Abstract
An 83-year-old gentleman with a history of 23-mm Hancock-II-bioprosthetic aortic valve (BAV) replacement ten-years prior presented with symptoms of dyspnea and lower extremity edema. During the preceding seven-years, he had been noted to have asymptomatic increased mean transvalvular gradients (MG; 36–50 mmHg) felt to be due to either early bioprosthetic degeneration, pannus formation, or patient–prosthesis mismatch. An echocardiogram at the time of symptom development demonstrated significant flow acceleration through the aortic valve, mild regurgitation, and severely increased MG (48 mmHg) with prolonged acceleration time (AT, 140 msec). A trial of warfarin anticoagulation resulted in dramatic improvement after only 6 weeks with laminar flow through the AV, near-total resolution of regurgitation, and a decrease in MG to 14 mmHg and AT to 114 msec. These findings strongly suggest that BAV thrombosis was the predominant mechanism responsible for the longstanding high MG. Our case highlights that BAV thrombosis should be considered in the differential of elevated gradients regardless of the age of prosthesis, and that a trial of warfarin anticoagulation may be beneficial even if elevated gradients have been present for a prolonged period. Valvular gradients are often abnormal long before a formal diagnosis; however, these may reverse quickly with anticoagulation therapy.
1. Introduction
Bioprosthetic aortic valve (BAV) thrombosis is an increasingly recognized complication of tissue valve prosthesis, and it is considered one of the major mechanisms responsible for valve dysfunction in addition to BAV degeneration, pannus formation, or patient–prosthesis mismatch [1,2]. Although once considered rare, discrepancies between the doppler and catheter gradients of aortic valve prosthesis have been reported since the early 1990s [3]. Chronically elevated valvular gradients are hallmark findings and are often present long before symptom development and formal diagnosis [4,5]. As such, BAV thrombosis may be categorized as either subclinical or clinical [6].
Subclinical thrombosis in bioprosthetic surgical aortic valves (SAVI) appears to occur significantly less frequently and later when compared with transcatheter valves (TAVI) [5,7]. Furthermore, subclinical BAV thrombosis was historically deemed to occur primarily within 12 months following the implantation [5,7,8]. Subsequent observations have shown that BAV thrombosis may occur years following the prosthesis implantation [9]. Here, we present a unique case of therapeutically confirmed BAV thrombosis ten years following implantation and with seven years of consistently elevated gradients, and with aortic valve (AV) hemodynamic parameters normalizing following a 6-week treatment course of vitamin-K antagonist (VKA).
2. Case Description
An 83-year-old Caucasian gentleman with a history of hypertension and hyperlipidemia underwent SAVI with 23-mm Hancock II BAV due to severe aortic stenosis and aorta to right coronary artery bypass grafting in May of 2004 at the age of 73 years. Mid-2007, three years post-procedure, the patient was noted to have increased mean transvalvular gradients (MG) ranging from 36 to 50 mmHg with increased velocity (Table 1; post-operative and prior to 2007, echocardiographic studies were unavailable). Since the patient’s left ventricular (LV) systolic function remained within normal limits and he remained asymptomatic (completing farm chores without exercise limitations), his prosthetic parameters were not further investigated. Instead, he was followed by serial transthoracic echocardiograms (TTE) with a working diagnosis of bioprosthetic degeneration versus pannus formation or patient–prosthesis mismatch. The entire time patient remained on aspirin, 81 mg daily.

Table 1.
Echocardiographic aortic valve parameters over a 7-year period and following a 6-week warfarin course.
In the spring of 2014, he developed insidiously worsening exertional dyspnea and peripheral edema. His primary care physician initiated furosemide, 40 mg daily, and referred him to his cardiologist. At that time, his vital signs were pertinent for blood pressure of 150/60 mmHg, a pulse of 76 beats/min, and oxygen saturation greater than 96%. On physical exam, he had a 2/6 systolic ejection murmur loudest at the right upper sternal border, which radiated to the carotids bilaterally and jugular venous pressure estimated at 10 cm of water. TTE was performed at the cardiology clinic and demonstrated significant flow acceleration through the aortic prosthesis, mild aortic regurgitation, and severely increased MG (48 mmHg) with prolonged acceleration time (AT; 140 msec) on continuous wave (CW) Doppler (Figure 1A–C). At that time, the diagnosis of BAV thrombosis was entertained, and the patient was initiated on a trial of warfarin anticoagulation with a target international normalized ratio (INR) of 2.5. The patient was scheduled for transesophageal echocardiography (TEE), which was delayed for 6 weeks owing to the patient’s wish to complete his spring planting. During this delay he continued warfarin therapy. Six weeks later TTE and TEE were completed and demonstrated a dramatic improvement in the prosthetic hemodynamics (the follow-up TTE was performed by the same echocardiography machine and was read by the same cardiologist as the initial TTE). There was laminar flow through the aortic valve, near-total resolution of aortic regurgitation, and a decrease of MG to 14 mmHg and AT to 114 msec (Figure 1D–F). Velocity was improved as well at 2.7 m/s. The patient’s symptoms had resolved, and he returned to his farm chores without limitations. These findings strongly suggest the predominant mechanism responsible for the longstanding high prosthetic gradients was indeed BAV thrombosis. The patient was continued on warfarin long term and remained asymptomatic until his decease a year later.
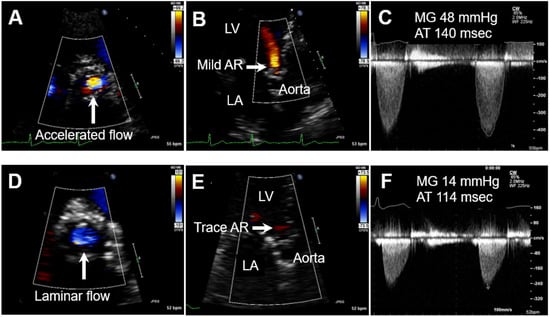
Figure 1.
Transthoracic echocardiogram with CW Doppler before (A–C) and after (D–F) warfarin course (note virtually identical CW Doppler settings). (A) Short axis view demonstrating significant flow acceleration through the aortic prosthesis; (B) apical-axis view demonstrating mild aortic regurgitation (LV: left ventricle, LA: left atrium); (C) severely increased mean transvalvular gradient (MG) of 48 mmHg with prolonged acceleration time (AT) 140 msec. (D) Laminar flow through the aortic valve; (E) near disappearance of aortic regurgitation; (F) decrease in MG to 14 mmHg and AT to 114 msec.
3. Discussion
The diagnosis of BAV thrombosis is challenging. Rather than direct visualization of the thrombus, diagnosis is usually based on echocardiographic identification of subtle morphologic and hemodynamic changes such as: increased cusp thickness, abnormal (decreased) leaflet motion, and 50% increases in prosthesis MG and AT [10]. These criteria have a high sensitivity and specificity for BAV thrombosis when seen in addition to the recently proposed Mayo Clinic diagnostic algorithm of decreased Doppler velocity index (DVI) of <0.25, and a more than 20% decrease in the effective orifice area (EOA) from the baseline [4].
Despite TTE being traditionally relied upon for structural and hemodynamic monitoring following prosthetic valve implantation, Makkar et al., in their paper resulting from the RESOLVE registry, reported that amongst TAVI patients the subtly reduced leaflet motion and change in hemodynamic parameters (commonly caused by subclinical BAV thrombosis) are better diagnosed by multidetector cardiac CT (CCT) imaging and TEE, and may be missed by TTE [8]. The mild increase in valvular gradients seen in subclinical BAV thrombosis are often within the expected echocardiographic range for BAV, and therefore may not be discernable by TTE [6]. CCT is highly accurate in assessing reduced leaflet motion (RELM) and leaflet morphology (specifically thickening), which is often referenced as hypoattenuating leaflet thickening (HALT). The use of CCT to evaluate subclinical BAV thrombosis, however, is not recommended outside clinical studies due to an unjustified exposure to radiation and contrast without evidence of treatment benefit in such cases [6,8]. Diagnosis of BAV can be made by pathological examination of the explanted prosthesis but is typically utilized only in patients with valve failure requiring repeated replacement [2,11]. Although embolic complications rarely occur in subclinical BAV thrombosis, valve thrombosis should be considered in any patient with a prosthetic heart valve presenting with an embolic event [12,13]. Furthermore, guidelines recommend yearly TTE beginning 5 years following implantation in asymptomatic patients [12,13].
The mechanism and risk factors for BAV thrombosis are not entirely certain. Immobile leaflets immediately following an aortic bioprosthetic valve implantation are frequently underrecognized and appear to be associated with early BAV dysfunction and thrombosis [2]. Early immobile leaflets are presumed to generate turbulent flow eddies across the prosthesis leaflets causing increased shear stress and premature deterioration of the valve, as well facilitating clot formation that may further impair leaflet mobility [2,14]. Increase of valve gradients immediately following SAVI have also been shown to be a predictor of BAV thrombosis, whereas following TAVI commissural misalignment was a reported predictor in some studies [15,16]. The impact of subclinical BAV thrombosis on postprocedural outcomes, valve durability and function, as well as the risk of mortality and stroke remains uncertain [6]. Recently, prolonged subclinical BAV thrombosis with increased gradients has been recognized as a cause of valve failure years following implantation [5,9]. There are data supporting that elevated valvular gradients are present months before BAV thrombosis is diagnosed clinically, thus yearly echocardiographic surveillance and increased awareness could lead to earlier diagnosis and more effective therapy [5].
With the intention to further investigate the time between BAV implantation and valve thrombosis diagnosis (time to BAV thrombosis), we performed a comprehensive literature search of the Medline database (National Library of Medicine, Bethesda, MD, USA) via the PubMed search engine from the inception until 1 July 2022. We used the following search keywords (combination of MeSH and non-MeSH terms): “bioprosthetic valve thrombosis“ AND “aorta OR aortic”. This search yielded 228 articles. We reviewed all surgical and transcatheter BAV thrombosis case reports and series, including cases reported in both observational and cohort studies, clinical trials, and systematic reviews that were diagnosed at least 1 year following implantation. Furthermore, the reference list of identified articles was manually screened to identify additional cases that could be included in our analysis. Results of time from valve implantation to BAV thrombosis diagnosis were displayed as a range (months). In articles without precise BAV data amongst other valves or valvular complications (in some papers valve thrombosis was represented as one of the reasons for valve failure), the highest value was used and was marked by the star in Table 2.

Table 2.
A literature search of bioprosthetic aortic valve thrombosis with the time of bioprosthetic aortic valve implantation to thrombosis diagnosis displayed as a range value or highest value.
We found that the majority of BAV thrombosis cases occurred within 6 years. There was one case of bioprosthetic valve thrombosis reported by Egbe et al. which occurred more than 9 years following implantation, although not the precise valve location [10]. Upon reflection, this report may well be our case given that it originated from the same institution. Hattori et al. reported a case of acute myocardial infarction caused by thrombus derived from a large aneurysm of the sinus of Valsalva and BAV 10 years following implantation [23]. The authors hypothesized that the severely dilated aneurysm of the sinus of Valsalva precipitated turbulent blood flow resulting in a hypercoagulable state, and the stent strut of the BAV contributed to thrombus formation. Basra et al. reported BAV thrombosis diagnosed by CCT in 32 patients 5 days to 130.9 months following TAVI or SAVI [32]. The mean and median times from implantation to diagnosis were 27.8 and 14.2 months, respectively; any detailed report about the patient diagnosed with BAV thrombosis nearly 11 years following implantation is lacking. Although our patient had documented elevated gradients 3 years following SAVI, BAV thrombosis was not diagnosed and therapeutically confirmed until 10 years following implantation. As such, our report is a unique case of late BAV thrombosis. Despite continuously elevated gradients for 7 years, our patient remained asymptomatic and complication free. Likewise, in many of the reviewed cases, increased valvular gradients could be retroactively found on TTE months prior to the BAV thrombosis being diagnosed. Our report also demonstrates that even when gradients have been elevated for several years, BAV thrombosis may resolve promptly with VKA
Recommendations for mitigation of BAV thrombosis have not been completely standardized. United States (US) and European guidelines highlight an increased risk of BAV thrombosis within the first 3 months following implantation [12,13]. In patients with other indications for anticoagulation, lifelong oral anticoagulation is recommended following bioprosthetic SAVI or TAVI [12]. In patients who are otherwise without an indication for anticoagulation, the optimal antithrombotic strategy following a BAV implantation remains controversial and without high-quality evidence [12]. The general recommendation following bioprosthetic SAVI is to consider low-dose aspirin or VKA for the first 3 months [12]. There are many studies that support early use of VKA to reduce the risk of thrombosis and embolic complications; however, there is also evidence of increased major bleeding with VKA use compared with low-dose aspirin without a reduction in the rate of death or thromboembolic events [12,55]. Until recently, following TAVI, both US and European guidelines recommended dual antiplatelet therapy (DAPT) for the first 3 to 6 months, followed by lifelong single antiplatelet therapy (SAPT); however, the 2021 European guidelines were updated to recommend only lifelong SAPT following TAVI [12,13].
With respect to BAV treatment, the 2021 ESC/EACTS guidelines recommend VKA and/or unfractionated heparin before considering re-intervention (class I, level C recommendation) [12]. Anticoagulation should also be considered in patients with leaflet thickening and reduced leaflet motion leading to elevated gradients, with anticoagulation continued at least until resolution (class IIa, level B recommendation) [12]. Previous studies concluded that anticoagulation with VKA should be considered the first-line therapy in hemodynamically stable patients, as it usually results in hemodynamic and clinical improvement with minimal risk [1,9]. In early subclinical BAV thrombosis, novel oral anticoagulants (NOACs) may be as effective as warfarin [7], but data are lacking for late thrombosis. DAPT was found by many studies to be suboptimal for both the prevention and treatment of BAV thrombosis [7,8]. Redo surgery or thrombolytic therapy are reserved for hemodynamically unstable patients requiring urgent treatment [1,12].
Increased awareness of this entity over the past decade, in addition to updated recommendations and diagnostic algorithms, have led to earlier diagnosis and initiation of appropriate treatment [4]. Despite successful medical therapy and restoration of valve hemodynamics, BAV thrombosis continues to be a risk factor for accelerated bioprosthetic valve failure and repeated BAV thrombosis may occur [1,9,11,26]. Therefore, indefinite anticoagulation should be considered after initial the treatment of BAV thrombosis [26].
4. Conclusions
Our case highlights that BAV thrombosis should be considered in the differential of elevated bioprosthetic gradients regardless of the prosthesis age, and that a trial of oral anticoagulation with VKA may be beneficial even if elevated gradients have been present for a prolonged period. Valvular gradients are often abnormal long before the diagnosis is established and may reverse quickly with anticoagulation therapy.
5. Limitations of the Study
Limitations of our study are inherent to the nature of this type of literature review and include selection bias as well as publication bias. An additional limitation of our literature review is that we have included only cases in the English language and ones that were published in journals that are indexed in the Medline database. Although these strict criteria were implemented to avoid low-quality case reports, we recognize that we might have missed some high-quality cases if they did not meet our pre-selection criteria.
Author Contributions
Conceptualization, M.R. and R.D.H.; methodology, writing—original draft preparation, M.R., C.W.N. and R.D.H.; writing—review and editing, M.R., C.W.N. and R.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written, informed consent for publication was obtained from the patient for the case report and imaging.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AT | acceleration time |
| AV | aortic valve |
| BAV | bioprosthetic aortic valve |
| CCT | cardiac CT |
| CW | continuous wave |
| DAPT | dual antiplatelet therapy |
| DVI | Doppler velocity index |
| EACTS | European Association for Cardio-Thoracic Surgery |
| EF | ejection fraction |
| EOA | effective orifice area |
| ESC | European Society of Cardiology |
| HALT | hypoattenuating leaflet thickening |
| INR | international normalized ratio |
| LV | left ventricle |
| MG | mean gradient |
| NOAC | novel oral anticoagulants |
| RELM | reduced leaflet motion |
| SAPT | single antiplatelet therapy |
| SAVI | surgical aortic valve implantation |
| TAVI | transcatheter aortic valve implantation |
| TEE | transesophageal echocardiogram |
| TTE | transthoracic echocardiogram |
| VKA | vitamin-K antagonist |
References
- Pislaru, S.; Hussain, I.; Pellikka, P.A.; Maleszewski, J.J.; Hanna, R.D.; Schaff, H.; Connolly, H.M. Misconceptions, diagnostic challenges and treatment opportunities in bioprosthetic valve thrombosis: Lessons from a case series. Eur. J. Cardio-Thoracic Surg. 2014, 47, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Naser, J.; Crestanello, J.; Nkomo, V.; Luis, S.; Thaden, J.; Geske, J.; Anderson, J.; Sinak, L.; Michelena, H.; Pislaru, S.; et al. Immobile Leaflets at Time of Bioprosthetic Valve Implantation: A Novel Risk Factor for Early Bioprosthetic Failure: A Novel Risk Factor for Early Bioprosthetic Failure. Heart Lung Circ. 2022, 31, 1166–1175. [Google Scholar] [CrossRef]
- Baumgartner, H.; Khan, S.; DeRobertis, M.; Czer, L.; Maurer, G. Discrepancies between Doppler and catheter gradients in aortic prosthetic valves in vitro. A manifestation of localized gradients and pressure recovery. Circulation 1990, 82, 1467–1475. [Google Scholar] [CrossRef]
- Roslan, A.B.; Naser, J.A.; Nkomo, V.T.; Padang, R.; Lin, G.; Pislaru, C.; Greason, K.L.; Pellikka, P.A.; Eleid, M.F.; Thaden, J.J.; et al. Performance of Echocardiographic Algorithms for Assessment of High Aortic Bioprosthetic Valve Gradients. J. Am. Soc. Echocardiogr. 2022, 35, 7. [Google Scholar] [CrossRef] [PubMed]
- A Naser, J.; Petrescu, I.; Ionescu, F.; Nkomo, V.T.; Pislaru, C.; Schaff, H.V.; A Pellikka, P.; Connolly, H.M.; Egbe, A.C.; Pislaru, S.V. Gradient changes in bioprosthetic valve thrombosis: Duration of anticoagulation and strategies to improve detection. Open Hear. 2021, 8, e001608. [Google Scholar] [CrossRef]
- Søndergaard, L. Does Subclinical Leaflet Thrombosis Impact the Durability of Bioprosthetic Aortic Valves? JACC Cardiovasc. Interv. 2022, 15, 1123–1125. [Google Scholar] [CrossRef]
- Chakravarty, T.; Søndergaard, L.; Friedman, J.; De Backer, O.; Berman, D.; Kofoed, K.F.; Jilaihawi, H.; Shiota, T.; Abramowitz, Y.; Jørgensen, T.H.; et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: An observational study. Lancet 2017, 389, 2383–2392. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.; Jilaihawi, H.; Chakravarty, T.; Kofoed, K.F.; De Backer, O.; Asch, F.M.; Ruiz, C.E.; Olsen, N.T.; Trento, A.; et al. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N. Engl. J. Med. 2015, 373, 2015–2024. [Google Scholar] [CrossRef]
- Egbe, A.C.; Connolly, H.M.; Pellikka, P.A.; Schaff, H.V.; Hanna, R.; Maleszewski, J.J.; Nkomo, V.T.; Pislaru, S.V. Outcomes of Warfarin Therapy for Bioprosthetic Valve Thrombosis of Surgically Implanted Valves: A Prospective Study. JACC Cardiovasc. Interv 2017, 10, 379–387. [Google Scholar] [CrossRef]
- Egbe, A.; Pislaru, S.V.; Ali, M.A.; Khan, A.R.; Boler, A.N.; Schaff, H.V.; Akintoye, E.; Connolly, H.M.; Nkomo, V.T.; Pellikka, P.A. Early Prosthetic Valve Dysfunction Due to Bioprosthetic Valve Thrombosis: The Role of Echocardiography. JACC Cardiovasc. Imaging 2018, 11, 951–958. [Google Scholar] [CrossRef]
- Egbe, A.C.; Pislaru, S.V.; Pellikka, P.A.; Poterucha, J.T.; Schaff, H.V.; Maleszewski, J.J.; Connolly, H.M. Bioprosthetic Valve Thrombosis Versus Structural Failure: Clinical and Echocardiographic Predictors. J. Am. Coll Cardiol. 2015, 66, 2285–2294. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Hear. J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2021, 162, e183–e353. [Google Scholar] [CrossRef]
- Imamura, E.; Ohteki, H.; Tsutsui, T.; Nishiya, Y.; Ishihara, S.; Koyanagi, H. Open versus closed position fixation of bioprosthesis. Comparative in vitro studies from the viewpoint of durability. J. Thorac. Cardiovasc. Surg. 1982, 83, 755. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pibarot, P.; Chambers, J.; Edvardsen, T.; Delgado, V.; Dulgheru, R.; Pepi, M.; Cosyns, B.; Dweck, M.R.; Garbi, M.; et al. Recommendations for the imaging assessment of prosthetic heart valves: A report from the European Association of Cardiovascular Imaging endorsed by the Chinese Society of Echocardiography, the Inter-American Society of Echocardiography, and the Brazilian Department of Cardiovascular Imaging†. Eur. Hear. J.-Cardiovasc. Imaging 2016, 17, 589–590. [Google Scholar] [CrossRef]
- Raschpichler, M.; Flint, N.; Yoon, S.-H.; Kaewkes, D.; Patel, C.; Singh, C.; Patel, V.; Kashif, M.; Borger, M.A.; Chakravarty, T.; et al. Commissural Alignment After Balloon-Expandable Transcatheter Aortic Valve Replacement Is Associated With Improved Hemodynamic Outcomes. JACC: Cardiovasc. Interv. 2022, 15, 1126–1136. [Google Scholar] [CrossRef]
- Nuis, R.J.; Yee, J.; Adrichem, R.; Hokken, T.W.; Lenzen, M.; Daemen, J.; de Jaegere, P.P.; Van Mieghem, N.M. Incidence and mechanisms of bioprosthetic dysfunction after transcatheter implantation of a mechanically-expandable heart valve. EuroIntervention 2022. [Google Scholar] [CrossRef]
- Bing, R.; Deutsch, M.-A.; Sellers, S.L.; Corral, C.A.; Andrews, J.P.; van Beek, E.J.; Bleiziffer, S.; Burchert, W.; Clark, T.; Dey, D.; et al. 18F-GP1 Positron Emission Tomography and Bioprosthetic Aortic Valve Thrombus. JACC: Cardiovasc. Imaging 2022, 15, 1107–1120. [Google Scholar] [CrossRef]
- Andrade, D.; E Vinck, E.; Zuluaga, J.F. Valve-sparing thrombectomy for aortic-valve bio-prosthetic thrombosis. Asian Cardiovasc. Thorac. Ann. 2021, 30, 211–212. [Google Scholar] [CrossRef]
- Kambeitz, C.; Kemp, W. Thrombosis of Bioprosthetic Valve Associated With Acute Myocardial Injury. Am. J. Forensic Med. Pathol. 2020, 42, e59–e60. [Google Scholar] [CrossRef] [PubMed]
- Bartus, K.; Litwinowicz, R.; Bilewska, A.; Stapor, M.; Bochenek, M.; Rozanski, J.; Sadowski, J.; Filip, G.; Kusmierczyk, M.; Kapelak, B. Final 5-year outcomes following aortic valve replacement with a RESILIA™ tissue bioprosthesis. Eur. J. Cardio-Thoracic Surg. 2021, 59, 434–441. [Google Scholar] [CrossRef]
- Leon, M.B.; Mack, M.J.; Hahn, R.T.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Alu, M.C.; Madhavan, M.V.; Chau, K.H.; Russo, M.; et al. Outcomes 2 Years After Transcatheter Aortic Valve Replacement in Patients at Low Surgical Risk. J. Am. Coll. Cardiol. 2021, 77, 1149–1161. [Google Scholar] [CrossRef]
- Hattori, T.; Yoshida, R.; Yoshida, Y.; Akita, S.; Kato, W.; Tajima, K.; Murohara, T. A case of acute myocardial infarction caused by a giant thrombus derived from an aneurysm of the sinus of valsalva and a bioprosthetic aortic valve. J. Echocardiogr. 2020, 19, 181–182. [Google Scholar] [CrossRef]
- Landes, U.; Webb, J.G.; De Backer, O.; Sondergaard, L.; Abdel-Wahab, M.; Crusius, L.; Kim, W.-K.; Hamm, C.; Buzzatti, N.; Montorfano, M.; et al. Repeat Transcatheter Aortic Valve Replacement for Transcatheter Prosthesis Dysfunction. J. Am. Coll. Cardiol. 2020, 75, 1882–1893. [Google Scholar] [CrossRef]
- A Chacon-Portillo, M.; Dhakal, B.; Janardhanan, R. Bioprosthetic aortic valve haemodynamic deterioration secondary to a thrombus. BMJ Case Rep. 2020, 13, e233400. [Google Scholar] [CrossRef]
- Petrescu, I.; Egbe, A.C.; Ionescu, F.; Nkomo, V.T.; Greason, K.L.; Pislaru, C.; Pellikka, P.A.; Connolly, H.M.; Pislaru, S.V. Long-Term Outcomes of Anticoagulation for Bioprosthetic Valve Thrombosis. J. Am. Coll. Cardiol. 2020, 75, 857–866. [Google Scholar] [CrossRef]
- Abdel-Wahab, M.; Landt, M.; Neumann, F.-J.; Massberg, S.; Frerker, C.; Kurz, T.; Kaur, J.; Toelg, R.; Sachse, S.; Jochheim, D.; et al. 5-Year Outcomes After TAVR With Balloon-Expandable Versus Self-Expanding Valves: Results From the CHOICE Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 1071–1082. [Google Scholar] [CrossRef]
- Kealhofer, J.V.; Markowitz, J.S.; Nijjar, P.S. Use of Computed Tomography to Distinguish Thrombus from Pannus on a Bioprosthetic Aortic Valve. Tex. Hear. Inst. J. 2019, 46, 219–221. [Google Scholar] [CrossRef]
- Bamford, P.; Rogers, J.; Bassin, L.; Kull, A. Large Bioprosthetic Aortic Valve Thrombi on DOACs. Hear. Lung Circ. 2019, 28, e139–e142. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Herman, B.; Koshy, G. Very late bioprosthetic aortic valve thrombosis. BMJ Case Rep. 2019, 12, e228871. [Google Scholar] [CrossRef]
- Leatherby, R.J.; Osman, M.; Birdi, I.; Serino, W. Early failure of a bioprosthetic aortic valve due to thrombus formation while on rivaroxaban. Eur. J. Cardio-Thoracic Surg. 2018, 55, 1231–1233. [Google Scholar] [CrossRef] [PubMed]
- Basra, S.S.; Gopal, A.; Hebeler, K.R.; Baumgarten, H.; Anderson, A.; Potluri, S.P.; Brinkman, W.T.; Szerlip, M.; Gopal, D.; Filardo, G.; et al. Clinical Leaflet Thrombosis in Transcatheter and Surgical Bioprosthetic Aortic Valves by Four-Dimensional Computed Tomography. Ann. Thorac. Surg. 2018, 106, 1716–1725. [Google Scholar] [CrossRef]
- Franzone, A.; Pilgrim, T.; Haynes, A.G.; Lanz, J.; Asami, M.; Praz, F.; Räber, L.; Roost, E.; Langhammer, B.; Windecker, S.; et al. Transcatheter aortic valve thrombosis: Incidence, clinical presentation and long-term outcomes. Eur. Hear. J. Cardiovasc. Imaging 2017, 19, 398–404. [Google Scholar] [CrossRef]
- Fan, J.; Lipatov, K.; Lane, W.; Mixon, T. Management of bioprosthetic cardiac valve thrombosis. Bayl. Univ. Med Cent. Proc. 2018, 31, 496–498. [Google Scholar] [CrossRef] [PubMed]
- O'Callaghan, M.; Chester, R.; Scheckel, C.; Lee, J.Z.; Fernandes, R.; Shamoun, F. Bioprosthetic Valve Thrombosis while on a Novel Oral Anticoagulant for Atrial Fibrillation. CASE 2018, 2, 54–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vollema, E.M.; Kong, W.K.F.; Katsanos, S.; Kamperidis, V.; van Rosendael, P.; Van Der Kley, F.; De Weger, A.; Marsan, N.A.; Delgado, V.; Bax, J.J. Transcatheter aortic valve thrombosis: The relation between hypo-attenuated leaflet thickening, abnormal valve haemodynamics, and stroke. Eur. Hear. J. 2017, 38, 1207–1217. [Google Scholar] [CrossRef]
- Couture, E.L.; Lepage, S.; Masson, J.-B.; Daneault, B. Very late transcatheter heart valve thrombosis. World J. Cardiol. 2017, 9, 196–199. [Google Scholar] [CrossRef]
- Dalén, M.; Sartipy, U.; Cederlund, K.; Franco-Cereceda, A.; Svensson, A.; Themudo, R.; Svenarud, P.; Brolin, E.B. Hypo-Attenuated Leaflet Thickening and Reduced Leaflet Motion in Sutureless Bioprosthetic Aortic Valves. J. Am. Hear. Assoc. 2017, 6, e005251. [Google Scholar] [CrossRef]
- Jose, J.; Sulimov, D.S.; El-Mawardy, M.; Sato, T.; Allali, A.; Holy, E.W.; Becker, B.; Landt, M.; Kebernik, J.; Schwarz, B.; et al. Clinical Bioprosthetic Heart Valve Thrombosis After Transcatheter Aortic Valve Replacement: Incidence, Characteristics, and Treatment Outcomes. JACC Cardiovasc. Interv. 2017, 10, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Regazzoli, D.; Ancona, M.B.; Mangieri, A.; Agricola, E.; Spagnolo, P.; Mussardo, M.; Colombo, A.; Latib, A. A Case of Very Late (3 Years) Transcatheter Heart Valve Thrombosis. JACC Cardiovasc. Interv. 2016, 9, e83–e84. [Google Scholar] [CrossRef] [PubMed]
- Del Trigo, M.; Muñoz-Garcia, A.J.; Wijeysundera, H.C.; Nombela-Franco, L.; Cheema, A.N.; Gutierrez, E.; Serra, V.; Kefer, J.; Amat-Santos, I.J.; Benitez, L.M.; et al. Incidence, Timing, and Predictors of Valve Hemodynamic Deterioration After Transcatheter Aortic Valve Replacement: Multicenter Registry. J. Am. Coll Cardiol. 2016, 67, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Gałąska, R.; Kulawiak-Gałąska, D.; Fijałkowski, M.; Szurowska, E.; Gruchała, M. Multidetector computed tomography to detect reversible subclinical aortic bioprosthetic valve thrombosis with high systolic gradients. Cardiol. J. 2016, 23, 411–412. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latib, A.; Naganuma, T.; Abdel-Wahab, M.; Danenberg, H.; Cota, L.; Barbanti, M.; Baumgartner, H.; Finkelstein, A.; Legrand, V.; de Lezo, J.S.; et al. Treatment and Clinical Outcomes of Transcatheter Heart Valve Thrombosis. Circ. Cardiovasc. Interv. 2015, 8, e001779. [Google Scholar] [CrossRef] [PubMed]
- Jander, N.; Sommer, H.; Pingpoh, C.; Kienzle, R.-P.; Martin, G.; Zeh, W.; Pache, G.; Siepe, M.; Beyersdorf, F.; Schumacher, M.; et al. The porcine valve type predicts obstructive thrombosis beyond the first three postoperative months in bioprostheses in the aortic position. Int. J. Cardiol. 2015, 199, 90–95. [Google Scholar] [CrossRef]
- Cremer, P.C.; Rodriguez, L.L.; Griffin, B.P.; Tan, C.D.; Rodriguez, E.R.; Johnston, D.R.; Pettersson, G.B.; Menon, V. Early Bioprosthetic Valve Failure: Mechanistic Insights via Correlation between Echocardiographic and Operative Findings. J. Am. Soc. Echocardiogr. 2015, 28, 1131–1148. [Google Scholar] [CrossRef] [PubMed]
- Orbach, A.; Karkabi, B.; Shiran, A. Reversible restenosis after transcatheter aortic valve implantation. Eur. Hear. J. Cardiovasc. Imaging 2013, 15, 350. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brown, M.L.; Park, S.J.; Sundt, T.M.; Schaff, H.V. Early thrombosis risk in patients with biologic valves in the aortic position. J. Thorac. Cardiovasc. Surg. 2012, 144, 108–111. [Google Scholar] [CrossRef]
- Jander, N.; Kienzle, R.-P.; Kayser, G.; Neumann, F.-J.; Gohlke-Baerwolf, C.; Minners, J. Usefulness of Phenprocoumon for the Treatment of Obstructing Thrombus in Bioprostheses in the Aortic Valve Position. Am. J. Cardiol. 2011, 109, 257–262. [Google Scholar] [CrossRef]
- Peeceeyen, S.; Cao, C.; Fermanis, G.; Manganas, C. Early stenosis of Medtronic Mosaic bioprosthesis in the aortic position. J. Thorac. Cardiovasc. Surg. 2011, 143, e13–e14. [Google Scholar] [CrossRef]
- Achouh, P.; Jemel, A.; Chaudeurge, A.; Redheuil, A.; Zegdi, R.; Fabiani, J.-N. Aortic Biological Valve Thrombosis in an HIV Positive Patient. Ann. Thorac. Surg. 2011, 91, e90–e91. [Google Scholar] [CrossRef]
- Ohnaka, M.; Nishimura, K.; Kurokawa, S. Flat Fibrin Thrombus Deposition on Tissue Valve After Aortic Valve Replacement. Ann. Thorac. Surg. 2010, 89, 2032–2034. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Fukuyama, O. "Reversible" late bioprosthetic aortic valve stenosis with spontaneous recovery. Hawaii Med J. 2009, 68. [Google Scholar]
- Juliard, J.M.; Paillole, C.; Dahan, M.; Steg, P.G.; Himbert, D.; Aumont, M.C. Late thrombotic obstruction of an aortic bioprosthetic valve: Successful treatment by oral anticoagulation. Clin. Cardiol. 1993, 16, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Schoen, F.J.; Mudge, G.H.; Collins, J.J. Thrombosis associated with a porcine bioprosthesis and ascending aortic graft in a patient with the Marfan syndrome. J. Thorac. Cardiovasc. Surg. 1983, 85, 27–33. [Google Scholar] [CrossRef]
- Rafiq, S.; Steinbrüchel, D.A.; Lilleør, N.B.; Møller, C.H.; Lund, J.T.; Thiis, J.J.; Køber, L.; Olsen, P.S. Antithrombotic therapy after bioprosthetic aortic valve implantation: Warfarin versus aspirin, a randomized controlled trial. Thromb. Res. 2016, 150, 104–110. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).