20 Years of Real-World Data to Estimate the Prevalence of Heart Failure and Its Subtypes in an Unselected Population of Integrated Care Units
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Settings
2.2. Study Definitions
2.2.1. Type 2 Diabetes Mellitus Definition
2.2.2. Heart Failure Definition
2.2.3. Heart Failure with Reduced LVEF Definition
2.2.4. Heart Failure with Mildly Reduced LVEF Definition
2.2.5. Heart Failure with Preserved LVEF Definition
2.2.6. Additional Comorbidities Definition
2.2.7. Medication Definitions
2.3. Statistical Analysis
3. Results
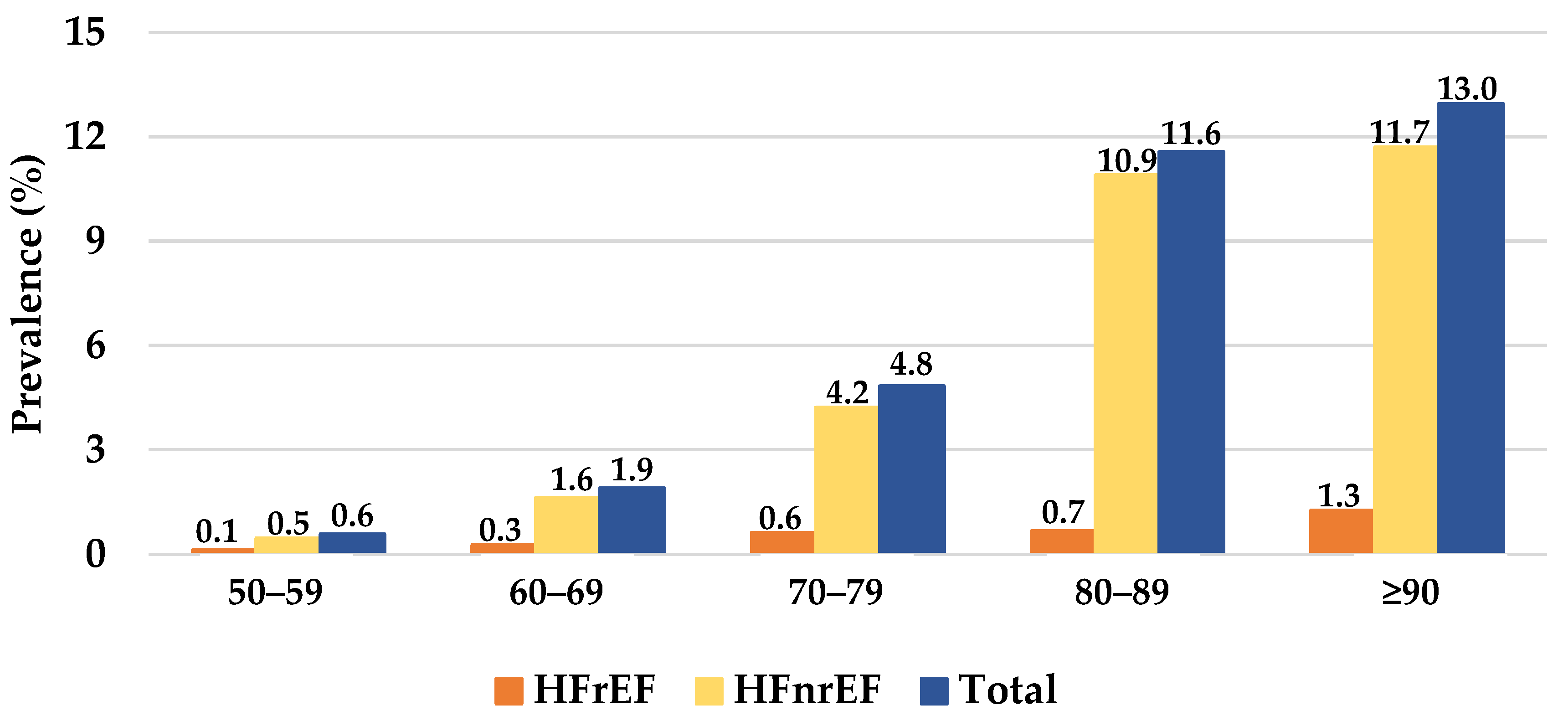
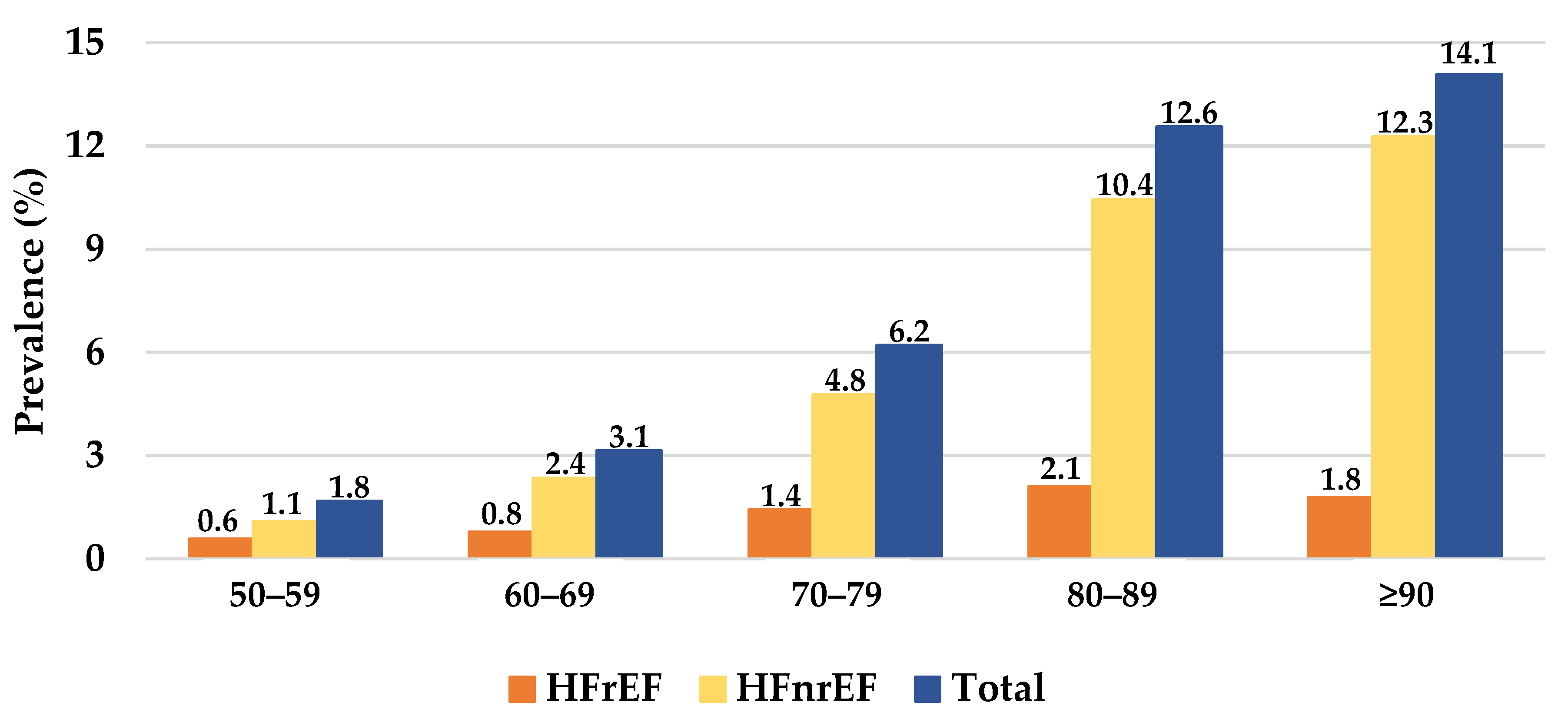
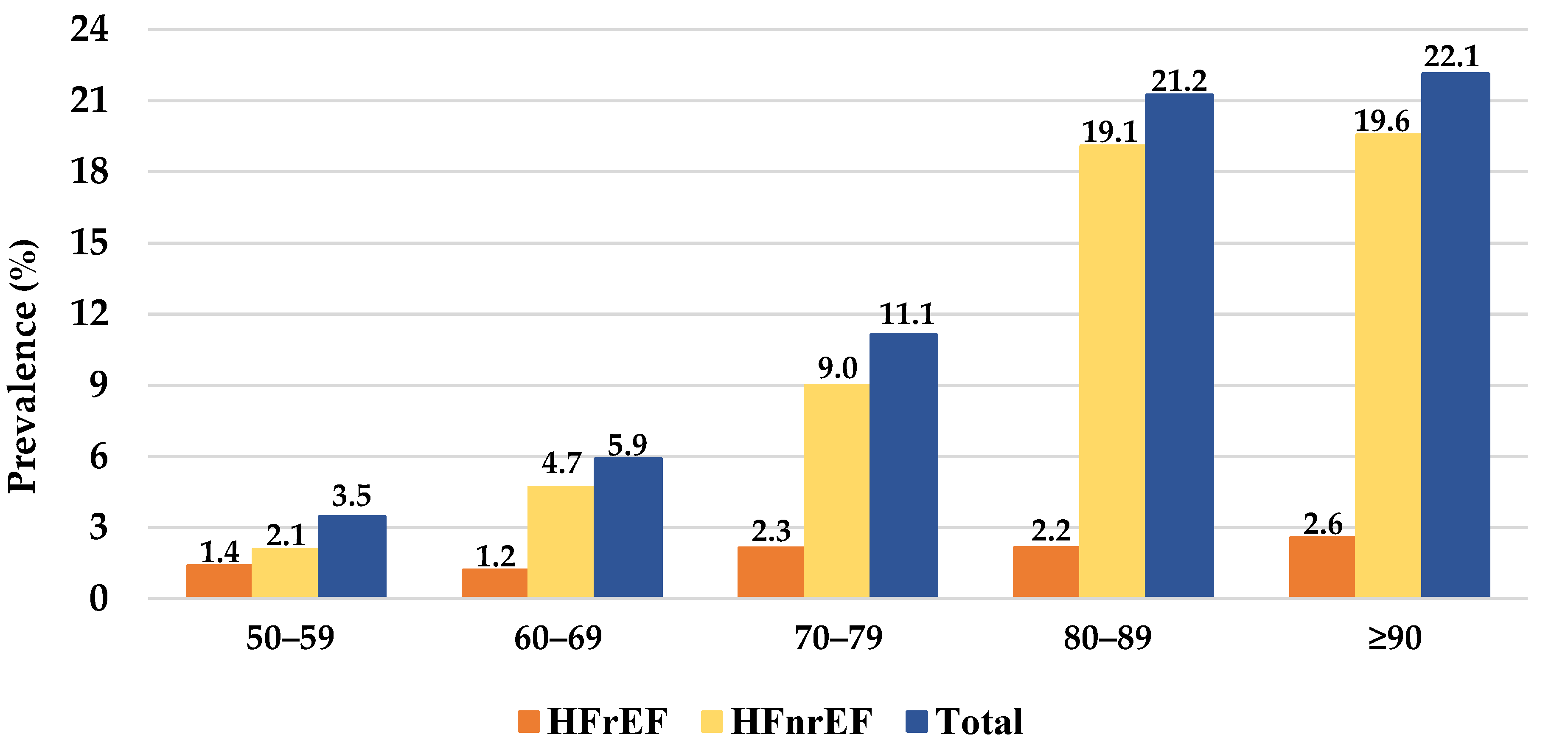
3.1. Characterization of Patients with HF
3.2. Characterization of HF Subtypes
3.3. Characterization of the Patients with HF and T2D
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Murphy, S.P.; Ibrahim, N.E.; Januzzi, J.L. Heart Failure with Reduced Ejection Fraction: A Review. JAMA 2020, 324, 488–504. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, C.; Brás, D.; Araújo, I.; Ceia, F. Insuficiência cardíaca em números: Estimativas para o século XXI em Portugal. Rev. Port. Cardiol. 2018, 37, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ceia, F.; Fonseca, C.; Mota, T.; Morais, H.; Matias, F.; de Sousa, A.; Oliveira, A.G.; on behalf of the EPICA Investigators. Prevalence of chronic heart failure in Southwestern Europe: The EPICA study. Eur. J. Heart Fail. 2002, 4, 531–539. [Google Scholar] [CrossRef]
- Meyer, S.; Brouwers, F.P.; Voors, A.A.; Hillege, H.L.; De Boer, R.A.; Gansevoort, R.T.; Van Der Harst, P.; Rienstra, M.; Van Gelder, I.C.; Van Veldhuisen, D.J.; et al. Sex differences in new-onset heart failure. Clin. Res. Cardiol. 2014, 104, 342–350. [Google Scholar] [CrossRef]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; Costanzo, S.; et al. Sex-Specific Epidemiology of Heart Failure Risk and Mortality in Europe. JACC Heart Fail. 2019, 7, 204–213. [Google Scholar] [CrossRef]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.V.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef] [Green Version]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef]
- McHugh, K.; DeVore, A.D.; Wu, J.; Matsouaka, R.A.; Fonarow, G.C.; Heidenreich, P.A.; Yancy, C.W.; Green, J.B.; Altman, N.; Hernandez, A.F. Heart Failure with Preserved Ejection Fraction and Diabetes. J. Am. Coll. Cardiol. 2019, 73, 602–611. [Google Scholar] [CrossRef]
- Lehrke, M.; Marx, N. Diabetes Mellitus and Heart Failure. Am. J. Med. 2017, 130, S40–S50. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; McMurray, J.J.V.; Michelson, E.L.; Olofsson, B.; Östergren, J.; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: The CHARM-Preserved Trial. Lancet 2003, 362, 777–781. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Junbo Ge, D.P.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, J.J.; Östergren, J.; Swedberg, K.; Granger, C.B.; Held, P.; Michelson, E.L.; Olofsson, B.; Yusuf, S.; Pfeffer, M.A. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: The CHARM-Added trial. Lancet 2003, 362, 767–771. [Google Scholar] [CrossRef]
- Pitt, B.; Pfeffer, M.A.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Claggett, B.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; et al. Spironolactone for Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2014, 370, 1383–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruschitzka, F.; Abraham, W.T.; Singh, J.P.; Bax, J.J.; Borer, J.S.; Brugada, J.; Dickstein, K.; Ford, I.; Gorcsan, J., III; Gras, D. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N. Engl. J. Med. 2013, 369, 1395–1405. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) With the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 79, e263–e421. [Google Scholar] [CrossRef]
- Oren, O.; Goldberg, S. Heart Failure with Preserved Ejection Fraction: Diagnosis and Management. Am. J. Med. 2017, 130, 510–516. [Google Scholar] [CrossRef]
- Framingham Heart Study. Available online: https://framinghamheartstudy.org/ (accessed on 21 April 2022).
- Strong Heart Study. Available online: https://strongheartstudy.org/ (accessed on 21 April 2022).
- The Cardiovascular Health Study. Available online: https://chs-nhlbi.org/ (accessed on 21 April 2022).
- Ilieșiu, A.M.; Hodorogea, A.S. Treatment of Heart Failure with Preserved Ejection Fraction. Adv. Exp. Med. Biol. 2018, 88, 67–87. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.-P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Mcmurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. PARADIGM-HF Investigators and Committees. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Kidney Foundation. Available online: https://www.kidney.org/ (accessed on 21 April 2022).
- Levey, A.S.; Coresh, J.; Greene, T.; Stevens, L.A.; Zhang, Y.L.; Hendriksen, S.; Kusek, J.W.; Van Lente, F.; Chronic Kidney Disease Epidemiology Collaboration. Using Standardized Serum Creatinine Values in the Modification of Diet in Renal Disease Study Equation for Estimating Glomerular Filtration Rate. Ann. Intern. Med. 2006, 145, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Zaharia, M.; Xin, R.S.; Wendell, P.; Das, T.; Armbrust, M.; Dave, A.; Meng, X.; Rosen, J.; Venkataraman, S.; Franklin, M.J.; et al. Apache Spark. Commun. ACM 2016, 59, 56–65. [Google Scholar] [CrossRef]
- Development Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 21 April 2022).
- Severino, P.; D’Amato, A.; Prosperi, S.; Cas, A.D.; Mattioli, A.V.; Cevese, A.; Novo, G.; Prat, M.; Pedrinelli, R.; Raddino, R.; et al. Do the Current Guidelines for Heart Failure Diagnosis and Treatment Fit with Clinical Complexity? J. Clin. Med. 2022, 11, 857. [Google Scholar] [CrossRef]
- Seferović, P.M.; Vardas, P.; Jankowska, E.A.; Maggioni, A.P.; Timmis, A.; Milinković, I.; Polovina, M.; Gale, C.P.; Lund, L.H.; Lopatin, Y.; et al. The Heart Failure Association Atlas: Heart Failure Epidemiology and Management Statistics. Eur. J. Heart Fail. 2021, 23, 906–914. [Google Scholar] [CrossRef]
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef]
- Gerber, Y.; Weston, S.A.; Redfield, M.M.; Chamberlain, A.M.; Manemann, S.M.; Jiang, R.; Killian, J.M.; Roger, V.L. A Contemporary Appraisal of the Heart Failure Epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern. Med. 2015, 175, 996–1004. [Google Scholar] [CrossRef] [Green Version]
- Bursi, F.; Weston, S.A.; Redfield, M.M.; Jacobsen, S.J.; Pakhomov, S.; Nkomo, V.T.; Meverden, R.A.; Roger, V.L. Systolic and Diastolic Heart Failure in the Community. JAMA 2006, 296, 2209–2216. [Google Scholar] [CrossRef] [Green Version]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef]
- Van Riet, E.E.; Hoes, A.W.; Wagenaar, K.P.; Limburg, A.; Landman, M.A.; Rutten, F.H. Epidemiology of heart failure: The prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur. J. Heart Fail. 2016, 18, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Filippatos, G.; Siddiqi, T.J.; Brueckmann, M.; Böhm, M.; Chopra, V.K.; Ferreira, J.P.; Januzzi, J.L.; Kaul, S.; Piña, I.L.; et al. Empagliflozin, Health Status, and Quality of Life in Patients with Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation 2022, 145, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Varadhan, A.; Stephan, K.; Gupta, R.; Vyas, A.V.; Ranchal, P.; Aronow, W.S.; Hawwa, N.; Lanier, G.M. Growing role of SGLT2i in heart failure: Evidence from clinical trials. Expert Rev. Clin. Pharmacol. 2022, 15, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.D.; Burns, J.; Freed, B.H.; Maurer, M.S.; Burkhoff, D.; Shah, S.J. Meta-Analysis Global Group in Chronic (MAGGIC) Heart Failure Risk Score: Validation of a Simple Tool for the Prediction of Morbidity and Mortality in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e009594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macdonald, M.R.; Petrie, M.C.; Hawkins, N.M.; Petrie, J.R.; Fisher, M.; McKelvie, R.; Aguilar, D.; Krum, H.; McMurray, J.J. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur. Heart J. 2008, 29, 1224–1240. [Google Scholar] [CrossRef]
- Aguilar, D. Heart Failure, Diabetes Mellitus, and Chronic Kidney Disease. Circ. Heart Fail. 2016, 9. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, D.C.; Stefánsson, B.V.; Jongs, N.; Chertow, G.M.; Greene, T.; Hou, F.F.; McMurray, J.J.V.; Correa-Rotter, R.; Rossing, P.; Toto, R.D.; et al. Effects of dapagliflozin on major adverse kidney and cardiovascular events in patients with diabetic and non-diabetic chronic kidney disease: A prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021, 9, 22–31. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes. JAMA Cardiol. 2021, 6, 148. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; Maestrini, V.; Mariani, M.V.; Birtolo, L.I.; Scarpati, R.; Mancone, M.; Fedele, F. Structural and myocardial dysfunction in heart failure beyond ejection fraction. Heart Fail. Rev. 2019, 25, 9–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, P.W.; Choy, J.B.; Nanda, N.C.; Becher, H. Left Ventricular Ejection Fraction and Volumes: It Depends on the Imaging Method. Echocardiography 2013, 31, 87–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellikka, P.A.; She, L.; Holly, T.A.; Lin, G.; Varadarajan, P.; Pai, R.G.; Bonow, R.O.; Pohost, G.M.; Panza, J.A.; Berman, D.S.; et al. Variability in Ejection Fraction Measured by Echocardiography, Gated Single-Photon Emission Computed Tomography, and Cardiac Magnetic Resonance in Patients with Coronary Artery Disease and Left Ventricular Dysfunction. JAMA Netw. Open 2018, 1, e181456. [Google Scholar] [CrossRef] [PubMed]
| Demographics | n | % |
| Patients (N) | 2700 | |
| Age (mean, SD) | 74.0 | 12.1 |
| Females (n, %) | 1394 | 51.6 |
| General Comorbidities | n | % |
| Type 2 diabetes mellitus | 1207 | 44.7 |
| Myocardial infarction | 836 | 31.0 |
| Atrial fibrillation | 1110 | 41.1 |
| Stroke | 779 | 28.9 |
| Peripheral artery disease | 199 | 7.4 |
| Microvascular disease | 499 | 18.5 |
| Chronic kidney disease | 1803 | 66.7 |
| eGFR [45, 60] mL/min | 530 | 19.6 |
| eGFR [30, 45] mL/min | 577 | 21.4 |
| eGFR [15, 30] mL/min | 495 | 18.3 |
| eGFR ≤ 15 mL/min | 201 | 7.4 |
| Cardiovascular Medication | n | % |
| Hypertension medications | 1619 | 60.0 |
| Low dose acetylsalicylic acid | 746 | 27.6 |
| Receptor P2Y12 antagonists | 307 | 11.4 |
| Statins | 1747 | 64.7 |
| Angiotensin-converting enzyme inhibitors | 985 | 36.5 |
| Angiotensin II receptor blockers | 389 | 14.4 |
| Beta blockers | 1620 | 60.0 |
| High ceiling diuretics | 1266 | 46.9 |
| Aldosterone antagonists | 405 | 15.0 |
| Warfarin | 123 | 4.6 |
| No medication | 353 | 13.1 |
| Clinical Assessment | Median | IQR |
| Systolic blood pressure (mmHg) | 137.0 | 23.0 |
| Diastolic blood pressure (mmHg) | 77.0 | 15.0 |
| Total body weight (kg) | 73.0 | 19.0 |
| Body mass index (kg/m2) | 27.7 | 6.4 |
| Body surface area (m2, DuBois D) | 1.8 | 0.3 |
| Waist circumference (cm) | 101.0 | 16.0 |
| Laboratory Test Results | Median | IQR |
| Hemoglobin (mg/dL) | 12.8 | 2.6 |
| Glycated hemoglobin (%) | 5.8 | 1.1 |
| LDL cholesterol (mg/dL) | 106.0 | 53.0 |
| HDL cholesterol (mg/dL) | 43.0 | 16.0 |
| Total cholesterol (mg/dL) | 179.0 | 59.0 |
| Triglycerides (mg/dL) | 111.0 | 71.0 |
| BNP (pg/mL) | 195.7 | 253.9 |
| Serum creatinine (mg/mL) | 1.2 | 42.3 |
| GFR (mL/min, MDRD) | 50.7 | 83.1 |
| Echocardiogram Results | Median | IQR |
| Left atrial volume (mL) | 42.0 | 8.0 |
| Left atrial volume, indexed (mL/m2) | 23.4 | 4.8 |
| Left atrial diameter (mm) | 51.0 | 8.0 |
| Interventricular septum thickness (mm) | 11.0 | 3.0 |
| Posterior ventricular wall thickness (mm) | 9.0 | 1.0 |
| Left ventricular ejection fraction (%) | 59.0 | 14.0 |
| Left ventricular mass (g) | 147.7 | 48.3 |
| Left ventricular mass indexed (g/m2) | 83.1 | 23.1 |
| Interventricular septum thickness (mm) | 11.0 | 3.0 |
| HFrEF n = 429 | HFnrEF n = 2199 | p | |||
|---|---|---|---|---|---|
| Age (Mean, SD) | 67.0 | 12.0 | 74.7 | 12.0 | <0.001 |
| Females (n, %) | 139 | 33.9 | 1209 | 55.0 | <0.001 |
| General Comorbidities | n | % | n | % | |
| Type 2 diabetes mellitus | 204 | 46.5 | 969 | 44.1 | 0.421 |
| Myocardial infarction | 200 | 45.6 | 624 | 28.4 | <0.001 |
| Atrial fibrillation | 177 | 40.3 | 871 | 39.6 | 0.759 |
| Stroke | 112 | 25.5 | 652 | 29.6 | 0.059 |
| Peripheral artery disease | 44 | 10.0 | 152 | 6.9 | 0.050 |
| Microvascular disease | 85 | 19.4 | 398 | 18.1 | 0.500 |
| Chronic kidney disease | 297 | 67.7 | 1483 | 67.4 | 1.000 |
| eGFR [45, 60] mL/min | 83 | 18.9 | 447 | 20.3 | 0.146 |
| eGFR [30, 45] mL/min | 105 | 23.9 | 462 | 21.0 | |
| eGFR [15, 30] mL/min | 85 | 19.4 | 403 | 18.3 | |
| eGFR ≤ 15 mL/min | 24 | 5.5 | 171 | 7.8 | |
| Cardiovascular Medications | n | % | n | % | p |
| Hypertension medications | 316 | 72.0 | 1273 | 57.9 | <0.001 |
| Low dose acetylsalicylic acid | 167 | 38.0 | 574 | 26.1 | <0.001 |
| Receptor P2Y12 antagonists | 71 | 16.2 | 233 | 10.6 | <0.001 |
| Statins | 322 | 73.3 | 1386 | 63.0 | <0.001 |
| Angiotensin-converting enzyme inhibitors | 244 | 55.6 | 719 | 32.7 | <0.001 |
| Angiotensin II receptor blockers | 65 | 14.8 | 314 | 14.3 | 0.816 |
| Beta blockers | 348 | 79.3 | 1227 | 55.8 | <0.001 |
| High ceiling diuretics | 248 | 56.5 | 979 | 44.5 | <0.001 |
| Aldosterone antagonists | 214 | 48.7 | 182 | 8.3 | <0.001 |
| Warfarin | 26 | 5.9 | 92 | 4.2 | 0.171 |
| No medication | 35 | 8.0 | 314 | 14.3 | <0.001 |
| Clinical Assessment | Median | IQR |
|---|---|---|
| Systolic blood pressure (mmHg) | 138.0 | 24.0 |
| Diastolic blood pressure (mmHg) | 76.0 | 15.0 |
| Total body weight (kg) | 75.0 | 19.0 |
| Body mass index (kg/m2) | 28.4 | 6.6 |
| Body surface area (m2, DuBois D) | 1.8 | 0.2 |
| Waist circumference(cm) | 103.0 | 16.0 |
| Diabetes Medications | n | % |
| Insulins | 323 | 26.8 |
| Metformin | 585 | 48.5 |
| SGLT-2 inhibitors | 105 | 8.7 |
| DPP-4 inhibitors | 501 | 41.5 |
| Sulfonylurea | 118 | 9.8 |
| GLP-1 receptor agonists | 21 | 1.7 |
| No medication | 343 | 28.4 |
| Lab Results | Median | IQR |
| Hemoglobin (mg/dL) | 12.5 | 2.6 |
| Glycated hemoglobin (%) | 6.6 | 1.6 |
| LDL cholesterol (mg/dL) | 104.0 | 53.0 |
| HDL cholesterol (mg/dL) | 40.0 | 15.0 |
| Total cholesterol (mg/dL) | 170.9 | 57.5 |
| Triglycerides (mg/dL) | 125.0 | 80.0 |
| BNP (pg/mL) | 202.9 | 304.5 |
| Serum creatinine (mg/mL) | 1.5 | 40.1 |
| GFR (mL/min, MDRD) | 38.1 | 71.9 |
| Echocardiogram Results | Median | IQR |
| Left atrial volume (mL) | 42.0 | 8.0 |
| Left atrial volume, indexed (mL/m2) | 23.6 | 4.8 |
| Left atrial diameter (mm) | 51.0 | 8.0 |
| Interventricular septum thickness (mm) | 11.0 | 3.0 |
| Posterior ventricular wall thickness (mm) | 10.0 | 1.0 |
| Left ventricular ejection fraction (%) | 58.0 | 14.0 |
| Left ventricular mass (g) | 153.0 | 48.3 |
| Left ventricular mass indexed (g/m2) | 85.2 | 23.1 |
| T2D n = 1207 | Non-T2D n = 1493 | p | |||
|---|---|---|---|---|---|
| Age (Mean, SD) | 75.3 | 10.2 | 73.0 | 13.4 | <0.001 |
| Females (n, %) | 617 | 51.1 | 777 | 52.0 | 0.912 |
| General Comorbidities | n | % | n | % | p |
| Myocardial infarction | 390 | 32.3 | 446 | 29.9 | 0.263 |
| Atrial fibrillation | 517 | 42.8 | 593 | 39.7 | 0.116 |
| Stroke | 395 | 32.7 | 384 | 25.7 | <0.001 |
| Peripheral artery disease | 119 | 9.9 | 80 | 5.4 | <0.001 |
| Microvascular disease | 427 | 35.4 | 72 | 4.8 | <0.001 |
| Chronic kidney disease | 944 | 78.2 | 836 | 56.0 | <0.001 |
| eGFR [45, 60] mL/min | 208 | 17.2 | 322 | 21.6 | <0.001 |
| eGFR [30, 45] mL/min | 302 | 25.0 | 265 | 17.7 | |
| eGFR [15, 30] mL/min | 288 | 23.9 | 200 | 13.4 | |
| eGFR ≤ 15 mL/min | 146 | 12.1 | 49 | 3.3 | |
| Cardiovascular Medications | n | % | n | % | p |
| Hypertension medications | 757 | 62.7 | 862 | 57.7 | 0.013 |
| Low dose acetylsalicylic acid | 377 | 31.2 | 369 | 24.7 | <0.001 |
| Receptor P2Y12 antagonists | 153 | 12.7 | 154 | 10.3 | 0.080 |
| Statins | 840 | 69.6 | 907 | 60.8 | <0.001 |
| Angiotensin-converting enzyme inhibitors | 458 | 37.9 | 527 | 35.3 | 0.165 |
| Angiotensin II receptor blockers | 190 | 15.7 | 199 | 13.3 | 0.087 |
| Beta blockers | 767 | 63.5 | 853 | 57.1 | <0.001 |
| High ceiling diuretics | 659 | 54.6 | 607 | 40.7 | <0.001 |
| Aldosterone antagonists | 198 | 16.4 | 207 | 13.9 | 0.051 |
| Warfarin | 46 | 3.8 | 77 | 5.2 | 0.058 |
| No medication | 115 | 9.5 | 238 | 15.9 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavina, C.; Carvalho, D.S.; Valente, F.; Bernardo, F.; Dinis-Oliveira, R.J.; Santos-Araújo, C.; Taveira-Gomes, T. 20 Years of Real-World Data to Estimate the Prevalence of Heart Failure and Its Subtypes in an Unselected Population of Integrated Care Units. J. Cardiovasc. Dev. Dis. 2022, 9, 149. https://doi.org/10.3390/jcdd9050149
Gavina C, Carvalho DS, Valente F, Bernardo F, Dinis-Oliveira RJ, Santos-Araújo C, Taveira-Gomes T. 20 Years of Real-World Data to Estimate the Prevalence of Heart Failure and Its Subtypes in an Unselected Population of Integrated Care Units. Journal of Cardiovascular Development and Disease. 2022; 9(5):149. https://doi.org/10.3390/jcdd9050149
Chicago/Turabian StyleGavina, Cristina, Daniel Seabra Carvalho, Filipa Valente, Filipa Bernardo, Ricardo Jorge Dinis-Oliveira, Carla Santos-Araújo, and Tiago Taveira-Gomes. 2022. "20 Years of Real-World Data to Estimate the Prevalence of Heart Failure and Its Subtypes in an Unselected Population of Integrated Care Units" Journal of Cardiovascular Development and Disease 9, no. 5: 149. https://doi.org/10.3390/jcdd9050149
APA StyleGavina, C., Carvalho, D. S., Valente, F., Bernardo, F., Dinis-Oliveira, R. J., Santos-Araújo, C., & Taveira-Gomes, T. (2022). 20 Years of Real-World Data to Estimate the Prevalence of Heart Failure and Its Subtypes in an Unselected Population of Integrated Care Units. Journal of Cardiovascular Development and Disease, 9(5), 149. https://doi.org/10.3390/jcdd9050149








