Independent Association of Thyroid Dysfunction and Inflammation Predicts Adverse Events in Patients with Heart Failure via Promoting Cell Death
Abstract
:1. Introduction
2. Materials and Methods
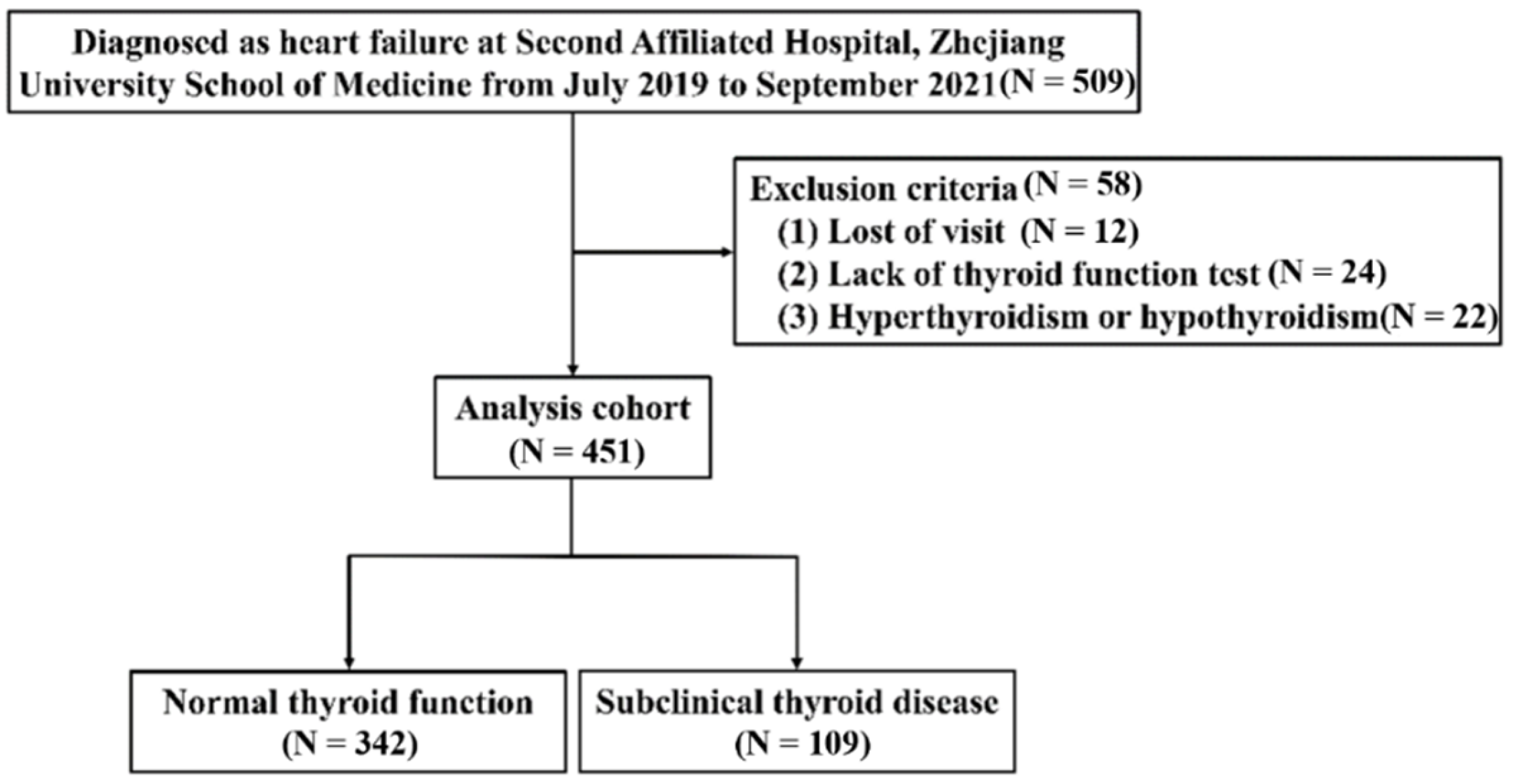
2.1. Study Population
2.2. Thyroid Hormone Sampling and Subgroups of Thyroid Status Definition
2.3. Data Collection and Clinical Outcome
2.4. Cell Culture
2.5. Cell Apoptosis Assay and Apoptosis Detection by Western Blot
2.6. Statistical Analysis
3. Results
3.1. Study Subjects and Baseline Characteristics
3.2. Correlation of Thyroid Function with Cardiac Parameters and Inflammation Values
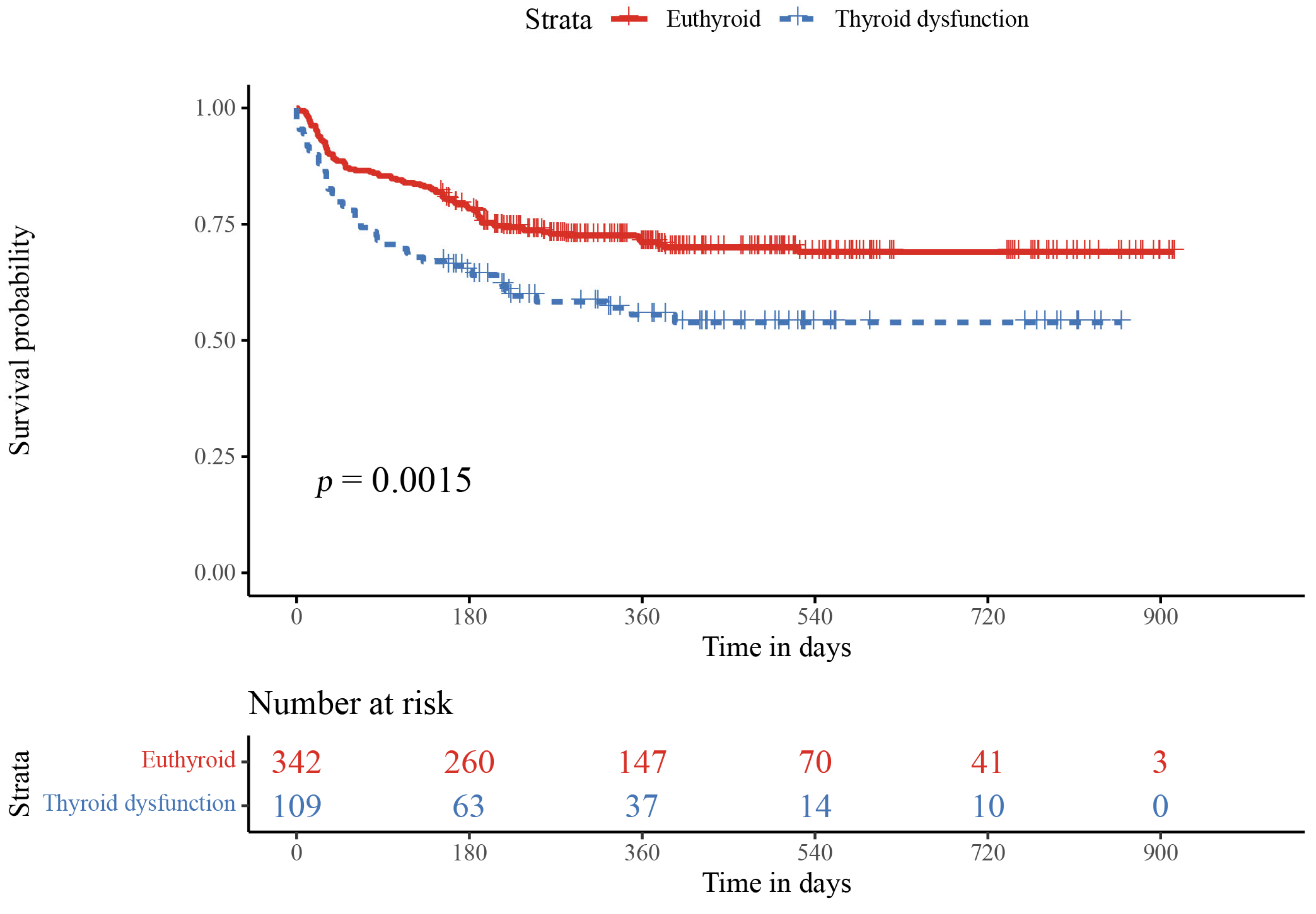
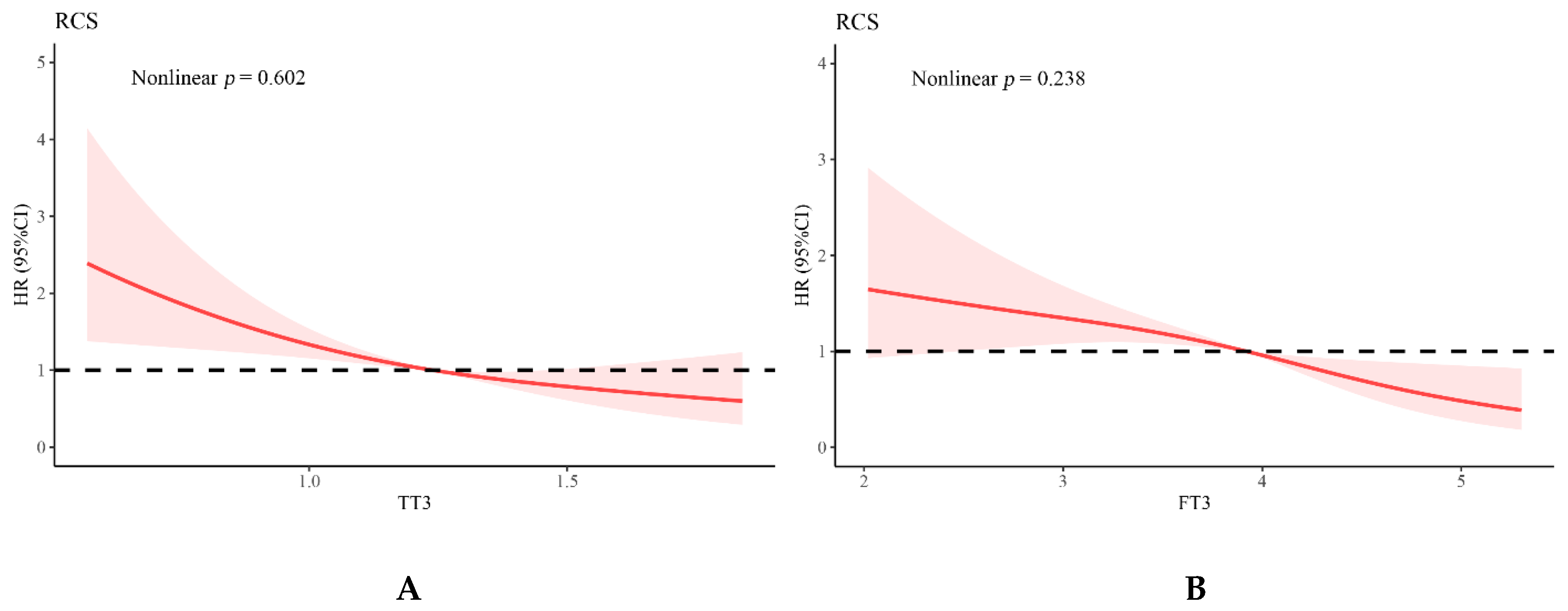
3.3. Relationship of Thyroid Function and Inflammation Values with Adverse Events
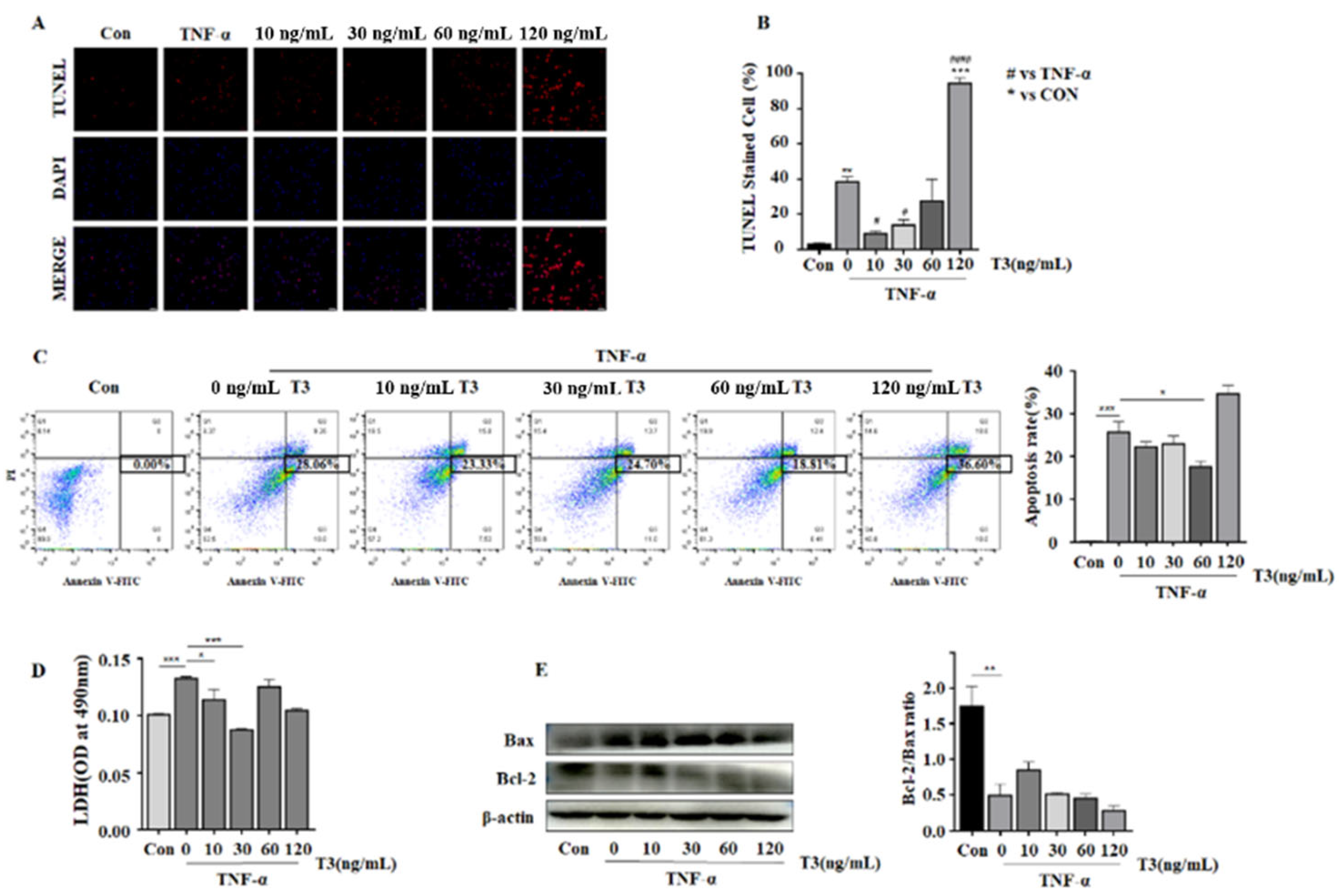
3.4. The Protecting Role of T3 in TNF-α Induced Neonatal Rat Ventricular Cardiomyocyte Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine transaminase |
| AF | Atrial fibrillation |
| ATF4 | Activating transcription factor 4 |
| BNP | Brain natriuretic peptide precursor |
| BMI | Body mass index; CRP, C-reactive protein |
| CK-MB | kinase isoenzyme-MB |
| cTnT | Cardiac troponin T |
| Cr | Creatinine |
| CAD | Coronary artery disease |
| CHOP | C/EBP homologous protein |
| ECLIA | Electrochemiluminescence Immunoassay |
| ERS | Endoplasmic reticulum stress |
| FT4 | Free thyroxine |
| FT3 | Free triiodothyronine |
| GLU | Glucose |
| HF | Heart failure |
| HFC | Heart Failure Collaboratory |
| Hb | Hemoglobin |
| HbA1c | Glycated hemoglobin |
| IVSd | Interventricular septal dimension |
| LVEF | Left ventricular ejection fraction |
| LA | Left atrium |
| LVEDV | Left ventricular end-diastolic volume |
| LVIDd | Left ventricular internal diameter in diastolic phase |
| LVPWd | Left ventricular posterior wall thickness in diastolic phase |
| MPO | Myeloperoxidase; NLR, Neutrophil-to-lymphocyte ratio |
| NT-proBNP | N-terminal pro-B type natriuretic peptide |
| RCS | Restricted cubic splines |
| SCH | Subclinical hypothyroidism |
| TH | Thyroid hormone |
| TNF-α | Tumor necrosis factor α |
| T4 | Thyroxine |
| T3 | Triiodothyronine |
| TG | Triglyceride |
| TT4 | Total thyroxine |
| TT3 | Total triiodothyronine |
| TSH | Thyrotropin |
| NYHA | New York Heart Association |
| 6MWT | The six minute walking test |
| NMR | Neutrophil to monocyte ratio |
| ACEI | Angiotensin-converting enzyme inhibitor |
| ARB | Angiotensin receptor blocker |
| ARNI | angiotensin receptor neprilysin inhibitor |
| HR | Hazard ratio |
| CI | Confidence interval |
References
- Gerdes, A.M.; Ojamaa, K. Thyroid Hormone and Cardioprotection. Compr. Physiol. 2016, 6, 1199–1219. [Google Scholar] [PubMed]
- Kalra, S.; Aggarwal, S.; Khandelwal, D. Thyroid Dysfunction and Dysmetabolic Syndrome: The Need for Enhanced Thyrovigilance Strategies. Int. J. Endocrinol. 2021, 2021, 9641846. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; Desai, A.S.; Medici, M.; Cooper, L.S.; Egan, D.; Sopko, G.; Fishman, G.I.; Goldman, S.; Cooper, D.S.; Mora, S.; et al. Thyroid and Cardiovascular Disease: Research Agenda for Enhancing Knowledge, Prevention, and Treatment. Thyroid 2019, 29, 760–777. [Google Scholar] [CrossRef]
- Mitchell, J.E.; Hellkamp, A.S.; Mark, D.B.; Anderson, J.; Johnson, G.W.; Poole, J.E.; Lee, K.L.; Bardy, G.H. Thyroid function in heart failure and impact on mortality. JACC Heart Fail. 2013, 1, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.D.M.; Pellizas, C.G. Thyroid Hormone Action on Innate Immunity. Front. Endocrinol. 2019, 10, 350. [Google Scholar] [CrossRef]
- De Vito, P.; Incerpi, S.; Pedersen, J.Z.; Luly, P.; Davis, F.B.; Davis, P.J. Thyroid hormones as modulators of immune activities at the cellular level. Thyroid 2011, 21, 879–890. [Google Scholar] [CrossRef]
- Dick, S.A.; Epelman, S. Chronic Heart Failure and Inflammation: What Do We Really Know? Circ. Res. 2016, 119, 159–176. [Google Scholar] [CrossRef]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef]
- Wang, L.; Liang, D.; Xu, X.; Jin, J.; Li, S.; Tian, G.; Gao, Z.; Liu, C.; He, Y. The prognostic value of neutrophil to lymphocyte and platelet to lymphocyte ratios for patients with lung cancer. Oncol. Lett. 2017, 14, 6449–6456. [Google Scholar] [CrossRef]
- Zhang, Y.; Bauersachs, J.; Langer, H.F. Immune mechanisms in heart failure. Eur. J. Heart Fail. 2017, 19, 1379–1389. [Google Scholar] [CrossRef] [Green Version]
- Keliher, E.J.; Ye, Y.X.; Wojtkiewicz, G.R.; Aguirre, A.D.; Tricot, B.; Senders, M.L.; Groenen, H.; Fay, F.; Perez-Medina, C.; Calcagno, C.; et al. Polyglucose nanoparticles with renal elimination and macrophage avidity facilitate PET imaging in ischaemic heart disease. Nat. Commun. 2017, 8, 14064. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.A.; Zagorski, J.; Gellar, M.A.; Stevinson, B.G.; Kline, J.A. Cardiac inflammation contributes to right ventricular dysfunction following experimental pulmonary embolism in rats. J. Mol. Cell. Cardiol. 2006, 41, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Thuren, T.; Zalewski, A.; Libby, P. Interleukin-1beta inhibition and the prevention of recurrent cardiovascular events: Rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS). Am. Heart J. 2011, 162, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Razvi, S.; Bensenor, I.M.; Azizi, F.; Pearce, E.N.; Peeters, R.P. Hypothyroidism. Nat. Rev. Dis. Primers 2022, 8, 30. [Google Scholar] [CrossRef]
- Hayashi, T.; Hasegawa, T.; Kanzaki, H.; Funada, A.; Amaki, M.; Takahama, H.; Ohara, T.; Sugano, Y.; Yasuda, S.; Ogawa, H.; et al. Subclinical hypothyroidism is an independent predictor of adverse cardiovascular outcomes in patients with acute decompensated heart failure. ESC Heart Fail. 2016, 3, 168–176. [Google Scholar] [CrossRef]
- Henderson, K.K.; Danzi, S.; Paul, J.T.; Leya, G.; Klein, I.; Samarel, A.M. Physiological replacement of T3 improves left ventricular function in an animal model of myocardial infarction-induced congestive heart failure. Circ. Heart Fail. 2009, 2, 243–252. [Google Scholar] [CrossRef]
- Forini, F.; Kusmic, C.; Nicolini, G.; Mariani, L.; Zucchi, R.; Matteucci, M.; Iervasi, G.; Pitto, L. Triiodothyronine prevents cardiac ischemia/reperfusion mitochondrial impairment and cell loss by regulating miR30a/p53 axis. Endocrinology 2014, 155, 4581–4590. [Google Scholar] [CrossRef]
- Writing Committee Members; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure. J. Card. Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef]
- Kannan, L.; Shaw, P.A.; Morley, M.P.; Brandimarto, J.; Fang, J.C.; Sweitzer, N.K.; Cappola, T.P.; Cappola, A.R. Thyroid Dysfunction in Heart Failure and Cardiovascular Outcomes. Circ. Heart Fail. 2018, 11, e005266. [Google Scholar] [CrossRef]
- Fiuzat, M.; Hamo, C.E.; Butler, J.; Abraham, W.T.; DeFilippis, E.M.; Fonarow, G.C.; Lindenfeld, J.; Mentz, R.J.; Psotka, M.A.; Solomon, S.D.; et al. Optimal Background Pharmacological Therapy for Heart Failure Patients in Clinical Trials: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2022, 79, 504–510. [Google Scholar] [CrossRef]
- Guo, X.; Yin, H.; Li, L.; Chen, Y.; Li, J.; Doan, J.; Steinmetz, R.; Liu, Q. Cardioprotective Role of Tumor Necrosis Factor Receptor-Associated Factor 2 by Suppressing Apoptosis and Necroptosis. Circulation 2017, 136, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Narasimhan, M.; Sakthivel, R.; Kumar, R.R.; Davidson, C.; Palaniappan, S.; Claybomb, W.W.; Hoidal, J.R.; Darley-Usmar, V.M.; Rajasekaran, N.S. A biphasic effect of TNF-alpha in regulation of the Keap1/Nrf2 pathway in cardiomyocytes. Redox. Biol. 2016, 9, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Landi, P.; Taddei, M.C.; Ripoli, A.; L’Abbate, A.; Iervasi, G. Triiodothyronine levels for risk stratification of patients with chronic heart failure. Am. J. Med. 2005, 118, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Bashkin, A.; Saleh, W.A.; Shehadeh, M.; Even, L.; Ronen, O. Subclinical hypothyroidism or isolated high TSH in hospitalized patients with chronic heart-failure and chronic renal-failure. Sci. Rep. 2021, 11, 10976. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Kroiss, M.; Berliner, D.; Seifert, M.; Allolio, B.; Guder, G.; Ertl, G.; Angermann, C.E.; Stork, S.; Fassnacht, M. Prognostic impact of subclinical thyroid dysfunction in heart failure. Int. J. Cardiol. 2013, 168, 300–305. [Google Scholar] [CrossRef]
- Almandoz, J.P.; Gharib, H. Hypothyroidism: Etiology, diagnosis, and management. Med. Clin. N. Am. 2012, 96, 203–221. [Google Scholar] [CrossRef]
- Lee, S.; Farwell, A.P. Euthyroid Sick Syndrome. Compr. Physiol. 2016, 6, 1071–1080. [Google Scholar]
- Iacoviello, M.; Guida, P.; Guastamacchia, E.; Triggiani, V.; Forleo, C.; Catanzaro, R.; Cicala, M.; Basile, M.; Sorrentino, S.; Favale, S. Prognostic role of sub-clinical hypothyroidism in chronic heart failure outpatients. Curr. Pharm. Des. 2008, 14, 2686–2692. [Google Scholar] [CrossRef]
- Kahana, L.; Keidar, S.; Sheinfeld, M.; Palant, A. Endogenous cortisol and thyroid hormone levels in patients with acute myocardial infarction. Clin. Endocrinol. 1983, 19, 131–139. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rhee, C.M.; Leung, A.M.; Braverman, L.E.; Brent, G.A.; Pearce, E.N. A review: Radiographic iodinated contrast media-induced thyroid dysfunction. J. Clin. Endocrinol. Metab. 2015, 100, 376–383. [Google Scholar] [CrossRef]
- de Luca, R.; Davis, P.J.; Lin, H.Y.; Gionfra, F.; Percario, Z.A.; Affabris, E.; Pedersen, J.Z.; Marchese, C.; Trivedi, P.; Anastasiadou, E.; et al. Thyroid Hormones Interaction with Immune Response, Inflammation and Non-thyroidal Illness Syndrome. Front. Cell Dev. Biol. 2020, 8, 614030. [Google Scholar] [CrossRef] [PubMed]
- Benites-Zapata, V.A.; Hernandez, A.V.; Nagarajan, V.; Cauthen, C.A.; Starling, R.C.; Tang, W.H. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. Am. J. Cardiol. 2015, 115, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Okada, H. Principles of immunology and its nuances in the central nervous system. Neuro Oncol. 2015, 17, 3–8. [Google Scholar] [CrossRef]
- Adelberg, H.M.; Siemsen, J.K.; Jung, R.C.; Nicoloff, J.T. Scintigraphic detection of pulmonary bacterial infections with labeled thyroid hormones and pertechnetate. Radiology 1971, 99, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Boelen, A.; Boorsma, J.; Kwakkel, J.; Wieland, C.W.; Renckens, R.; Visser, T.J.; Fliers, E.; Wiersinga, W.M. Type 3 Deiodinase Is Highly Expressed in Infiltrating Neutrophilic Granulocytes in Response to Acute Bacterial Infection. Thyroid 2008, 18, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- van der Spek, A.H.; Jim, K.K.; Karaczyn, A.; van Beeren, H.C.; Ackermans, M.T.; Darras, V.M.; Vandenbroucke-Grauls, C.M.J.E.; Hernandez, A.; Brouwer, M.C.; Fliers, E.; et al. The Thyroid Hormone Inactivating Type 3 Deiodinase Is Essential for Optimal Neutrophil Function: Observations from Three Species. Endocrinology 2018, 159, 826–835. [Google Scholar] [CrossRef]
- Pyzik, A.; Grywalska, E.; Matyjaszek-Matuszek, B.; Rolinski, J. Immune Disorders in Hashimoto’s Thyroiditis: What Do We Know So Far? J. Immunol. Res. 2015, 2015, 979167. [Google Scholar] [CrossRef]
- van der Spek, A.H.; Fliers, E.; Boelen, A. Thyroid Hormone and Deiodination in Innate Immune Cells. Endocrinology 2021, 162, 1–15. [Google Scholar] [CrossRef]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef]
- Uthamalingam, S.; Patvardhan, E.A.; Subramanian, S.; Ahmed, W.; Martin, W.; Daley, M.; Capodilupo, R. Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am. J. Cardiol. 2011, 107, 433–438. [Google Scholar] [CrossRef]
- Acet, H.; Ertas, F.; Bilik, M.Z.; Akil, M.A.; Ozyurtlu, F.; Aydin, M.; Oylumlu, M.; Polat, N.; Yuksel, M.; Yildiz, A.; et al. The relationship between neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and thrombolysis in myocardial infarction risk score in patients with ST elevation acute myocardial infarction before primary coronary intervention. Postepy Kardiol Interwencyjnej 2015, 11, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Sobolewska, A.; Wlodarczyk, M.; Stec-Michalska, K.; Fichna, J.; Wisniewska-Jarosinska, M. Mean Platelet Volume in Crohn’s Disease Patients Predicts Sustained Response to a 52-Week Infliximab Therapy: A Pilot Study. Dig. Dis. Sci. 2016, 61, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Park, J.; Shin, J.H.; Oh, Y.L.; Jung, H.A.; Chung, M.K.; Choe, J.H.; Ahn, Y.C.; Kim, S.W.; Chung, J.H.; et al. Prognostic Value of the Neutrophil-to-Lymphocyte Ratio before and after Radiotherapy for Anaplastic Thyroid Carcinoma. Cancers 2021, 13, 1913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liao, Y.; Yang, H.; Tao, J.; Ma, L.; Zuo, X. Irigenin reduces the expression of caspase-3 and matrix metalloproteinases, thus suppressing apoptosis and extracellular matrix degradation in TNF-alpha-stimulated nucleus pulposus cells. Chem. Biol. Interact. 2021, 349, 109681. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagen, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fan, H.; Wang, S.; Tang, G.; Zhai, C.; Shen, L. Ferroptosis: A Novel Therapeutic Target for Ischemia-Reperfusion Injury. Front. Cell Dev. Biol. 2021, 9, 688605. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, R.N.; Wu, F.; Zhai, L.J.; Wang, K.F.; Xiao, M.; Xie, T.; Sui, X. Non-apoptotic cell death in malignant tumor cells and natural compounds. Cancer Lett. 2018, 420, 210–227. [Google Scholar]
- Fearnhead, H.O.; Vandenabeele, P.; Berghe, T.V. How do we fit ferroptosis in the family of regulated cell death? Cell Death Differ. 2017, 24, 1991–1998. [Google Scholar] [CrossRef]
- Shimada, K.; Skouta, R.; Kaplan, A.; Yang, W.S.; Hayano, M.; Dixon, S.J.; Brown, L.M.; Valenzuela, C.A.; Wolpaw, A.J.; Stockwell, B.R. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016, 12, 497–503. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid Redox Signal 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Liang, B.; Liu, L.; Huang, H.; Li, L.; Zhou, J. High T3 Induces beta-Cell Insulin Resistance via Endoplasmic Reticulum Stress. Mediat. Inflamm. 2020, 2020, 5287108. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, H.; Zhuang, Y.; Zhang, J.; Ding, X.; Zhan, L.; Luo, S.; Zhang, Q.; Sun, F.; Zhang, M.; et al. LncRNA ACART protects cardiomyocytes from apoptosis by activating PPAR-gamma/Bcl-2 pathway. J. Cell. Mol. Med. 2020, 24, 737–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Euthyroid (N = 342) | Thyroid Abnormal (N = 109) | p-Value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years) | 64.79 [54.11; 72.77] | 68.99 [58.44; 78.10] | 0.004 |
| Male, n (%) | 236 (69.01%) | 77 (70.64%) | 0.839 |
| Smoke, n (%) | 62 (18.13%) | 18 (16.51%) | 0.810 |
| Drink, n (%) | 60 (17.54%) | 14 (12.84%) | 0.315 |
| Physical examination and Coexisting conditions | |||
| BMI (kg/m2) | 23.88 [21.78; 26.57] | 22.04 [19.43; 24.38] | <0.001 |
| Heart rate (bpm) | 78.00 [69.00; 87.00] | 75.00 [65.00; 87.00] | 0.113 |
| NYHA class | 0.002 | ||
| I | 42 (12.28%) | 6 (5.50%) | |
| II | 223 (65.20%) | 60 (55.05% | |
| III | 70 (20.47%) | 36 (33.03%) | |
| IV | 7 (2.05%) | 7 (6.42%) | |
| Hypertension, n (%) | 160 (46.78%) | 49 (44.95%) | 0.823 |
| Diabetes, n (%) | 93 (27.19%) | 33 (30.28%) | 0.616 |
| Coronary artery disease, n (%) | 113 (33.04%) | 36 (33.03%) | 1.000 |
| AF, n (%) | 115 (33.63%) | 37 (33.94%) | 1.000 |
| Stroke, n (%) | 22 (6.43%) | 14 (12.84%) | 0.051 |
| 6MWT (m) | 408.00 [285.00; 485.00] | 340.00 [100.00; 441.25] | <0.001 |
| Laboratory examination | |||
| FT3 (pmol/L) | 4.12 [3.67; 4.45] | 3.07 [2.48; 3.65] | <0.001 |
| FT4 (pmol/L) | 13.68 [12.57; 14.97] | 13.12 [11.98; 14.96] | 0.039 |
| TSH (mIU/L) | 1.61 [1.11; 2.34] | 2.33 [0.74; 5.49] | 0.003 |
| TT4 (nmol/L) | 103.46 (18.03) | 93.49 (24.11) | <0.001 |
| TT3 (nmol/L) | 1.31 (0.23) | 0.96 (0.36) | <0.001 |
| TT3/TT4 (×10−2) | 1.30 (0.27) | 1.05 (0.35) | <0.001 |
| TPOAb (IU/mL) | 0.55 [0.50; 1.09] | 0.75 [0.50; 1.55] | 0.019 |
| BNP (pg/mL) | 494.00 [189.60; 1177.00] | 908.60 [459.55; 3386.80] | 0.001 |
| NT-proBNP (pg/mL) | 1027.00 [491.50; 2271.75] | 2474.00 [932.00; 6501.50] | <0.001 |
| GLU (mmol/L) | 5.56 [4.93; 6.70] | 5.64 [4.93; 6.81] | 0.674 |
| cTnT (ug/L) | 0.02 [0.02; 0.04] | 0.04 [0.02; 0.12] | <0.001 |
| CRP (mg/L) | 5.35 [5.00; 11.60] | 7.55 [5.00; 14.78] | 0.080 |
| Neutrophils (×109/L) | 4.24 [3.21; 5.50] | 4.15 [3.08; 5.58] | 0.655 |
| Lymphocytes (×109/L) | 1.41 [1.09; 1.75] | 1.17 [0.90; 1.60] | 0.001 |
| Monocytes (×109/L) | 0.44 [0.34; 0.57] | 0.43 [0.34; 0.57] | 0.930 |
| NLR | 3.08 [2.13; 4.29] | 3.41 [2.29; 5.09] | 0.110 |
| NMR | 9.69 [7.34; 11.86] | 9.35 [7.39; 11.88] | 0.833 |
| Creatinine (umol/L) | 80.00 [67.00; 97.00] | 90.00 [74.00; 122.00] | <0.001 |
| Hb (g/L) | 137.00 [121.00; 150.00] | 125.00 [106.00; 142.50] | <0.001 |
| ALT (U/L) | 26.00 [21.00; 33.75] | 31.00 [22.00; 43.00] | 0.016 |
| HbA1c (%) | 6.10 [5.70; 6.80] | 6.10 [5.80; 6.68] | 0.851 |
| TG (mmol/L) | 1.11 [0.83; 1.48] | 0.97 [0.80; 1.29] | 0.045 |
| CK-MB (U/l) | 12.00 [8.00; 15.00] | 11.00 [9.00; 15.75] | 0.615 |
| Medications | |||
| ACEI/ABR/ARNI, n (%) | 289 (84.50%) | 78 (71.56%) | 0.004 |
| Beta-blocker, n (%) | 270 (78.95%) | 72 (66.06%) | 0.009 |
| Digoxin, n (%) | 61 (17.84%) | 24 (22.02%) | 0.406 |
| Amiodarone, n (%) | 68 (19.88%) | 13 (11.93%) | 0.082 |
| CCB, n (%) | 27 (7.89%) | 8 (7.34%) | 1.000 |
| Statin, n (%) | 199 (58.19%) | 58 (53.21%) | 0.422 |
| HFC score | 4.00 [2.00; 4.00] | 3.00 [2.00; 4.00] | 0.180 |
| Echocardiogram parameters | |||
| LVEDV (mL) | 158.60 [120.50; 208.00] | 147.00 [105.40; 189.00] | 0.067 |
| LVEF (%) | 33.55 [26.00; 42.38] | 34.00 [27.10; 46.20] | 0.190 |
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-Value | Model 1 | Model 2 | |||
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | |||
| Sex | 1.090 (0.767–1.540) | 0.636 | 1.084 (0.756–1.552) | 0.662 | 1.116 (0.778–1.601) | 0.551 |
| Age | 1.020 (1.000–1.030) | 0.008 | 1.015 (1.001–1.029) | 0.033 | 1.015 (1.001–1.029) | 0.037 |
| BMI | 0.959 (0.920–1.000) | 0.048 | 0.992 (0.950–1.349) | 0.719 | 0.993 (0.951–1.037) | 0.758 |
| Smoke | 0.771 (0.490–1.210) | 0.262 | ||||
| Drink | 0.737 (0.455–1.190) | 0.215 | ||||
| Amiodarone | 0.845 (0.541–1.320) | 0.459 | 0.853 (0.539–1.349) | 0.496 | 0.840 (0.531–1.328) | 0.456 |
| FT3 | 0.597 (0.493–0.724) | <0.001 | 0.677 (0.551–0.832) | <0.001 | ||
| FT4 | 1.020 (0.937–1.120) | 0.605 | ||||
| TSH | 1.130 (1.060–1.190) | <0.001 | ||||
| TT4 | 0.995 (0.987–1.000) | 0.220 | ||||
| TT3 | 0.224 (0.131–0.382) | <0.001 | 0.320 (0.181–0.565) | <0.001 | ||
| TPOAb | 0.995 (0.989–1.000) | 0.108 | ||||
| Neutrophils | 1.110 (1.030–1.200) | 0.010 | ||||
| Lymphocytes | 0.662 (0.481–0.911) | 0.011 | ||||
| Monocytes | 1.450 (0.697–3.020) | 0.319 | ||||
| NLR | 1.090 (1.060–1.130) | <0.001 | 1.073 (1.036–1.111) | <0.001 | 1.072 (1.035–1.110) | <0.001 |
| NMR | 1.030 (0.997–1.070) | 0.069 | ||||
| HFC score | 1.075 (0.963–1.199) | 0.197 | ||||
| LVEDV | 1.000 (0.997–1.000) | 0.871 | ||||
| LVEF | 0.990 (0.978–1.003) | 0.150 | 0.987 (0.973–1.001) | 0.075 | 0.988 (0.973–1.002) | 0.091 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Chen, G.; Su, S.; Zhao, C.; Ma, H.; Xiang, M. Independent Association of Thyroid Dysfunction and Inflammation Predicts Adverse Events in Patients with Heart Failure via Promoting Cell Death. J. Cardiovasc. Dev. Dis. 2022, 9, 290. https://doi.org/10.3390/jcdd9090290
Shen Y, Chen G, Su S, Zhao C, Ma H, Xiang M. Independent Association of Thyroid Dysfunction and Inflammation Predicts Adverse Events in Patients with Heart Failure via Promoting Cell Death. Journal of Cardiovascular Development and Disease. 2022; 9(9):290. https://doi.org/10.3390/jcdd9090290
Chicago/Turabian StyleShen, Yimin, Guanzhong Chen, Sheng’an Su, Chenchen Zhao, Hong Ma, and Meixiang Xiang. 2022. "Independent Association of Thyroid Dysfunction and Inflammation Predicts Adverse Events in Patients with Heart Failure via Promoting Cell Death" Journal of Cardiovascular Development and Disease 9, no. 9: 290. https://doi.org/10.3390/jcdd9090290
APA StyleShen, Y., Chen, G., Su, S., Zhao, C., Ma, H., & Xiang, M. (2022). Independent Association of Thyroid Dysfunction and Inflammation Predicts Adverse Events in Patients with Heart Failure via Promoting Cell Death. Journal of Cardiovascular Development and Disease, 9(9), 290. https://doi.org/10.3390/jcdd9090290






