT5 (Figure 1) in normal PBMC when reacted with exogenous GM-CSF [26]. This indicated that the anti-GM-CSF autoantibodies in this C. gattii infected patient were biologically active. The GM-CSF autoantibody titer in the plasma of one healthy volunteer was significantly higher than the base level found in other healthy volunteers but lower than that of the C. gattii infected patient. Not surprisingly, the plasma of GM-CSF autoantibody positive healthy volunteer caused only partial inhibition of STAT5 phosphorylation compared to the plasma of the C. gattii patient with GM-CSF autoantibody [26].
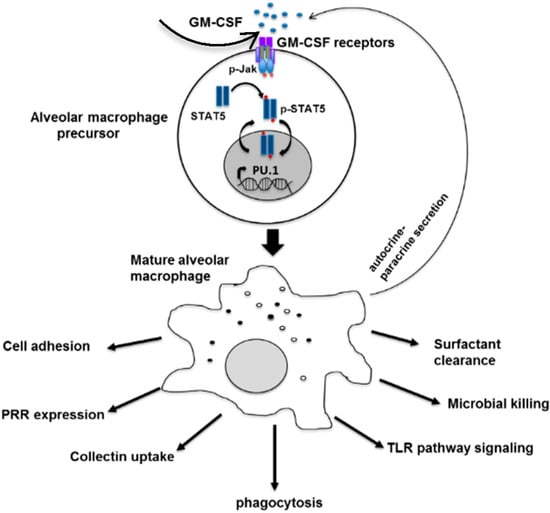
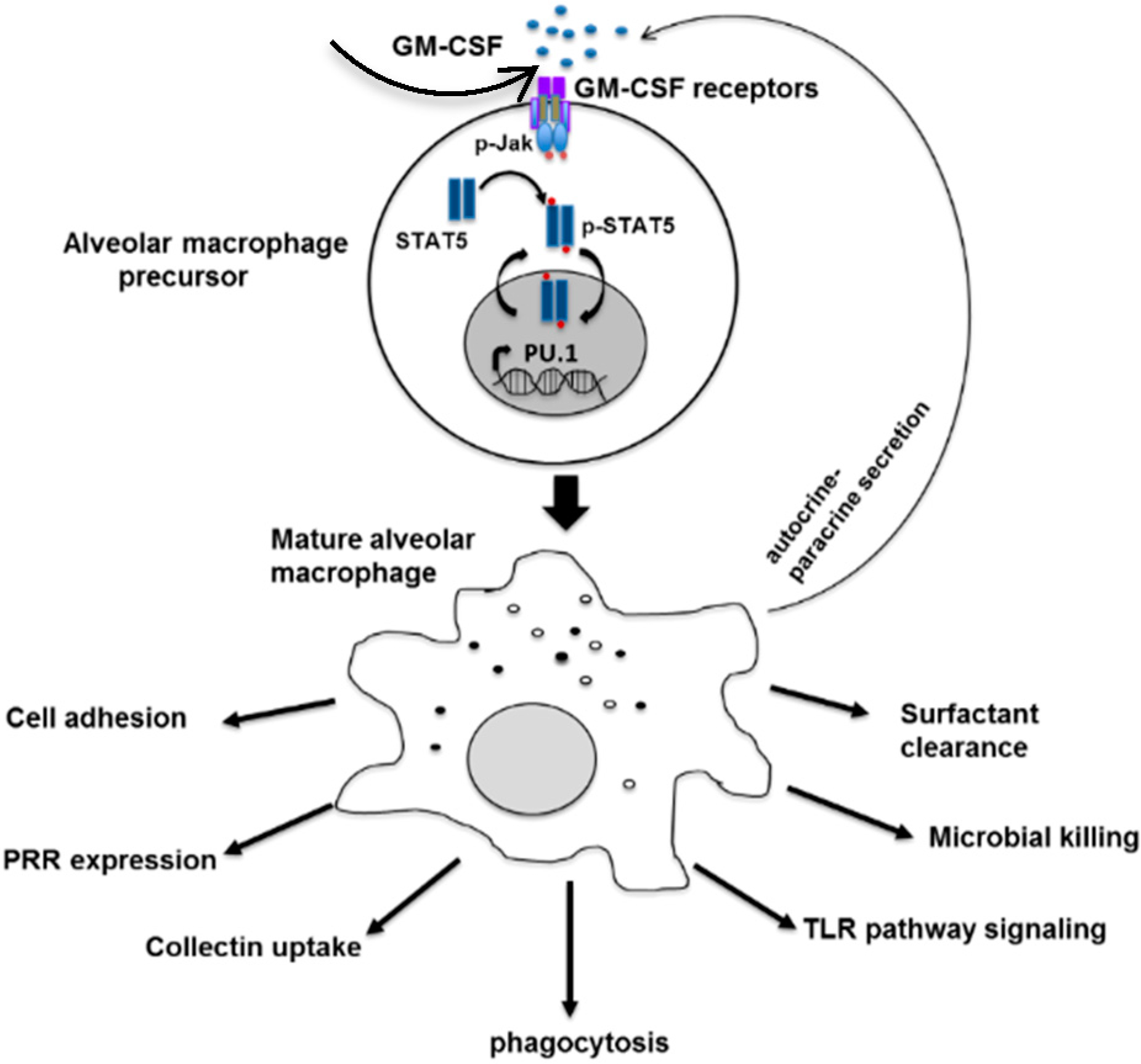
Figure 1.
The role of GM-CSF in differentiation and innate immune function of the alveolar macrophage. Matured macrophages also secrete GM-CSF via autocrine and paracrine signaling. Adapted with permission from Shibata et al., copyright Elsevier, 1982.
Figure 1.
The role of GM-CSF in differentiation and innate immune function of the alveolar macrophage. Matured macrophages also secrete GM-CSF via autocrine and paracrine signaling. Adapted with permission from Shibata et al., copyright Elsevier, 1982.
This finding associating the GM-CSF autoantibodies with C. gattii infection but not with C. neoformans infection among Chinese patients (Table 1) prompted us to determine the species status of the three strains listed as Cryptococcus without species identification shown in Table 2.

Table 2.
The etiologic agents of cryptococcosis reported in the seven patients with anti-GM-CSF autoantibodies by Rosen et al. [25] and the species confirmation by Saijo et al. [26].
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Origin | S. CA a | S. CA | Thailand | S. CA | NA b | NJ c | NJ |
| Infection | CNS/Lung | CNS/Lung | CNS/Lung | CNS/Lung Blood, Skin | CNS | CNS/Lung | CNS/Lung |
| Species | C. neof. | C. neof. | C. neof. | C. gattii | Cryptococcus | Cryptococcus | Cryptococcus |
| Species Confirmation | N.A d | N.A | N.A | N.A | C. gattii VGlll | C. gattii VGl | C. gattii VGl |
a Southern California; b Information not available; c New Jersey, d Culture not available.
We found all three strains to be C. gattii strains of either VGlll or VGl molecular type [26]. This suggested that among the 67 apparently immunocompetent patients whose plasma had been archived, only three had been GM-CSF autoantibody positive and all had been infected by C. gattii while the antibodies were lacking in the plasma of the remaining 64 patients who had been infected by C. neoformans. The three strains originally reported as C. neoformans by Rosen et al. [25] but were not available to confirm their identification were most likely C. gattii instead of C. neoformans. They had originated either from Thailand or Southern California, regions endemic for C. gattii [28,29]. Most clinical laboratories designate the etiologic agents of cryptococcosis as C. neoformans without distinguishing between the two species. This is due to the application of the same therapy for both pathogens [30] and thus separation between the two species has not been widely practiced.
In order to confirm anti-GM-CSF autoantibodies as a higher risk factor for C. gattii CNS infection than for C. neoformans infection, we turned our attention to otherwise healthy C. gattii infected patients in Australia for two reasons. Australia is known for the high incidence of C. gattii infection in healthy host [13,15,23] and unlike the Chinese patients, Australian patients comprise multiple ethnic groups, which would eliminate any particular ethnicity-associated trait in the analysis. Nine plasma samples, eight from C. gattii infected and one from C. neoformans infected otherwise healthy Australian patients with meningoencephalitis were obtained and screened for GM-CSF autoantibodies. As shown in Table 3, six of eight (75%) otherwise healthy C. gattii patients had anti-GM-CSF autoantibodies in their plasma while the antibody was not detected from the immunocompetent patient with C. neoformans infection. These results clearly demonstrated that the GM-CSF neutralizing antibodies predispose healthy hosts to C. gattii infection.
Does this imply that GM-CSF neutralizing antibodies are a risk factor exclusively for infection due to C. gattii but not C. neoformans? Not likely since autoantibodies against GM-CSF have been known as a risk factor for other fungal infections, which are initiated by inhalation. Moreover, we have identified one C. neoformans VNl strain that had been isolated from a GM-CSF autoantibody positive, otherwise healthy cryptococcosis patient (unpublished data). The reason for higher association of GM-CSF autoantibodies with C. gattii infection than with C. neoformans infection is not clear. However, the higher prevalence of C. gattii than C. neoformans infection among patients with anti-GM-CSF autoantibodies could offer some clues to address the major differences in host’s immune responses to these two etiologic agents of cryptococcosis.

Table 3.
Detection of GM-CSF autoantibodies in plasma of Australian immunocompetent otherwise healthy cryptococcosis patients with CNS infection [26].
| Patients | Ethnicity | Etiologic Agent | Anti-GM-CSF Ab | Mol. Types |
|---|---|---|---|---|
| 1 | Caucasian | C. gattii | + | VGl |
| 2 | Caucasian | C. gattii | + | VGl |
| 3 | Caucasian | C. gattii | + | VGl |
| 4 | Aborigine | C. gattii | + | VGl |
| 5 | Caucasian | C. gattii | − | VGl |
| 6 | Asian | C. neoformans | − | NA |
| 7 | Asian | C. gattii | + | VGl |
| 8 | Caucasian | C. gattii | + | VGll |
| 9 | Asian | C. gattii | − | VGl |
3. GM-CSF Neutralizing Antibody and Susceptibility to Cryptococcal Infection
Granulocyte-macrophage colony stimulating factor (GM-CSF) is one of the family of glycoprotein cytokines that mediates the survival, proliferation, differentiation and function of hematopoietic cells [31,32,33]. In human and mice, GM-CSF is involved in terminal differentiation of monocytes to alveolar macrophages [34,35], regulation of neutrophil functions [36], differentiation of dendritic cells [37] and augments innate immunity mostly through PU.1 [38,39] (Figure 1). The importance of GM-CSF in host defense against C. neoformnans has been recognized since the early 1990s when GM-CSF neutralizing antibody increased mortality of C. neoformans infected mice with rapid progression of meningoencephalitis [40]. Corroborating this observation, GM-CSF knockout mice (GM−/− mice) failed to clear the fungus, which led to higher cryptococcal burdens in pulmonary cryptococcosis because GM-CSF was required for early influx of macrophages, CD4 and CD8 cells into the lung [41]. Additionally, GM-CSF enhanced the anticryptococcal activity of human monocytes, neutrophils and macrophages and showed a synergistic effect with azoles suggesting a therapeutic implication for cryptococcosis [42,43].
Furthermore, cryptococcosis is reported more frequently than other fungal disease in patients with acquired pulmonary alveolar proteinosis (PAP) [44]. When autoantibodies block the GM-CSF pathway, differentiation and function of alveolar macrophages are impaired and the ability to clear surfactants is diminished [44,45,46] (Figure 1). As a result, pulmonary alveoli eventually accumulate periodic acid-Schiff (PAS)-positive proteinaceous surfactant components [44,47,48]. This feature is characteristic of acquired pulmonary alveolar proteinosis (PAP) originally termed by Rosen et al., as “idiopathic PAP” [49]. This differentiates the acquired PAP from the primary PAP, which results from mutations in the genes encoding the GM-CSF receptor [50], surfactant protein B or C, and from the secondary PAP. Secondary PAP develops in association with functional impairment or reduced number of alveolar macrophages such as due to some hematologic cancers, inhalation of inorganic dusts or toxic fumes and pharmacologic immunosuppression [51]. While idiopathic PAP was eventually identified as an autoimmune disease caused by GM-CSF autoantibodies [51,52,53], the primary stimulus leading to the production of GM-CSF autoantibodies remains unknown [54,55]. To advance the understanding of PAP pathogenesis, exogenous GM-CSF was administered to patients with acquired PAP, which appeared to benefit patients with therapeutic efficacy [56,57].
Since autoantibodies to GM-CSF cause defects in chemotaxis, adhesion, phagocytosis, microbicidal activity and phagolysosome fusion of alveolar macrophages [51], patients with PAP are at risk for infections from a variety of respiratory microorganisms including fungal species [25,44,58,59]. Among the 27 acquired PAP patients first reported by Rosen in 1958, one (patient 24) had been infected by Cryptococcus. The etiologic agent of cryptococcosis in this patient could have been C. gattii rather than C. neoformans. The patient was working in a lumberyard in Massachusetts when he was diagnosed with cryptococcosis [49]. The patients were likely exposed to high concentrations of wood dust. Massachusetts is not known as a region endemic to C. gattii but the lumberyard could have been handling trees imported from regions where C. gattii is endemic. This case is reminiscent of the first autochthonous case of C. gattii infection in Germany in an apparently immunocompetent healthy male who had been working in sawmills and woodworking factories and exposed to high levels of wood dust, including that of imported tropical trees [60]. This case report had appeared at least five years prior to the first discovery of Eucalyptus trees as the environmental source of C. gattii in Australia [61].
Although less frequently reported than cryptococcosis [25,26,49,62,63,64,65], a literature survey revealed five other fungal pathogens that caused infection in patients with acquired PAP. They are all mycoses controlled by macrophages upon inhalation: four cases due to Aspergillus fumigatus [58,59,66,67,68], one possible Blastomyces dermatitidis case [69], one Coccidioides case [70], one Mucorales case [48] and four cases due to Histoplasma capsulatum [49,71]. GM-CSF is reported as a critical cytokine in the generation of an optimal protective immune response against pulmonary challenge of mice with H. capsulatum [72]. Neutralization of GM-CSF resulted in an increase in fungal burden and higher mortality suggesting that the GM-CSF enhances antimicrobial defenses against intracellular pathogens such as H. capsulatum. The mode of anti-Histoplasma action enhanced by GM-CSF in mice was reported as the sequestration of labile Zn in infected macrophages by inducing metallothioneins in a STAT3 and STAT5 dependent manner [73,74]. Since Zn is a basic element essential for organismal growth, Zn deprived Histoplasma fails to replicate within the macrophages leading to fungal clearance [73,74]. GM-CSF also promoted resistance to Aspergillus conidia by maintaining proinflammatory response by alveolar macrophages [75].
Why do GM-CSF autoantibodies pose a higher risk for C. gattii than C. neoformans infection? Before attempting to address this question, it is necessary to investigate whether anti-GM-CSF autoantibodies are also prevalent in pulmonary C. gattii infection without CNS involvement since evaluation of GM-CSF autoantibodies and cryptococcosis thus far has only focused on patients with CNS infection. Pulmonary infection without CNS involvement is more prevalent with C. gattii than with C. neoformans infection [12,16,76]. If GM-CSF autoantibodies are prevalent only among the C. gattii patients with CNS involvement, it suggests that GM-CSF may play a more important role in dissemination of C. gattii than C. neoformans to the brain from the lung and/or brain immunity against C.gattii than C. neoformans. Little is known about the role of GM-CSF in dissemination of Cryptococci to the brain or brain immunity against the fungus. Our study with GM-CSF−/− mice suggested that a lack of the cytokine function enhances dissemination of both the C. neoformans strain H99 and the C. gattii VG1 strain isolated from the GM-CSF antibody positive Chinese patient [26] from the lung to the brain (unpublished data). However, the effect was significantly greater with the VGI strain compared to H99. Investigation of the immunological mechanism responsible for this difference between dissemination of H99 vs. the VGI strain is currently underway in our laboratory and should shed more light as to the role of GM-CSF in dissemination and brain immunity against C. gattii. Although GM-CSF does not appear to influence brain microglial killing of C. neoformans serotype A strains [77], there has not been any study on killing of C. gattii by immune cells in the brain.
4. Could C. gattii Infection Have Induced Production of GM-CSF Neutralizing Antibodies?
Before GM-CSF autoantibody was known as the cause of PAP, a hypothesis that PAP is an abnormal pulmonary response to an unusual infectious agent such as Pneumocyctis carinii [49,78,79] (now called P. jirovecii) or C. neoformans [80] gained some support [44]. However, the lung lavage fluids of a vast majority of PAP patients have been found to be free of pathogens and it has been recognized that most infectious cases in PAP patients are secondary in origin [44]. Among the first 27 cases of PAP patients reported, there were only two patients with superimposed fungal infections (one case each of histoplasmosis and cryptococcosis), which indicates that production of GM-CSF autoantibodies resulting in PAP was not due to fungal pathogens [49]. In the case of the seven GM-CSF autoantibody positive cryptococcosis patients reported by Rosen and colleagues [25] as well as the seven patients reported by Saijo et al. [26], none of them were diagnosed with PAP at the presentation of cryptococcal CNS infection. Only one patient developed symptomatic PAP two years later and another patient developed radiographic and cytopathologic changes without symptoms [25]. This suggests that the GM-CSF autoantibody positive cryptococcosis patients could eventually develop PAP but that cryptococcosis was not the cause of PAP. This also suggests that PAP symptoms were not necessary for GM-CSF autoantibody-associated C. gattii infection. There is a possibility that pulmonary infection with C. gattii stimulates GM-CSF production more than C. neoformans and the high cytokine production by C. gattii for a long period of time can cause induction of functional antibodies against the cytokine. Could C. gattii also exacerbate the production of GM-CSF autoantibodies more than C. neoformans in the patients who already are antibody positive? This question cannot be answered since the anti-GM-CSF antibody titers in these patients prior to C. gattii infection are unknown and there are no longitudinal studies on the GM-CSF autoantibody titers during the infection in such patients. Monitoring the health status and the GM-CSF autoantibody titers over time in the healthy Chinese volunteer positive for the cytokine antibodies [26] could offer valuable information concerning this question.
5. Conclusions
Anti-GM-CSF autoantibodies are apparently an important underlying risk factor for meningoencephalitis caused by Cryptococcus in otherwise healthy individuals. For some unknown reason, the neutralizing antibodies against GM-CSF appear to be a greater risk for cryptococcosis due to C. gattii than C. neoformans. This etiologic agent’s difference in the prevalence of GM-CSF neutralizing antibodies among otherwise healthy cryptococcosis patients may offer an important clue in order to address the differences in the host’s immunological response to the two pathogens. It is important to also include the GM-CSF autoantibody test during immunological profiling of cryptococcosis patients who appear to be otherwise healthy. This is not only for the assessment of subtle underlying immune disorders but also for better clinical management for those positive for GM-CSF antibodies since they may eventually develop PAP after meningoencephalitis.
Acknowledgments
This work was supported by funds from the intramural program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. We thank Mike Davis for his help in composing Figure 1.
Author Contributions
Both authors are responsible for writing the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kavanaugh, L.A.; Fraser, J.A.; Dietrich, F.S. Recent evolution of the human pathogen Cryptococcus neoformans by intervarietal transfer of a 14-gene fragment. Mol. Biol. Evol. 2006, 23, 1879–1890. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Bennett, J.E. Medical Mycology; Lea & Febiger: Philadelphia, PA, USA, 1992. [Google Scholar]
- Casadevall, A.; Perfect, J.R. Cryptococcus neoformans; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Dromer, F.; Mathoulin, S.; Dupont, B.; Laporte, A. Epidemiology of cryptococcosis in France: A 9-year survey (1985–1993). French Cryptococcosis Study Group. Clin. Infect. Dis. 1996, 23, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Varma, A.; Diaz, M.R.; Litvintseva, A.P.; Wollenberg, K.K.; Kwon-Chung, K.J. Cryptococcus neoformans strains and infection in apparently immunocompetent patients, China. Emerg. Infect. Dis. 2008, 14, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Ngamskulrungroj, P.; Varma, A.; Sionov, E.; Hwang, S.M.; Carriconde, F.; Meyer, W.; Litvintseva, A.P.; Lee, W.G.; Shin, J.H.; et al. Prevalence of the VNIc genotype of Cryptococcus neoformans in non-HIV-associated cryptococcosis in the Republic of Korea. FEMS Yeast Res. 2010, 10, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Mihara, T.; Izumikawa, K.; Kakeya, H.; Ngamskulrungroj, P.; Umeyama, T.; Takazono, T.; Tashiro, M.; Nakamura, S.; Imamura, Y.; Miyazaki, T.; et al. Multilocus sequence typing of Cryptococcus neoformans in non-HIV associated cryptococcosis in Nagasaki, Japan. Med. Mycol. 2013, 51, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; McCarthy, K.M.; Gould, S.; Fan, K.; Arthington-Skaggs, B.; Iqbal, N.; Stamey, K.; Hajjeh, R.A.; Brandt, M.E. Cryptococcus gattii infection: Characteristics and epidemiology of cases identified in a South African province with high HIV seroprevalence, 2002–2004. Clin. Infect. Dis. 2006, 43, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Lizarazo, J.; Escandon, P.; Agudelo, C.I.; Firacative, C.; Meyer, W.; Castaneda, E. Retrospective study of the epidemiology and clinical manifestations of Cryptococcus gattii infections in Colombia from 1997–2011. PLoS Negl. Trop. Dis. 2014, 8, e3272. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Dyavaiah, M.; Larsen, R.A.; Chaturvedi, V. Cryptococcus gattii in AIDS patients, southern California. Emerg. Infect. Dis. 2005, 11, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Speed, B.; Dunt, D. Clinical and host differences between infections with the two varieties of Cryptococcus neoformans. Clin. Infect. Dis. 1995, 21, 28–34, discussion 35–26. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, T.C.; Chen, S.C.A.; Phillips, P.; Marr, K.A. Clinical perspectives on Cryptococcus neoformans and Cryptococcus gattii: Implications for diagnosis and management. In Cryptococcus: From Human Pathogen to Model Yeast; Heitman, J., Kozel, T.R., Kwon-Chung, K.J., Perfect, J.R., Casadevall, A., Eds.; ASM Press: Washington, DC, USA, 2011; pp. 595–606. [Google Scholar]
- Chen, S.C.; Slavin, M.A.; Heath, C.H.; Playford, E.G.; Byth, K.; Marriott, D.; Kidd, S.E.; Bak, N.; Currie, B.; Hajkowicz, K.; et al. Clinical manifestations of Cryptococcus gattii infection: Determinants of neurological sequelae and death. Clin. Infect. Dis. 2012, 55, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Seaton, R.A.; Naraqi, S.; Wembri, J.P.; Warrell, D.A. Predictors of outcome in Cryptococcus neoformans var. gattii meningitis. QJM 1996, 89, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.H.; Sorrell, T.C.; Allworth, A.M.; Heath, C.H.; McGregor, A.R.; Papanaoum, K.; Richards, M.J.; Gottlieb, T. Cryptococcal disease of the CNS in immunocompetent hosts: Influence of cryptococcal variety on clinical manifestations and outcome. Clin. Infect. Dis. 1995, 20, 611–616. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, L.; Fyfe, M.; Romney, M.; Starr, M.; Galanis, E. Risk factors for Cryptococcus gattii infection, British Columbia, Canada. Emerg. Infect. Dis. 2011, 17, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Harrison, T.S. HIV-associated cryptococcal meningitis. AIDS 2007, 21, 2119–2129. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, J.N.; Casazza, J.P.; Stone, H.H.; Meintjes, G.; Lawn, S.D.; Levitz, S.M.; Harrison, T.S.; Koup, R.A. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J. Infect. Dis. 2013, 207, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Kwon-Chung, K.J.; Polacheck, I.; Bennett, J.E. Improved diagnostic medium for separation of Cryptococcus neoformans var. neoformans (serotypes A and D) and Cryptococcus neoformans var. gattii (serotypes B and C). J. Clin. Microbiol. 1982, 15, 535–537. [Google Scholar]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.L.; Wang, Z.; Janbon, G.; Idnurm, A.; Bahn, Y.S. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. In Human Fungal Pathogens; Casadevall, A., Mitchell, A.P., Berman, J., Kwon-Chung, K.J., Perfect, J.R., Heitman, J., Eds.; Cold Spring Harbor Press: New York, NY, USA, 2015; pp. 385–411. [Google Scholar]
- Chang, Y.C. Differences between Cryptococcus neoformans and Cryptococcus gattii in the molecular mechanisms governing utilization of D-amino acids as the sole nitrogen source. PLoS ONE 2015, e0131865. [Google Scholar] [CrossRef] [PubMed]
- Ngamskulrungroj, P.; Chang, Y.; Sionov, E.; Kwon-Chung, K.J. The primary target organ of Cryptococcus gattii is different from that of Cryptococcus neoformans in a murine model. MBio 2012, 3, e103–e112. [Google Scholar] [CrossRef] [PubMed]
- Sorrell, T.C. Cryptococcus neoformans variety gattii. Med. Mycol. 2001, 39, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Galanis, E.; Macdougall, L.; Kidd, S.; Morshed, M. Epidemiology of Cryptococcus gattii, British Columbia, Canada, 1999–2007. Emerg. Infect. Dis. 2010, 16, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Rosen, L.B.; Freeman, A.F.; Yang, L.M.; Jutivorakool, K.; Olivier, K.N.; Angkasekwinai, N.; Suputtamongkol, Y.; Bennett, J.E.; Pyrgos, V.; Williamson, P.R.; et al. Anti-GM-CSF autoantibodies in patients with cryptococcal meningitis. J. Immunol. 2013, 190, 3959–3966. [Google Scholar] [CrossRef] [PubMed]
- Saijo, T.; Chen, J.; Chen, S.C.; Rosen, L.B.; Yi, J.; Sorrell, T.C.; Bennett, J.E.; Holland, S.M.; Browne, S.K.; Kwon-Chung, K.J. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. MBio 2014, 5, e912–e914. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Gilgado, F.; Ngamskulrungroj, P.; Trilles, L.; Hagen, F.; Castaneda, E.; Boekhout, T. Molecular typing of the Cryptococcus neoformans/Cryptococcus gattii species complex. In Cryptococcus: From Human Pathogen to Model Yeast; ASM Press: Washington, DC, USA, 2011; pp. 327–357. [Google Scholar]
- Kwon-Chung, K.J.; Bennett, J.E. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am. J. Epidemiol. 1984, 120, 123–130. [Google Scholar] [PubMed]
- Kwon-Chung, K.J.; Bennett, J.E. High prevalence of Cryptococcus neoformans var. gattii in tropical and subtropical regions. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 1984, 257, 213–218. [Google Scholar] [PubMed]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.H.; et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of america. Clin. Infect. Dis. 2010, 50, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Gasson, J.C. Molecular physiology of granulocyte-macrophage colony-stimulating factor. Blood 1991, 77, 1131–1145. [Google Scholar] [PubMed]
- Stanley, E.; Lieschke, G.J.; Grail, D.; Metcalf, D.; Hodgson, G.; Gall, J.A.; Maher, D.W.; Cebon, J.; Sinickas, V.; Dunn, A.R. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl Acad. Sci. USA 1994, 91, 5592–5596. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, D. Control of granulocytes and macrophages: Molecular, cellular, and clinical aspects. Science 1991, 254, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Akagawa, K.S.; Kamoshita, K.; Tokunaga, T. Effects of granulocyte-macrophage colony-stimulating factor and colony-stimulating factor-1 on the proliferation and differentiation of murine alveolar macrophages. J. Immunol. 1988, 141, 3383–3390. [Google Scholar] [PubMed]
- Chen, B.D.; Mueller, M.; Chou, T.H. Role of granulocyte/macrophage colony-stimulating factor in the regulation of murine alveolar macrophage proliferation and differentiation. J. Immunol. 1988, 141, 139–144. [Google Scholar] [PubMed]
- Uchida, K.; Beck, D.C.; Yamamoto, T.; Berclaz, P.Y.; Abe, S.; Staudt, M.K.; Carey, B.C.; Filippi, M.D.; Wert, S.E.; Denson, L.A.; et al. GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N. Engl. J. Med. 2007, 356, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Van de Laar, L.; Coffer, P.J.; Woltman, A.M. Regulation of dendritic cell development by GM-CSF: Molecular control and implications for immune homeostasis and therapy. Blood 2012, 119, 3383–3393. [Google Scholar]
- Bonfield, T.L.; Raychaudhuri, B.; Malur, A.; Abraham, S.; Trapnell, B.C.; Kavuru, M.S.; Thomassen, M.J. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L1132–L1136. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Berclaz, P.Y.; Chroneos, Z.C.; Yoshida, M.; Whitsett, J.A.; Trapnell, B.C. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001, 15, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Collins, H.L.; Bancroft, G.J. Cytokine enhancement of complement-dependent phagocytosis by macrophages: Synergy of tumor necrosis factor-alpha and granulocyte-macrophage colony-stimulating factor for phagocytosis of Cryptococcus neoformans. Eur. J. Immunol. 1992, 22, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Olszewski, M.A.; McDonald, R.A.; Wells, J.C.; Paine, R.; Huffnagle, G.B.; Toews, G.B. Role of granulocyte macrophage colony-stimulating factor in host defense against pulmonary Cryptococcus neoformans infection during murine allergic bronchopulmonary mycosis. Am. J. Pathol. 2007, 170, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Chiller, T.; Farrokhshad, K.; Brummer, E.; Stevens, D.A. Effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor on polymorphonuclear neutrophils, monocytes or monocyte-derived macrophages combined with voriconazole against Cryptococcus neoformans. Med. Mycol. 2002, 40, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Tascini, C.; Vecchiarelli, A.; Preziosi, R.; Francisci, D.; Bistoni, F.; Baldelli, F. Granulocyte-macrophage colony-stimulating factor and fluconazole enhance anti-cryptococcal activity of monocytes from AIDS patients. AIDS 1999, 13, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Presneill, J.J. Pulmonary alveolar proteinosis: Progress in the first 44 years. Am. J. Respir. Crit. Care Med. 2002, 166, 215–235. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Katyal, S.L.; Bedrossian, C.W.; Rogers, R.M. Pulmonary alveolar proteinosis. Staining for surfactant apoprotein in alveolar proteinosis and in conditions simulating it. Chest 1983, 83, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, B.C.; Whitsett, J.A. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu. Rev. Physiol. 2002, 64, 775–802. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, B.C.; Carey, B.C.; Uchida, K.; Suzuki, T. Pulmonary alveolar proteinosis, a primary immunodeficiency of impaired GM-CSF stimulation of macrophages. Curr. Opin. Immunol. 2009, 21, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.M.; Macleod, W.M. Pulmonary alveolar proteinosis. Br. J. Dis. Chest 1969, 63, 13–28. [Google Scholar] [CrossRef]
- Rosen, S.H.; Castleman, B.; Liebow, A.A. Pulmonary alveolar proteinosis. N. Engl. J. Med. 1958, 258, 1123–1142. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.; Trapnell, B.C. The molecular basis of pulmonary alveolar proteinosis. Clin. Immunol. 2010, 135, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, B.C.; Whitsett, J.A.; Nakata, K. Pulmonary alveolar proteinosis. N. Engl. J. Med. 2003, 349, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Watanabe, J.; Kitamura, T.; Yamada, Y.; Kanegasaki, S.; Nakata, K. Lungs of patients with idiopathic pulmonary alveolar proteinosis express a factor which neutralizes granulocyte-macrophage colony stimulating factor. FEBS Lett. 1999, 442, 246–250. [Google Scholar] [CrossRef]
- Kitamura, T.; Tanaka, N.; Watanabe, J.; Uchida; Kanegasaki, S.; Yamada, Y.; Nakata, K. Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1999, 190, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.L.; Hansell, D.; Lawson, P.R.; Reid, K.B.; Morgan, C. Pulmonary alveolar proteinosis: Clinical aspects and current concepts on pathogenesis. Thorax 2000, 55, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Thomson, C.A.; Allan, L.L.; Jackson, L.M.; Olson, M.; Hercus, T.R.; Nero, T.L.; Turner, A.; Parker, M.W.; Lopez, A.L.; et al. Characterization of pathogenic human monoclonal autoantibodies against GM-CSF. Proc. Natl. Acad. Sci. USA 2013, 110, 7832–7837. [Google Scholar] [CrossRef] [PubMed]
- Kavuru, M.S.; Sullivan, E.J.; Piccin, R.; Thomassen, M.J.; Stoller, J.K. Exogenous granulocyte-macrophage colony-stimulating factor administration for pulmonary alveolar proteinosis. Am. J. Respir. Crit. Care Med. 2000, 161, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.F.; Presneill, J.J.; Schoch, O.D.; Downie, G.H.; Moore, P.E.; Doyle, I.R.; Vincent, J.M.; Nakata, K.; Kitamura, T.; Langton, D.; et al. Therapeutic efficacy of granulocyte-macrophage colony-stimulating factor in patients with idiopathic acquired alveolar proteinosis. Am. J. Respir. Crit. Care Med. 2001, 163, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Prakash, U.B.S.; Barham, S.S.; Carpenter, H.A.; Dines, D.E.; Marsh, H.M. Pulmonary alveolar phospholipoproteinosis: Experience with 34 cases and a review. Mayo Clin. Proc. 1987, 62, 499–518. [Google Scholar] [CrossRef]
- Bedrossian, C.W.; Luna, M.A.; Conklin, R.H.; Miller, W.C. Alveolar proteinosis as a consequence of immunosuppression. A hypothesis based on clinical and pathologic observations. Hum. Pathol. 1980, 11, 527–535. [Google Scholar] [PubMed]
- Kohl, K.H.; Hof, H.; Schrettenbrunner, A.; Seeliger, H.P.; Kwon-Chung, K.J. Cryptococcus neoformans var. gattii in Europe. Lancet 1985, 1, 1515. [Google Scholar] [CrossRef]
- Ellis, D.H.; Pfeiffer, T.J. Natural habitat of Cryptococcus neoformans var. gattii. J. Clin. Microbiol. 1990, 28, 1642–1644. [Google Scholar] [PubMed]
- Linell, F.; Magnusson, B.; Norden, A. Cryptococcosis; review and report of a case. Acta Derm. Venereol. 1953, 33, 103–122. [Google Scholar] [PubMed]
- Sunderland, W.A.; Campbell, R.A.; Edwards, M.J. Pulmonary alveolar proteinosis and pulmonary cryptococcosis in an adolescent boy. J. Pediatr. 1972, 80, 450–456. [Google Scholar] [CrossRef]
- McCook, T.A.; Kirks, D.R.; Merten, D.F.; Osborne, D.R.; Spock, A.; Pratt, P.C. Pulmonary alveolar proteinosis in children. Am. J. Roentgenol. 1981, 137, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Chew, G.T.; Robinson, B.W. Pulmonary and meningeal cryptococcosis in pulmonary alveolar proteinosis. Aust. N. Z. J. Med. 1999, 29, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Bjorkholm, B.; Elgefors, B. Cerebellar aspergilloma. Scand. J. Infect. Dis. 1986, 18, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Von Egidy, H.; Bassler, R.; Tilling, W. Contribution to the alveolar proteinosis of the lungs. Beitr. Klin. Erforsch. Tuberk. Lungenkr. 1967, 134, 365–380. [Google Scholar]
- Groniowski, J.; Walski, M.; Szymanska, D. Electron microscopic observations on pulmonary alveolar lipoproteinosis. Ann. Med. Sect. Pol. Acad. Sci. 1974, 19, 109–110. [Google Scholar] [PubMed]
- Kellar, S.L.; Harshfield, D.L.; Grigg, K.G. Radiological case of the month. Pulmonary alveolar proteinosis. J. Ark. Med. Soc. 1995, 92, 307–308. [Google Scholar] [PubMed]
- Herger, P.C. A case study: Anesthetic considerations for pulmonary lavage. AANA J. 1975, 43, 398–400. [Google Scholar] [PubMed]
- Hartung, M.; Salfelder, K. Pulmonary alveolar proteinosis and histoplasmosis: Report of three cases. Virchows Arch. A Pathol. Anat. Histol. 1975, 368, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Deepe, G.S., Jr.; Gibbons, R.; Woodward, E. Neutralization of endogenous granulocyte-macrophage colony-stimulating factor subverts the protective immune response to Histoplasma capsulatum. J. Immunol. 1999, 163, 4985–4993. [Google Scholar] [PubMed]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S., Jr. Zinc sequestration: Arming phagocyte defense against fungal attack. PLoS Pathog. 2013, 9, e1003815. [Google Scholar]
- Subramanian Vignesh, K.; Landero Figueroa, J.A.; Porollo, A.; Caruso, J.A.; Deepe, G.S., Jr. Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 2013, 39, 697–710. [Google Scholar]
- Brummer, E.; Kamberi, M.; Stevens, D.A. Regulation by granulocyte-macrophage colony-stimulating factor and/or steroids given in vivo of proinflammatory cytokine and chemokine production by bronchoalveolar macrophages in response to Aspergillus conidia. J. Infect. Dis. 2003, 187, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.; Galanis, E.; MacDougall, L.; Chong, M.Y.; Balshaw, R.; Cook, V.J.; Bowie, W.; Steiner, T.; Hoang, L.; Morshed, M.; et al. Longitudinal clinical findings and outcome among patients with Cryptococcus gattii infection in British Columbia. Clin. Infect. Dis. 2015, 60, 1368–1376. [Google Scholar] [PubMed]
- Lipovsky, M.M.; Juliana, A.E.; Gekker, G.; Hu, S.; Hoepelman, A.I.; Peterson, P.K. Effect of cytokines on anticryptococcal activity of human microglial cells. Clin. Diagn. Lab. Immunol. 1998, 5, 410–411. [Google Scholar] [PubMed]
- De Sanctis, P.N. Pulmonary alveolar proteinosis. A review of the findings and theories to date, with a digression on Pneumocystis carinii pneumonia. BMQ 1962, 13, 19–35. [Google Scholar]
- Plenk, H.P.; Swift, S.A.; Chambers, W.L.; Peltzer, W.E. Pulmonary alveolar proteinosis-a new disease? Radiology 1960, 74, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Bergman, F.; Linell, F. Cryptococcosis as a cause of pulmonary alveolar proteinosis. Acta Pathol. Microbiol. Scand. 1961, 53, 217–224. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
