Telemonitoring Potential of Wearable Cardioverter-Defibrillators during the Follow-Up of Patients with Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
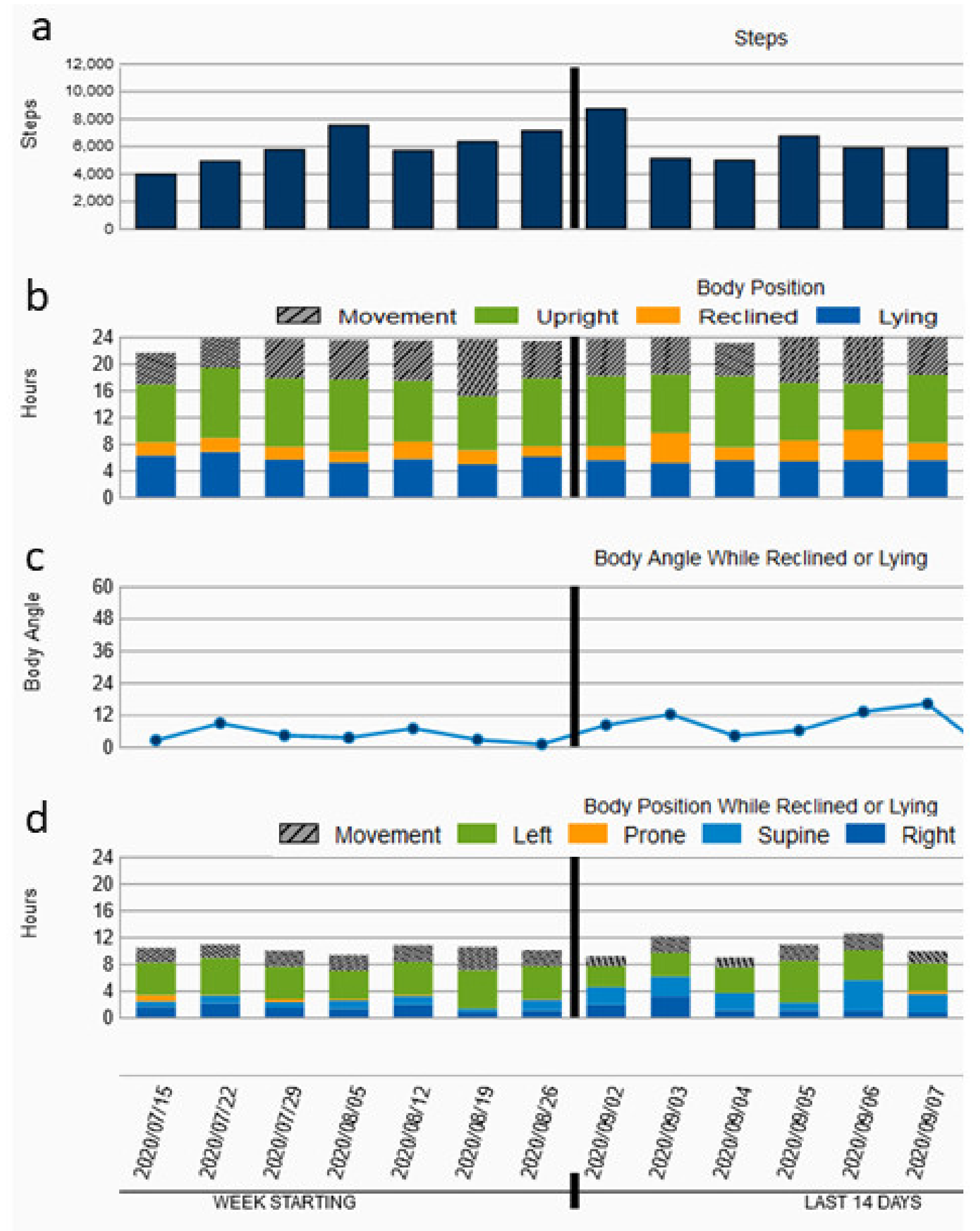
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zipes, D.P.; Wellens, H.J. Sudden cardiac death. Circulation 1998, 98, 2334–2351. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L., Jr. Heart Failure with Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Bohm, M.; Burri, H.; Butler, J.; Celutkiene, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Wassnig, N.K.; Gunther, M.; Quick, S.; Pfluecke, C.; Rottstadt, F.; Szymkiewicz, S.J.; Ringquist, S.; Strasser, R.H.; Speiser, U. Experience with the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation 2016, 134, 635–643. [Google Scholar] [CrossRef]
- Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Wranicz, J.; Malik, R.; Morin, D.P.; Zweibel, S.; Buxton, A.E.; Elayi, C.S.; Chung, E.H.; et al. Wearable Cardioverter-Defibrillator after Myocardial Infarction. N. Engl. J. Med. 2018, 379, 1205–1215. [Google Scholar] [CrossRef]
- Blockhaus, C.; List, S.; Waibler, H.P.; Gulker, J.E.; Klues, H.; Bufe, A.; Seyfarth, M.; Koektuerk, B.; Shin, D.I. Wearable Cardioverter-Defibrillator Used as a Telemonitoring System in a Real-Life Heart Failure Unit Setting. J. Clin. Med. 2021, 10, 5435. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Piercy, K.L.; Troiano, R.P.; Ballard, R.M.; Carlson, S.A.; Fulton, J.E.; Galuska, D.A.; George, S.M.; Olson, R.D. The Physical Activity Guidelines for Americans. JAMA 2018, 320, 2020–2028. [Google Scholar] [CrossRef]
- Saint-Maurice, P.F.; Troiano, R.P.; Bassett, D.R., Jr.; Graubard, B.I.; Carlson, S.A.; Shiroma, E.J.; Fulton, J.E.; Matthews, C.E. Association of Daily Step Count and Step Intensity with Mortality Among US Adults. JAMA 2020, 323, 1151–1160. [Google Scholar] [CrossRef]
- Burch, A.E.; D’Souza, B.; Gimbel, J.R.; Rohrer, U.; Masuda, T.; Sears, S.; Scherr, D. Physical activity is reduced prior to ventricular arrhythmias in patients with a wearable cardioverter defibrillator. Clin. Cardiol. 2020, 43, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Tripp, C.; Burch, A.E.; Erath, J.W.; Hain, A.; Sears, S.F. Physical Activity in Adults with Wearable Cardioverter Defibrillators in the Post-Myocardial Infarction Period. J. Cardiopulm. Rehabil. Prev. 2020, 40, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Olgin, J.E.; Lee, B.K.; Vittinghoff, E.; Morin, D.P.; Zweibel, S.; Rashba, E.; Chung, E.H.; Borggrefe, M.; Hulley, S.; Lin, F.; et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J. Cardiovasc. Electrophysiol. 2020, 31, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Dontje, M.L.; van der Wal, M.H.; Stolk, R.P.; Brugemann, J.; Jaarsma, T.; Wijtvliet, P.E.; van der Schans, C.P.; de Greef, M.H. Daily physical activity in stable heart failure patients. J. Cardiovasc. Nurs. 2014, 29, 218–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romiti, G.F.; Recchia, F.; Zito, A.; Visioli, G.; Basili, S.; Raparelli, V. Sex and Gender-Related Issues in Heart Failure. Heart Fail. Clin. 2020, 16, 121–130. [Google Scholar] [CrossRef]
- Savarese, G.; D’Amario, D. Sex Differences in Heart Failure. Adv. Exp. Med. Biol. 2018, 1065, 529–544. [Google Scholar] [CrossRef]
- Rodriguez, F.; Wang, Y.; Johnson, C.E.; Foody, J.M. National patterns of heart failure hospitalizations and mortality by sex and age. J. Card. Fail. 2013, 19, 542–549. [Google Scholar] [CrossRef]
- De Koninck, J.; Lorrain, D.; Gagnon, P. Sleep positions and position shifts in five age groups: An ontogenetic picture. Sleep 1992, 15, 143–149. [Google Scholar] [CrossRef] [Green Version]
- Fujita, M.; Miyamoto, S.; Tambara, K.; Budgell, B. Trepopnea in patients with chronic heart failure. Int. J. Cardiol. 2002, 84, 115–118. [Google Scholar] [CrossRef]
- Ozeke, O.; Ertan, C.; Demir, A.D. Sleep apnea, heart failure, and sleep position. Sleep Breath. 2012, 16, 933–935. [Google Scholar] [CrossRef]
- Bayraktar, M.F.; Ozeke, O. Serial echocardiographic changes with different body positions and sleeping side preference in heart failure patients. Echocardiography 2018, 35, 1132–1137. [Google Scholar] [CrossRef]
- Joho, S.; Oda, Y.; Hirai, T.; Inoue, H. Impact of sleeping position on central sleep apnea/Cheyne-Stokes respiration in patients with heart failure. Sleep Med. 2010, 11, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Basoglu, O.K.; Keskin, B.; Tasbakan, M.S.; Gurgun, C. Effect of Semirecumbent Sleep Position on Severity of Obstructive Sleep Apnea in Patients with Heart Failure. J. Card. Fail. 2015, 21, 842–847. [Google Scholar] [CrossRef]
- OrDonnell, J.; Velardo, C.; Shah, S.A.; Khorshidi, G.S.; Salvi, D.; Rahimi, K.; Tarassenko, L. Physical Activity and Sleep Analysis of Heart Failure Patients using Multi-sensor Patches. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2018, 2018, 6092–6095. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]


| n = 140 | |
|---|---|
| Sex (female) | 32 (22.86%) |
| Age (years) | 57.91 ± 12.76 |
| Diagnosis (ICM/DCM) | ICM: 78 (55.71%) DCM: 62 (44.29%) |
| First diagnosis | 104 (74.28%) |
| Ejection fraction (%) | 26.9 ± 7.35 |
| NYHA stage | 2.80 ± 0.86 |
| BMI | 29.29 ± 7.04 |
| CAD | 92 (65.71%) |
| ATH | 89 (63.57%) |
| History of stroke | 16 (11.43%) |
| Diabetes mellitus II | 38 (27.14%) |
| OSAS | 17 (12.14%) |
| COPD | 19 (13.57%) |
| History AF | 40 (28.57%) |
| Creatinine (mg/dL) | 1.09 ± 0.49 |
| BNP (pg/mL) | 4798.34 ± 6769.94 |
| n = 140 | |
|---|---|
| Betablocker | 140 (100%) |
| ACE-I/AT | 99 (70.71%) |
| ATR | 127 (90.71%) |
| ARNI | 40 (28.57%) |
| Ivabradin | 11 (7.87%) |
| Digitalis | 13 (9.28%) |
| OAC | 53 (37.85%) |
| n = 140 | |
|---|---|
| EF after follow-up | 37.05 ± 9.12 |
| ICD/CRT-D | 39 (27.85%) |
| Compliance (h/d) | 21.39 ± 3.98 |
| Duration of WCD use (days) | 59.78 ± 35.72 |
| Average heart rate (bpm) | 70.06 ± 11.89 |
| Average daily steps | 6027.45 ± 3564.38 |
| Average upright position (%) | 52.15 ± 15.67 |
| Average reclined position (%) | 17.64 ± 10.51 |
| Average flat position (%) | 30.15 ± 14.93 |
| Average left position (%) | 22.59 ± 14.53 |
| Average prone position (%) | 9.31 ± 13.38 |
| Average right position (%) | 21.23 ± 14.58 |
| Average supine position (%) | 46.91 ± 20.45 |
| Coefficient | 95% CI | p-Value | |
|---|---|---|---|
| Age (years) | −59 | −111; −7 | 0.027 |
| BMI | −117 | −186; −47 | 0.001 |
| Sex (m vs. f) | 1347 | 95; 2599 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blockhaus, C.; Guelker, J.-E.; Feyen, L.; Bufe, A.; Seyfarth, M.; Shin, D.-I. Telemonitoring Potential of Wearable Cardioverter-Defibrillators during the Follow-Up of Patients with Heart Failure. J. Cardiovasc. Dev. Dis. 2022, 9, 175. https://doi.org/10.3390/jcdd9060175
Blockhaus C, Guelker J-E, Feyen L, Bufe A, Seyfarth M, Shin D-I. Telemonitoring Potential of Wearable Cardioverter-Defibrillators during the Follow-Up of Patients with Heart Failure. Journal of Cardiovascular Development and Disease. 2022; 9(6):175. https://doi.org/10.3390/jcdd9060175
Chicago/Turabian StyleBlockhaus, Christian, Jan-Erik Guelker, Ludger Feyen, Alexander Bufe, Melchior Seyfarth, and Dong-In Shin. 2022. "Telemonitoring Potential of Wearable Cardioverter-Defibrillators during the Follow-Up of Patients with Heart Failure" Journal of Cardiovascular Development and Disease 9, no. 6: 175. https://doi.org/10.3390/jcdd9060175
APA StyleBlockhaus, C., Guelker, J.-E., Feyen, L., Bufe, A., Seyfarth, M., & Shin, D.-I. (2022). Telemonitoring Potential of Wearable Cardioverter-Defibrillators during the Follow-Up of Patients with Heart Failure. Journal of Cardiovascular Development and Disease, 9(6), 175. https://doi.org/10.3390/jcdd9060175





