Changes in Serum IgG Glycosylation Patterns for Abdominal Aortic Aneurysm Patients
Abstract
1. Introduction
2. Methods
2.1. Patients and Samples
2.2. Lectin Microarray Analysis
2.3. Lectin Blot Verification
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Lectin Microarray Results
3.3. Lectin Blot Analysis for AAA Patients
3.4. Significant Clinical Relevance of Lectins
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davis, F.M.; Daugherty, A.; Lu, H.S. Updates of Recent Aortic Aneurysm Research. Arter. Thromb. Vasc. Biol. 2019, 39, e83–e90. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J. Abdominal aortic aneurysm: Update on pathogenesis and medical treatments. Nat. Rev. Cardiol. 2018, 16, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Quintana, R.A.; Taylor, W.R. Cellular Mechanisms of Aortic Aneurysm Formation. Circ. Res. 2019, 124, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef]
- Stather, P.W.; Sidloff, D.A.; Dattani, N.; Gokani, V.J.; Choke, E.; Sayers, R.D.; Bown, M. Meta-analysis and meta-regression analysis of biomarkers for abdominal aortic aneurysm. Br. J. Surg. 2014, 101, 1358–1372. [Google Scholar] [CrossRef]
- Tilson, M.D. Decline of the atherogenic theory of the etiology of the abdominal aortic aneurysm and rise of the autoimmune hypothesis. J. Vasc. Surg. 2016, 64, 1523–1525. [Google Scholar] [CrossRef][Green Version]
- Furusho, A.; Aoki, H.; Ohno-Urabe, S.; Nishihara, M.; Hirakata, S.; Nishida, N.; Ito, S.; Hayashi, M.; Imaizumi, T.; Hiromatsu, S.; et al. Involvement of B Cells, Immunoglobulins, and Syk in the Pathogenesis of Abdominal Aortic Aneurysm. J. Am. Heart Assoc. 2018, 7, e007750. [Google Scholar] [CrossRef]
- Ando, T.; Iizuka, N.; Sato, T.; Chikada, M.; Kurokawa, M.S.; Arito, M.; Okamoto, K.; Suematsu, N.; Makuuchi, H.; Kato, T. Autoantigenicity of carbonic anhydrase 1 in patients with abdominal aortic aneurysm, revealed by proteomic surveillance. Hum. Immunol. 2013, 74, 852–857. [Google Scholar] [CrossRef]
- Li, H.; Bai, S.; Ao, Q.; Wang, X.; Tian, X.; Li, X.; Tong, H.; Hou, W.; Fan, J. Modulation of Immune-Inflammatory Responses in Abdominal Aortic Aneurysm: Emerging Molecular Targets. J. Immunol. Res. 2018, 2018, 7213760. [Google Scholar] [CrossRef]
- Quast, I.; Peschke, B.; Lünemann, J.D. Regulation of antibody effector functions through IgG Fc N-glycosylation. Cell. Mol. Life Sci. 2017, 74, 837–847. [Google Scholar] [CrossRef]
- Plomp, R.; Dekkers, G.; Rombouts, Y.; Visser, R.; Koeleman, C.A.; Kammeijer, G.S.; Jansen, B.C.; Rispens, T.; Hensbergen, P.J.; Vidarsson, G.; et al. Hinge-Region O-Glycosylation of Human Immunoglobulin G3 (IgG3). Mol. Cell. Proteom. 2015, 14, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Yamada, M.; Kuno, A.; Tateno, H. Lectin microarrays: Concept, principle and applications. Chem. Soc. Rev. 2013, 42, 4443–4458. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Kuno, A.; Tateno, H. Development and Applications of the Lectin Microarray. SialoGlyco Chem. Biol. II 2014, 367, 105–124. [Google Scholar] [CrossRef]
- Dang, K.; Zhang, W.; Jiang, S.; Lin, X.; Qian, A. Application of Lectin Microarrays for Biomarker Discovery. ChemistryOpen 2020, 9, 285–300. [Google Scholar] [CrossRef]
- Erbel, R.; Aboyans, V.; Boileaul, C.; Bossone, E.; Bartolomeo, R.D.; Eggebrecht, H.; Evangelista, A.; Falk, V.; Frank, H.; Gaemperli, O.; et al. ESC Guidelines on the diagnosis and treatment of aortic diseases: Document Covering Acute and Chronic Aortic Diseases of the Thoracic and Abdominal Aorta of the Adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2873–2926. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, L.; Gu, Y.; Xin, A.; Wu, B.; Zhou, S.; Guo, S.; Liu, Y.; Diao, H.; Shi, H.; et al. A Human Lectin Microarray for Sperm Surface Glycosylation Analysis. Mol. Cell. Proteom. 2016, 15, 2839–2851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, S.; Tang, S.; Li, X.; Zhang, G.; Li, M.; Zeng, X.; Hu, C. Changes of Serum IgG Glycosylation Patterns in Primary Biliary Cholangitis Patients. Front. Immunol. 2021, 12, 669137. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y. B lymphocytes in abdominal aortic aneurysms. Atherosclerosis 2015, 242, 311–317. [Google Scholar] [CrossRef]
- Russell, A.; Adua, E.; Ugrina, I.; Laws, S.; Wang, W. Unravelling Immunoglobulin G Fc N-Glycosylation: A Dynamic Marker Potentiating Predictive, Preventive and Personalised Medicine. Int. J. Mol. Sci. 2018, 19, 390. [Google Scholar] [CrossRef]
- De Haan, N.; Falck, D.; Wuhrer, M. Monitoring of immunoglobulin N- and O-glycosylation in health and disease. Glycobiology 2019, 30, 226–240. [Google Scholar] [CrossRef]
- Chu, T.H.; Patz, E.F., Jr.; Ackerman, M.E. Coming together at the hinges: Therapeutic prospects of IgG3. mAbs 2021, 13, 1882028. [Google Scholar] [CrossRef] [PubMed]
- Stümer, J.; Biermann, M.H.C.; Knopf, J.; Magorivska, I.; Kastbom, A.; Svärd, A.; Janko, C.; Bilyy, R.; Schett, G.; Sjöwall, C.; et al. Altered glycan accessibility on native immunoglobulin G complexes in early rheumatoid arthritis and its changes during therapy. Clin. Exp. Immunol. 2017, 189, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Rokosh, R.S.; Wu, W.W.; Schermerhorn, M.; Chaikof, E.L. Society for Vascular Surgery implementation of clinical practice guidelines for patients with an abdominal aortic aneurysm: Postoperative surveillance after abdominal aortic aneurysm repair. J. Vasc. Surg. 2021, 74, 1438–1439. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Uh, H.-W.; Beekman, M.; Koeleman, C.A.M.; Hokke, C.H.; Westendorp, R.G.J.; Wuhrer, M.; Houwing-Duistermaat, J.J.; Slagboom, P.E.; Deelder, A.M. Decreased Levels of Bisecting GlcNAc Glycoforms of IgG Are Associated with Human Longevity. PLoS ONE 2010, 5, e12566. [Google Scholar] [CrossRef]
- Menni, C.; Gudelj, I.; Macdonald-Dunlop, E.; Mangino, M.; Zierer, J.; Bešić, E.; Joshi, P.K.; Trbojević-Akmačić, I.; Chowienczyk, P.J.; Spector, T.D.; et al. Glycosylation Profile of Immunoglobulin G Is Cross-Sectionally Associated with Cardiovascular Disease Risk Score and Subclinical Atherosclerosis in Two Independent Cohorts. Circ. Res. 2018, 122, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Tibuakuu, M.; Fashanu, O.E.; Zhao, D.; Otvos, J.D.; Brown, T.T.; Haberlen, S.A.; Guallar, E.; Budoff, M.J.; Palella, F.J.; Martinson, J.J.; et al. GlycA, a novel inflammatory marker, is associated with subclinical coronary disease. AIDS 2019, 33, 547–557. [Google Scholar] [CrossRef]
- Dashti, H.; Porras, M.A.P.; Mora, S. Glycosylation and Cardiovascular Diseases. Adv. Exp. Med. Biol. 2021, 325, 307–319. [Google Scholar] [CrossRef]
- Lu, J.-P.; Knežević, A.; Wang, Y.-X.; Rudan, I.; Campbell, H.; Zou, Z.-K.; Lan, J.; Lai, Q.-X.; Wu, J.-J.; He, Y.; et al. Screening Novel Biomarkers for Metabolic Syndrome by Profiling Human Plasma N-Glycans in Chinese Han and Croatian Populations. J. Proteome Res. 2011, 10, 4959–4969. [Google Scholar] [CrossRef]
- Marvar, P.J.; Thabet, S.R.; Guzik, T.J.; Lob, H.E.; McCann, L.A.; Weyand, C.; Gordon, F.J.; Harrison, D.G. Central and Peripheral Mechanisms of T-Lymphocyte Activation and Vascular Inflammation Produced by Angiotensin II–Induced Hypertension. Circ. Res. 2010, 107, 263–270. [Google Scholar] [CrossRef]



| No. (%) or Mean ± SD | AAA (n = 75) | TA (n = 22) | RA (n = 22) | APS (n = 24) | HC (n = 100) |
|---|---|---|---|---|---|
| Male, n% | 61 (81.3) | 18 (81.8) | 18 (81.8) | 20 (83.3) | 81 (81) |
| Age (years) | 69.9 ± 8.6 | 34.5 ± 8.1 | 61.0 ± 10.9 | 37.4 ± 12.2 | 66.5 ± 6.8 |
| Hypertension, n% | 60 (80) | 10 (45.5) | 59 (59) | 4 (16.7) | 59 (59) |
| Dyslipidemia, n% | 42 (56) | 0 | 3 (13.6) | 0 | 48 (48) |
| Diabetes, n% | 10 (13.3) | 0 | 4 (18.2) | 2 (8.3) | 22 (22) |
| CAD, n% | 29 (38.7) | 0 | 2 (9.1) | 3 (12.5) | NA |
| Stroke, n% | 11 (14.7) | 2 (9.1) | 0 | 5 (20.8) | NA |
| Ever smoked, n% | 42 (56) | 2 (9.1) | 10 (45.5) | 11 (45.8) | NA |
| Total glycerol (mmol/L) | 1.5 ± 0.8 | 0.7 ± 0.3 | NA | 5.3 ± 0.9 | 1.5 ± 0.8 |
| LDL cholesterol (mmol/L) | 2.6 ± 1.0 | 2.3 ± 0.9 | NA | 3.5 ± 0.6 | 3.0 ± 3.1 |
| HDL cholesterol (mmol/L) | 1.0 ± 0.2 | 1.2 ± 0.4 | NA | NA | 1.4 ± 0.6 |
| Total cholesterol (mmol/L) | 4.2 ± 1.2 | 4.1 ± 0.8 | NA | 2.1 ± 0.9 | 4.6 ± 1.1 |
| HCY (μmol/L) | 17.4 ± 8.2 | 13.8 ± 0.5 | NA | NA | 14.4 ± 3.2 |
| hsCRP (mg/L) | 6.1 ± 19.3 | 19.4 ± 38.2 | 12.0 ± 21.2 | 3.9 ± 7.2 | 1.6 ± 3.6 |
| ESR (mm/h) | 17.2 ± 15.4 | 21.5 ± 32.1 | 25.6 ± 24.3 | 14.3 ± 22.4 | NA |
| Blood creatinine (μmol/L) | 89.2 ± 33.3 | 79.8 ± 25.8 | 66.2 ± 14.1 | NA | 82.8 ± 21.4 |
| Blood uric acid (μmol/L) | 378.4 ± 80.9 | 307.9 ± 94.5 | NA | NA | 353.6 ± 84.8 |
| FBG (mmol/L) | 5.4 ± 1.3 | 4.9 ± 0.4 | NA | NA | 6.1 ± 1.7 |
| HbA1c, % | 6.1 ± 1.2 | NA | NA | NA | 5.9 ± 0.9 |
| Medication, n% | |||||
| Statin | 36 (48) | NA | NA | NA | NA |
| Antiplatelet | 56 (74.7) | NA | NA | NA | NA |
| Antihypertensive | 37 (49.3) | NA | NA | NA | NA |
| No. (%) or Mean ± SD | All (n = 75) | Hypertension (n = 60) | Dyslipidemia (n = 42) | DM (n = 10) |
|---|---|---|---|---|
| Diameter, mm | 52.5 ± 13.7 | 52.2 ± 13.3 | 50.4 ± 12.6 | 49.2 ± 10.1 |
| Aneurysm diameter >5 cm, n% | 42 (56) | 35 (58.3) | 19 (45.2) | 5 (50) |
| ILT, n% | 52 (69.3) | 43 (71.7) | 26 (61.9) | 7 (70) |
| Symptom | 21 (28) | 15 (25) | 14 (33.3) | 4 (40) |
| Iliac aneurysm, n% | 33 (44) | 27 (45) | 15 (35.7) | 3 (30) |
| HbA1c, % | 6.1 ± 1.2 | 6.1 ± 1.3 | 6.1 ± 0.8 | 6.9 ± 1.8 |
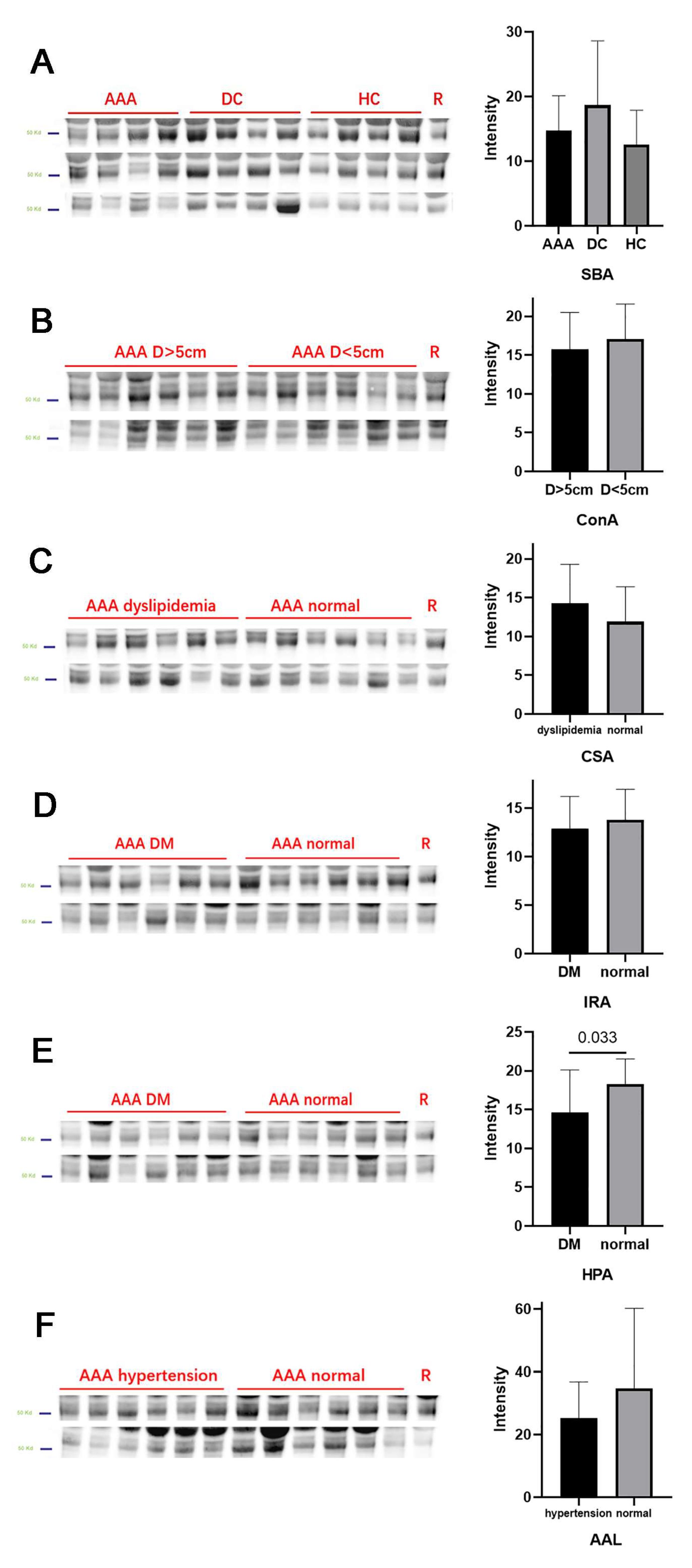
| Groups with Significant Change | Lectin Full Name | Monosaccharide Specificity | |
|---|---|---|---|
| SBA | AAA vs. DC/HC, decrease | Soybean agglutinin | GalNAc |
| Con A | AAA > 5 cm vs. <5 cm, decrease | Concanavalin A | Mannose |
| CSA | AAA dyslipidemia vs. normal, increase | Cytisus sscoparius agglutinin | GalNAc |
| IRA | AAA DM vs. normal, decrease | Iris hybrid agglutinin | GalNAc |
| HPA | AAA DM vs. normal, decrease | Helix pomatia agglutinin | GalNAc |
| AAL | AAA hypertension vs. normal, decrease | Aleuria aurantia lectin | Fucose |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Meng, J.; Lv, Y.; Wang, Q.; Tian, X.; Li, M.; Zeng, X.; Hu, C.; Zheng, Y. Changes in Serum IgG Glycosylation Patterns for Abdominal Aortic Aneurysm Patients. J. Cardiovasc. Dev. Dis. 2022, 9, 291. https://doi.org/10.3390/jcdd9090291
Li S, Meng J, Lv Y, Wang Q, Tian X, Li M, Zeng X, Hu C, Zheng Y. Changes in Serum IgG Glycosylation Patterns for Abdominal Aortic Aneurysm Patients. Journal of Cardiovascular Development and Disease. 2022; 9(9):291. https://doi.org/10.3390/jcdd9090291
Chicago/Turabian StyleLi, Siting, Jingjing Meng, Yanze Lv, Qian Wang, Xinping Tian, Mengtao Li, Xiaofeng Zeng, Chaojun Hu, and Yuehong Zheng. 2022. "Changes in Serum IgG Glycosylation Patterns for Abdominal Aortic Aneurysm Patients" Journal of Cardiovascular Development and Disease 9, no. 9: 291. https://doi.org/10.3390/jcdd9090291
APA StyleLi, S., Meng, J., Lv, Y., Wang, Q., Tian, X., Li, M., Zeng, X., Hu, C., & Zheng, Y. (2022). Changes in Serum IgG Glycosylation Patterns for Abdominal Aortic Aneurysm Patients. Journal of Cardiovascular Development and Disease, 9(9), 291. https://doi.org/10.3390/jcdd9090291







