The Perivascular Fat Attenuation Index Improves the Diagnostic Performance for Functional Coronary Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Coronary Computed Tomography Angiography Acquisition
2.3. Coronary Plaque Analysis
2.4. Perivascular Fat Attenuation Index Acquisition
2.5. Invasive Coronary Angiography and Fractional Flow Reserve Measurements
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
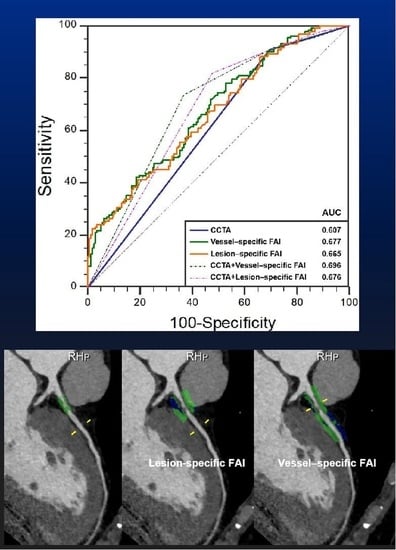
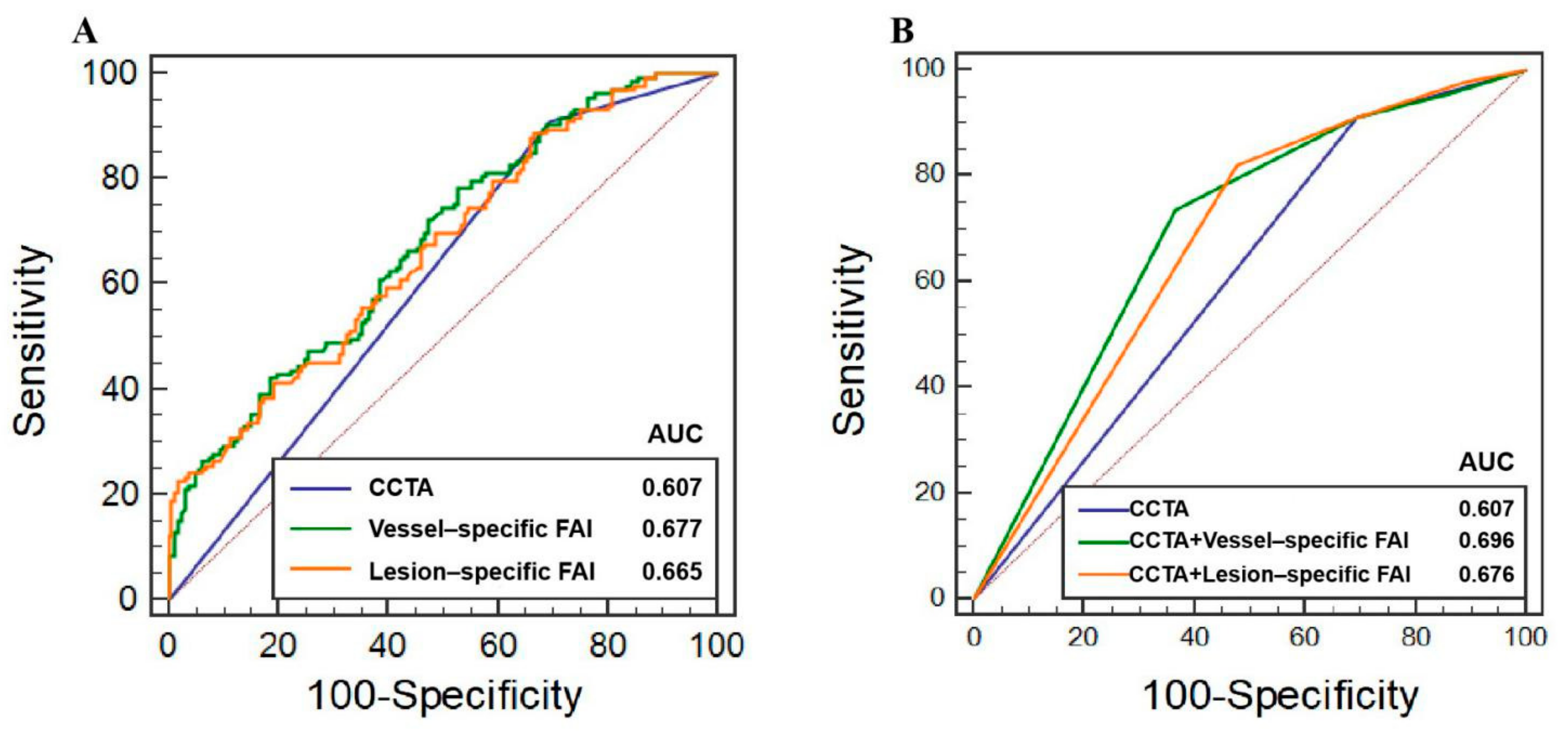
3.2. Discrimination of Ischemia
3.3. Additive Values of FAI to CCTA
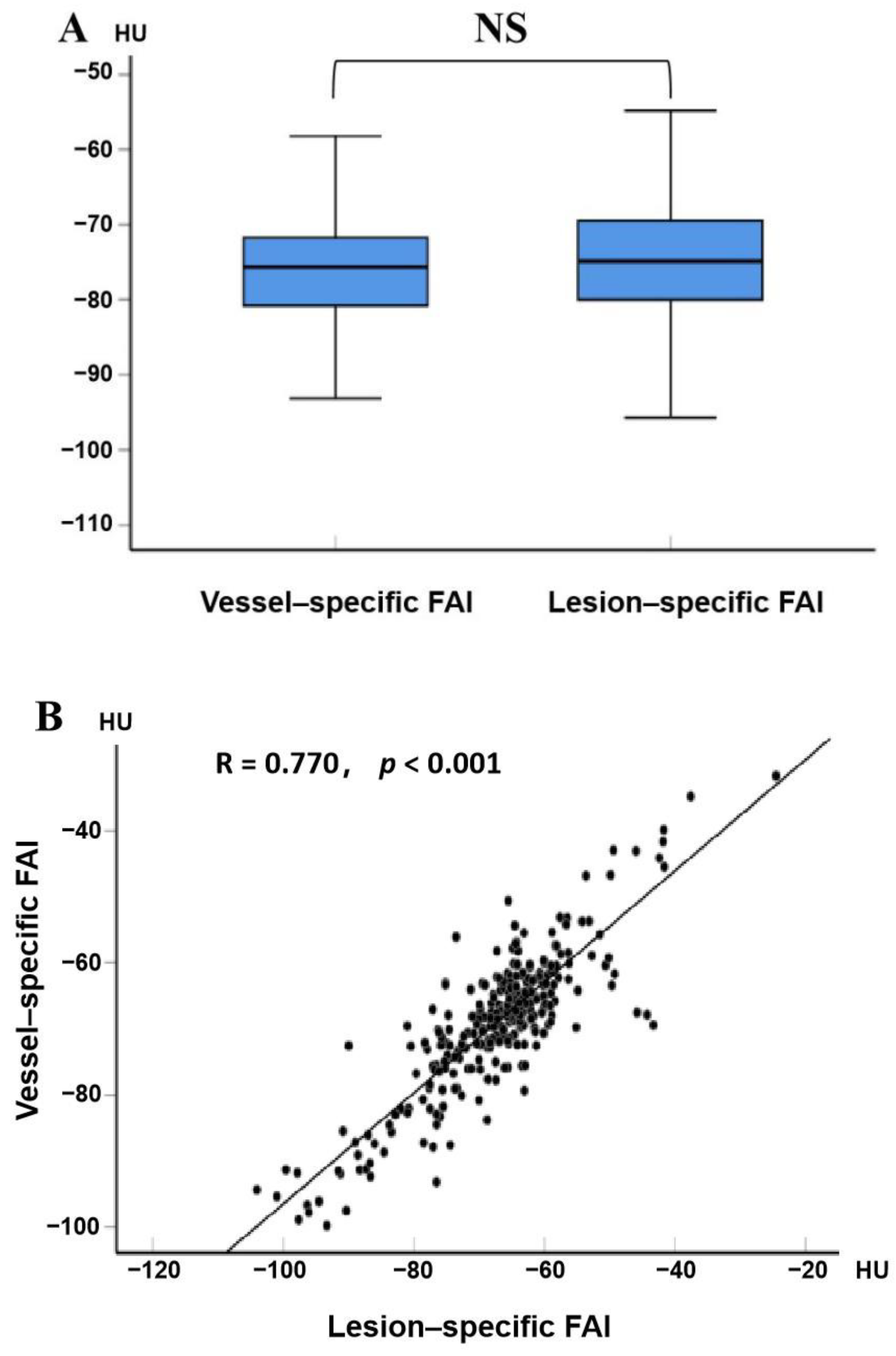
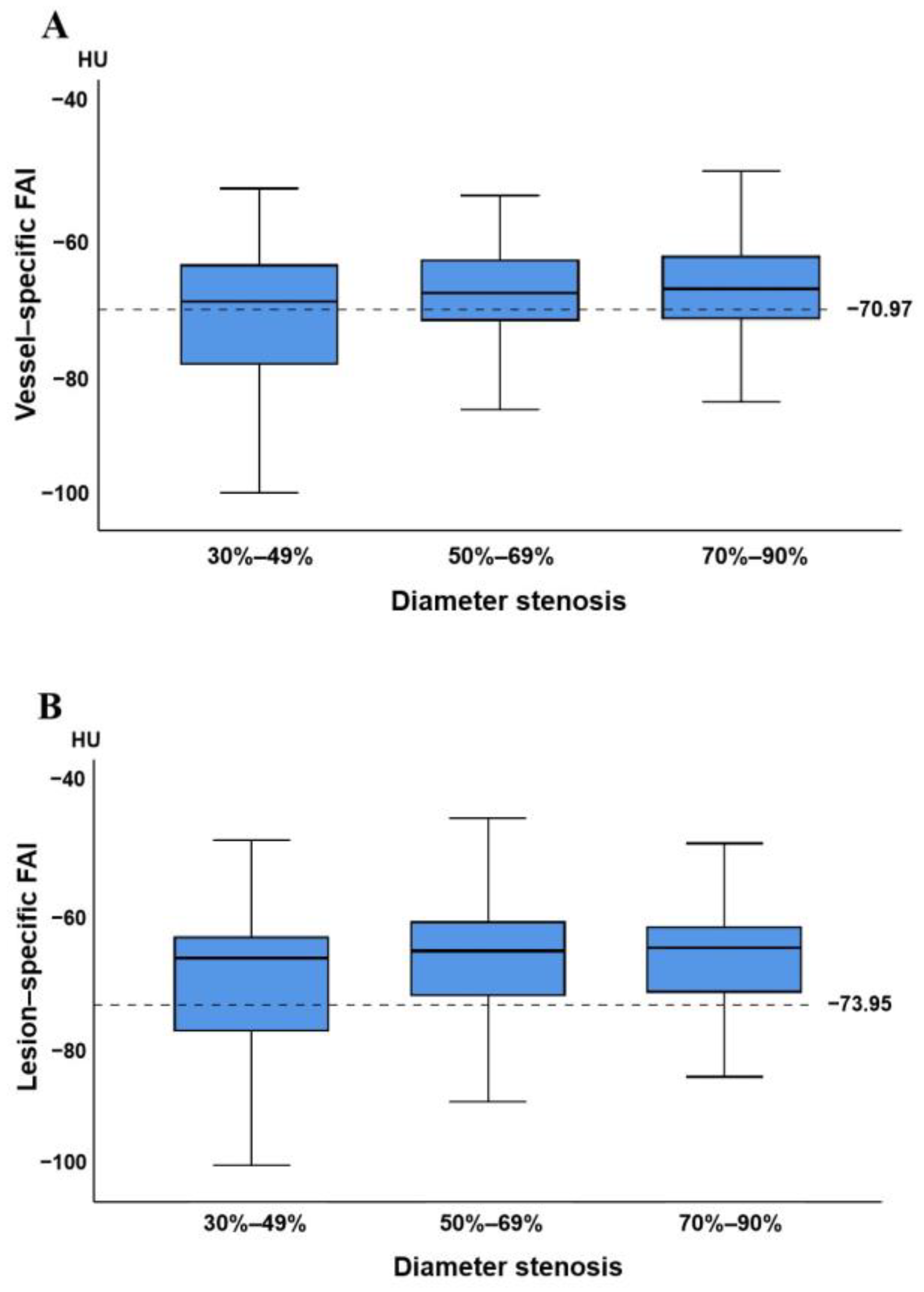
3.4. Relationship between Vessel-Specific FAI, Lesion-Specific FAI, and Coronary Stenosis
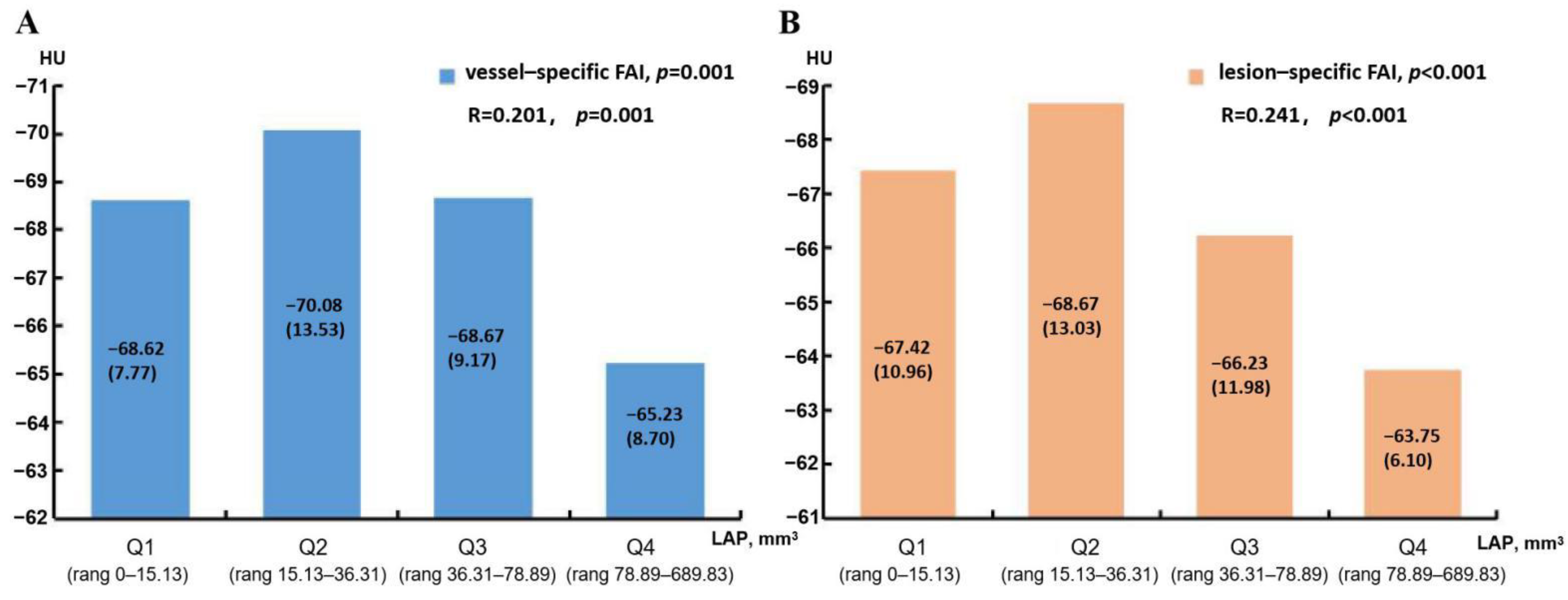
3.5. Predictors of Vessel-Specific FAI and Lesion-Specific FAI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Disease Burden and Mortality Estimates; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Moss, A.J.; Williams, M.C.; Newby, D.E.; Nicol, E.D. The Updated NICE Guidelines: Cardiac CT as the First-Line Test for Coronary Artery Disease. Curr. Cardiovasc. Imaging Rep. 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijboom, W.B.; Meijs, M.F.; Schuijf, J.; Cramer, M.J.; Mollet, N.R.; van Mieghem, C.A.; Nieman, K.; van Werkhoven, J.M.; Pundziute, G.; Weustink, A.C.; et al. Diagnostic Accuracy of 64-Slice Computed Tomography Coronary Angiography: A Prospective, Multicenter, Multivendor Study. J. Am. Coll. Cardiol. 2008, 52, 2135–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Un-dergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar] [PubMed] [Green Version]
- Meijboom, W.B.; Van Mieghem, C.A.G.; van Pelt, N.; Weustink, A.; Pugliese, F.; Mollet, N.R.; Boersma, E.; Regar, E.; van Geuns, R.J.; de Jaegere, P.J.; et al. Comprehensive assessment of coronary artery stenoses: Computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J. Am. Coll. Cardiol. 2008, 52, 636–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaw, L.J.; Berman, D.S.; Maron, D.J.; Mancini, G.B.J.; Hayes, S.W.; Hartigan, P.M.; Weintraub, W.S.; O’Rourke, R.A.; Dada, M.; Spertus, J.A.; et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: Results from the Clinical Outcomes Utilizing Revascularization and Aggres-sive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008, 117, 1283–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erne, P.; Schoenenberger, A.W.; Burckhardt, D.; Zuber, M.; Kiowski, W.; Buser, P.T.; Dubach, P.; Resink, T.J.; Pfisterer, M. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: The SWISSI II randomized controlled trial. JAMA 2007, 297, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, M.; Sanna, F.; Lazaros, G.; Akoumianakis, I.; Sheena, P.; Antonopoulos, A.S.; Duke, C.; Herdman, L.; Psarros, C.; Oikonomou, E.K.; et al. Predictive value of telomere length on outcome following acute myocardial in-farction: Evidence for contrasting effects of vascular vs. blood oxidative stress. Eur. Heart J. 2017, 38, 3094–3104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomog-raphy and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [Green Version]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.-M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef] [Green Version]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared With Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, M.; Yang, S.; Sugiyama, T.; Zhang, J.; Kanaji, Y.; Yamaguchi, M.; Hada, M.; Sumino, Y.; Horie, T.; Nogami, K.; et al. Peri-coronary inflammation is associated with findings on coronary computed tomography angiography and fractional flow reserve. J. Cardiovasc. Comput. Tomogr. 2020, 14, 483–489. [Google Scholar] [CrossRef]
- Yu, M.; Dai, X.; Deng, J.; Lu, Z.; Shen, C.; Zhang, J. Diagnostic performance of perivascular fat attenuation index to predict hemodynamic signifi-cance of coronary stenosis: A preliminary coronary computed tomography angiography study. Eur. Radiol. 2020, 30, 673–681. [Google Scholar] [CrossRef]
- Wen, D.; Li, J.; Ren, J.; Zhao, H.; Li, J.; Zheng, M. Pericoronary adipose tissue CT attenuation and volume: Diagnostic performance for hemody-namically significant stenosis in patients with suspected coronary artery disease. Eur. J. Radiol. 2021, 140, 109740. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Chen, X.; Ma, Y.; Liu, H.; Zhang, J.; Xu, L.; Wang, Y.; Liu, T.; Wang, K.; Yang, J.; et al. Lesion-Specific Peri-Coronary Fat Attenuation Index Is Associated With Functional Myocardial Ischemia Defined by Abnormal Fractional Flow Reserve. Front. Cardiovasc. Med. 2021, 8, 755295. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed to-mography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-B.; Heo, R.; Hartaigh, B.; Cho, I.; Gransar, H.; Nakazato, R.; Leipsic, J.; Mancini, G.B.J.; Koo, B.-K.; Otake, H.; et al. Atherosclerotic plaque characteristics by CT angiography identify coronary lesions that cause ischemia: A direct comparison to fractional flow reserve. JACC Cardiovasc. Imaging 2015, 8, 1–10. [Google Scholar] [CrossRef]
- Pijls, N.H.; van Schaardenburgh, P.; Manoharan, G.; Boersma, E.; Bech, J.-W.; Veer, M.V.; Bär, F.; Hoorntje, J.; Koolen, J.; Wijns, W.; et al. Percutaneous Coronary Intervention of Functionally Nonsignificant Stenosis: 5-Year Follow-Up of the DEFER Study. J. Am. Coll. Cardiol. 2007, 49, 2105–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonino, P.A.L.; De Bruyne, B.; Pijls, N.H.J.; Siebert, U.; Ikeno, F.; van Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. Fractional Flow Reserve versus Angiography for Guiding Percutaneous Coronary Intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef] [Green Version]
- De Bruyne, B.; Pijls, N.H.; Kalesan, B.; Barbato, E.; Tonino, P.A.; Piroth, Z.; Jagic, N.; Mobius-Winckler, S.; Rioufol, G.; Witt, N.; et al. Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease. N. Engl. J. Med. 2012, 367, 991–1001. [Google Scholar] [CrossRef] [Green Version]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Dunn, O.J. Multiple Comparisons Using Rank Sums. Technometrics 1964, 6, 241–252. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino, R.B., Sr.; D’Agostino, R.B., Jr.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Paynter, N.P. Performance of reclassification statistics in comparing risk prediction models. Biom. J. 2011, 53, 237–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis is an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Lavi, S.; McConnell, J.P.; Rihal, C.S.; Prasad, A.; Mathew, V.; Lerman, L.O.; Lerman, A. Local production of lipoprotein-associated phospholipase A2 and lysophosphati-dylcholine in the coronary circulation: Association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation 2007, 115, 2715–2721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmadi, N.; Ruiz-García, J.; Hajsadeghi, F.; Azen, S.; Mack, W.; Hodis, H.; Lerman, A. Impaired coronary artery distensibility is an endothelium-dependent process and is associated with vulnerable plaque composition. Clin. Physiol. Funct. Imaging 2014, 36, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Yang, L.; Liu, Y.; Yang, B.; Lv, S.; Hu, C.; Zhu, Y.; Zhang, H.; Ma, Q.; Wang, Z.; et al. Relation between quantity and quality of peri-coronary epicardial adipose tissue and its underlying hemodynamically significant coronary stenosis. BMC Cardiovasc. Disord. 2020, 20, 226. [Google Scholar] [CrossRef]
- Goeller, M.; Tamarappoo, B.K.; Kwan, A.C.; Cadet, S.; Commandeur, F.; Razipour, A.; Slomka, P.J.; Gransar, H.; Chen, X.; Otaki, Y.; et al. Relationship between changes in pericoronary adipose tissue attenuation and coronary plaque burden quantified from coronary computed tomography angiography. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Lavi, S.; Yang, E.H.; Prasad, A.; Mathew, V.; Barsness, G.W.; Rihal, C.S.; Lerman, L.O.; Lerman, A. The Interaction Between Coronary Endothelial Dysfunction, Local Oxidative Stress, and Endogenous Nitric Oxide in Humans. Hypertension 2008, 51, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Cereda, A.; Toselli, M.; Palmisano, A.; Vignale, D.; Khokhar, A.; Campo, G.; Bertini, M.; Loffi, M.; Andreini, D.; Pontone, G.; et al. Coronary calcium score as a predictor of outcomes in the hypertensive COVID-19 population: Results from the Italian (S) Core-COVID-19 Registry. Hypertens. Res. 2021, 45, 333–343. [Google Scholar] [CrossRef]
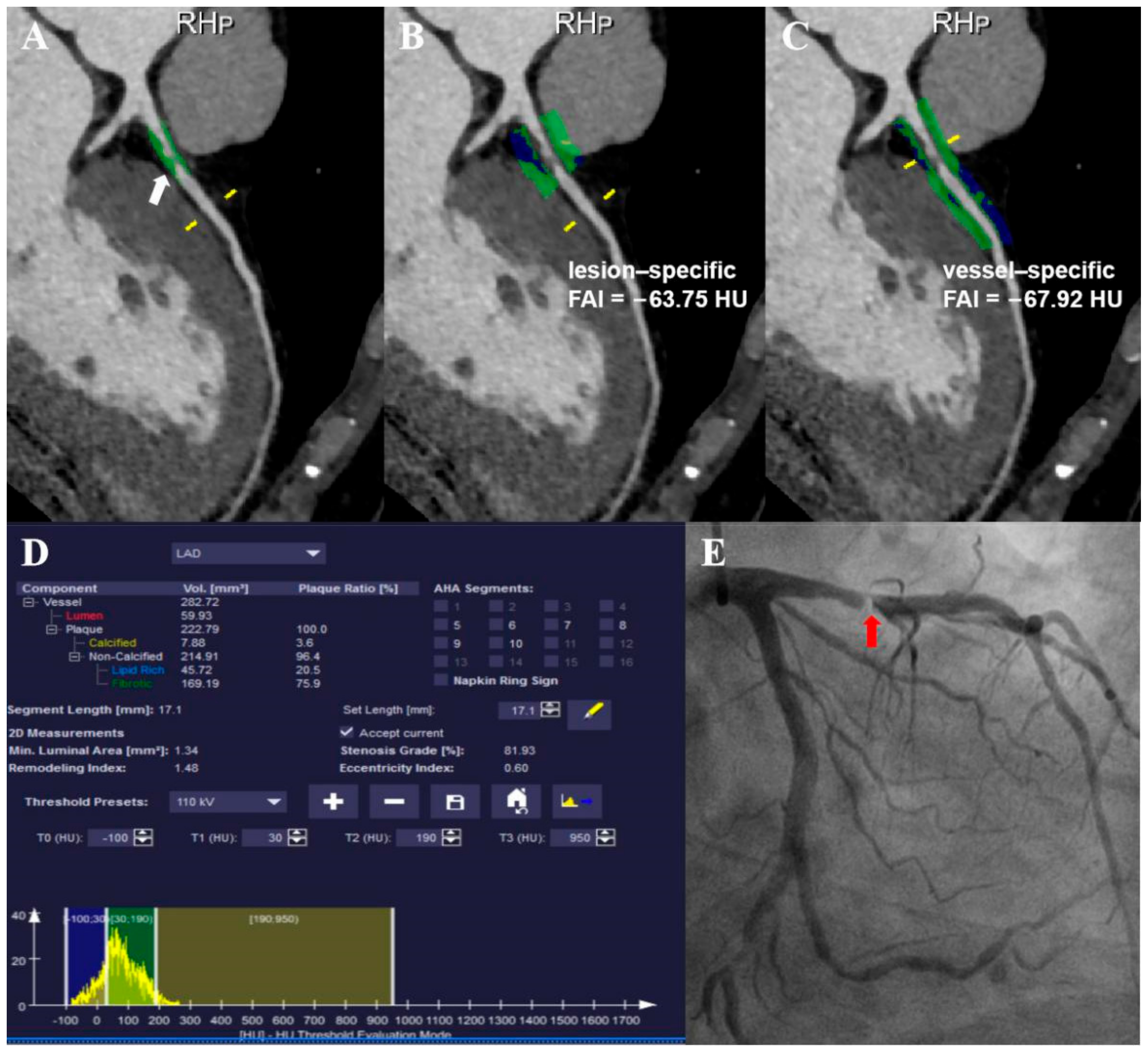
| Characteristic | Value |
|---|---|
| Number of patients, n | 261 |
| Number of lesion vessels, n | 294 |
| Age, year | 56.3 ± 8.7 |
| Male, n (%) | 188 (72.03) |
| Body mass index, kg/m2 | 25.9 ± 3.1 |
| Risk factors | |
| Hypertension, n (%) | 153 (58.6) |
| Diabete, n (%) | 83 (31.8) |
| Dyslipidemia, n (%) | 222 (85.1) |
| Smoker, n (%) | 140 (53.6) |
| Family history of CAD, n (%) | 47 (18.0) |
| Distribution of lesion | |
| LAD, n (%) | 210 (71.4) |
| LCX, n (%) | 35 (11.9) |
| RCA, n (%) | 49 (16.7) |
| Calcification score | 63.00 (3.18–208.45) |
| <400, n (%) | 136 (52.1) |
| ≥400, n (%) | 125 (47.9) |
| CCTA stenosis | |
| Stenosis between 30–49%, n (%) | 64 (21.77) |
| Stenosis between 50–69%, n (%) | 114 (38.78) |
| Stenosis between 70–90%, n (%) | 119 (40.48) |
| Invasive FFR | 0.80 ± 0.11 |
| Vessels with FFR ≤ 0.80, n (%) | 133 (45.2) |
| RCA with FFR ≤ 0.80, n (%) | 12 (4.1) |
| LAD with FFR ≤ 0.80, n (%) | 46 (15.6) |
| LCX with FFR ≤ 0.80, n (%) | 75 (25.5) |
| True Positive a | True Negative a | False Positive a | False Negative a | % Accuracy | % Sensitivity | % Specificity | % PPV | % NPV | AUC | |
|---|---|---|---|---|---|---|---|---|---|---|
| CCTA | 121 | 49 | 112 | 12 | 57.82 (54.71–60.83) | 90.98 (84.77–95.25) | 30.44 (23.44–38.17) | 51.93 (49.05–54.80) | 80.33 (69.40–88.03) | 0.607 (0.549–0.663) |
| Vessel-specific FAI ≥ −70.97 HU | 104 | 75 | 86 | 29 | 60.88 (0.578–0.639) | 78.20 (70.21–84.89) | 46.58 (38.70–54.60) | 54.74 (50.50–58.90) | 72.12 (64.30–78.79) | 0.677 (0.620–0.730) |
| Lesion-specific FAI ≥ −73.95 HU | 118 | 53 | 108 | 15 | 58.16 (55.12–61.22) | 88.72 (82.08–93.55) | 32.92 (25.73–40.75) | 52.21 (49.11–55.29) | 77.94 (67.64–85.66) | 0.665 (0.608–0.719) |
| AUC (95% CI) | Difference with CCTA (95% CI) | p | NRI (95% CI) | p | IDI (95% CI) | p | |
|---|---|---|---|---|---|---|---|
| CCTA | 0.607 (0.549–0.663) | - | - | - | - | - | - |
| CCTA + Vessel-specific FAI | 0.696 (0.640–0.748) | 0.089 (0.050–0.127) | <0.001 | 49.6% (28.1–70.4%) | <0.001 | 5.9% (3.3–8.4%) | <0.001 |
| CCTA + Lesion-specific FAI | 0.676 (0.619–0.729) | 0.069 (0.036–0.102) | <0.001 | 43.3% (25.2–61.4%) | <0.001 | 5.4% (2.9–7.8%) | <0.001 |
| Vessel-Specific FAI | Lesion-Specific FAI | |||||
|---|---|---|---|---|---|---|
| ≥−70.97 HU (n = 190) | <−70.97 HU (n = 104) | p | ≥−73.95 HU (n = 226) | <−73.95 HU (n = 68) | p | |
| Age, year | 56.4 ± 8.8 | 56.2 ± 8.6 | 0.822 | 55.9 ± 8.8 | 57.9 ± 8.3 | 0.097 |
| Male, n (%) a | 146 (76.84) | 67 (64.42) | 0.023 | 178 (78.76) | 38 (55.88) | <0.001 |
| Body mass index, kg/m2 | 25.86 ± 3.15 | 25.90 ± 3.02 | 0.911 | 25.86 ± 3.15 | 25.90 ± 2.96 | 0.920 |
| hsCRP, mg/L | 1.10 (1.57) | 1.08 (1.74) | 0.980 | 1.04 (1.57) | 1.10 (1.75) | 0.871 |
| Hypertension, n (%) a | 113 (59.47) | 60 (64.42) | 0.767 | 133 (58.85) | 40 (58.82) | 0.997 |
| Diabete, n (%) a | 56 (29.47) | 34 (32.69) | 0.567 | 66 (29.20) | 24 (35.29) | 0.339 |
| Dyslipidemia, n (%) a | 160 (84.21) | 89 (85.58) | 0.756 | 193 (85.40) | 56 (82.35) | 0.541 |
| Smoker, n (%) a | 109 (57.37) | 50 (48.08) | 0.126 | 130 (57.52) | 29 (42.65) | 0.031 |
| Family history of CAD, n (%) a | 36 (18.95) | 17 (16.35) | 0.579 | 41 (18.14) | 12 (17.65) | 0.926 |
| TPV, mm3 | 282.21 (215.82) | 231.95 (218.76) | 0.012 | 267.21 (216.64) | 252.89 (234.30) | 0.182 |
| CP volume, mm3 | 17.38 (53.67) | 15.89 (37.15) | 0.963 | 15.53 (49.13) | 18.41 (45.85) | 0.308 |
| IAP volume, mm3 | 182.83 (135.15) | 168.87 (157.20) | 0.112 | 182.32 (132.45) | 173.34 (168.22) | 0.442 |
| LAP volume, mm3 | 49.70 (89.30) | 30.65 (35.59) | 0.002 | 44.63 (77.36) | 28.60 (36.02) | 0.002 |
| CS | 57.00 (212.35) | 64.50 (154.15) | 0.782 | 57.00 (188.58) | 82.00 (314.40) | 0.125 |
| Plaque length, mm | 24.28 (18.98) | 18.71 (18.52) | 0.010 | 23.59 (18.16) | 18.29 (23.88) | 0.054 |
| PR, n (%) a | 57 (30.00) | 30 (28.85) | 0.836 | 70 (30.97) | 17 (25.00) | 0.344 |
| Univariable Analysis | ||||
|---|---|---|---|---|
| Vessel-Specific FAI | Lesion-Specific FAI | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| Age | 1.003 (0.976–1.031) | 0.821 | 0.973 (0.943–1.005) | 0.097 |
| Sex | 1.832 (1.085–3.095) | 0.024 | 2.708 (1.530–4.796) | 0.001 |
| Body mass index | 0.996 (0.922–1.076) | 0.911 | 0.995 (0.912–1.087) | 0.919 |
| hsCRP | 1.025 (0.937–1.122) | 0.586 | 1.067 (0.951–1.198) | 0.267 |
| Hypertension | 1.076 (0.663–1.748) | 0.767 | 1.001 (0.577–1.737) | 0.997 |
| Diabete | 0.860 (0.514–1.440) | 0.567 | 0.756 (0.426–1.343) | 0.340 |
| Dyslipidemia | 0.899 (0.459–1.760) | 0.756 | 1.253 (0.607–2.587) | 0.541 |
| Smoker | 1.453 (0.899–2.349) | 0.127 | 1.821 (1.053–3.151) | 0.032 |
| Family history of CAD | 1.196 (0.635–2.255) | 0.579 | 1.034 (0.509–2.102) | 0.926 |
| TPV | 1.002 (1.001–1.004) | 0.009 | 1.001 (1.000–1.003) | 0.111 |
| CP volume | 1.002 (0.997–1.006) | 0.405 | 0.999 (0.995–1.004) | 0.789 |
| IAP volume | 1.002 (1.000–1.004) | 0.063 | 1.001 (0.999–1.004) | 0.292 |
| LAP volume | 1.009 (1.004–1.014) | 0.001 | 1.010 (1.003–1.016) | 0.003 |
| CS | 1.000 (1.000–1.001) | 0.548 | 0.999 (0.999–1.000) | 0.137 |
| Plaque length | 1.018 (1.001–1.036) | 0.034 | 1.011 (0.992–1.029) | 0.258 |
| PR | 1.057 (0.625–1.788) | 0.836 | 1.346 (0.726–2.495) | 0.345 |
| Multivariable Analysis | ||||
| Vessel-Specific FAI | Lesion-Specific FAI | |||
| OR (95% CI) | p | OR (95% CI) | p | |
| Sex | 1.541 (0.897–2.650) | 0.117 | 1.478 (0.780–2.800) | 0.231 |
| Smoker | - | - | 1.104 (0.616–1.980) | 0.739 |
| TPV | 1.000 (0.997–1.002) | 0.818 | - | - |
| LAP volume | 1.008 (1.001–1.014) | 0.016 | 1.008 (1.003–1.014) | 0.002 |
| Plaque length | 1.010 (0.989–1.032) | 0.338 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, H.; Zhao, N.; Geng, W.; Hou, Z.; Gao, Y.; Lu, B. The Perivascular Fat Attenuation Index Improves the Diagnostic Performance for Functional Coronary Stenosis. J. Cardiovasc. Dev. Dis. 2022, 9, 128. https://doi.org/10.3390/jcdd9050128
Yan H, Zhao N, Geng W, Hou Z, Gao Y, Lu B. The Perivascular Fat Attenuation Index Improves the Diagnostic Performance for Functional Coronary Stenosis. Journal of Cardiovascular Development and Disease. 2022; 9(5):128. https://doi.org/10.3390/jcdd9050128
Chicago/Turabian StyleYan, Hankun, Na Zhao, Wenlei Geng, Zhihui Hou, Yang Gao, and Bin Lu. 2022. "The Perivascular Fat Attenuation Index Improves the Diagnostic Performance for Functional Coronary Stenosis" Journal of Cardiovascular Development and Disease 9, no. 5: 128. https://doi.org/10.3390/jcdd9050128
APA StyleYan, H., Zhao, N., Geng, W., Hou, Z., Gao, Y., & Lu, B. (2022). The Perivascular Fat Attenuation Index Improves the Diagnostic Performance for Functional Coronary Stenosis. Journal of Cardiovascular Development and Disease, 9(5), 128. https://doi.org/10.3390/jcdd9050128