Abstract
Myocarditis is an inflammatory disease of the myocardium that is troublesome to diagnose and manage, especially in children. Since the introduction of endomyocardial biopsy (EMB), new diagnostic tools have provided useful data. Especially when enhanced with immunohistochemistry and polymerase chain reaction (PCR) studies, EMB remains the gold standard for the diagnosis. Notably, cardiac magnetic resonance (MRI) is a non-invasive tool that can confirm the diagnosis and has a particular usefulness during the follow-up. The causes of myocarditis are heterogeneous (mostly viral in children). The course and outcome of the illness in the pediatric population represent a complex interaction between etiologic agents and the immune system, which is still not fully understood. The clinical presentation and course of myocarditis vary widely from paucisymptomatic illness to acute heart failure refractory to therapy, arrhythmias, angina-like presentation and sudden cardiac death. In this setting, cardiac biomarkers (i.e., troponins and BNP), although unspecific, can be used to support the diagnosis. Finally, the efficacy of therapeutic strategies is controversial and not confirmed by clinical trials. In this review, we summarized the milestones in diagnosis and provided an overview of the therapeutic options for myocarditis in children.
1. Introduction
Myocarditis represents a challenge especially in children, despite being studied since the second half of the 20th century with sensible progress in the diagnostic field over time.
Clinical manifestations of the disease can vary widely and encompass paucisymptomatic illness, acute heart failure sometimes requiring mechanical circulatory support (MCS), arrhythmias, angina-like presentation and sudden death. Biomarkers can be useful to support the diagnosis, but are not specific [1].
The main cause of myocarditis in children is viral, but many other agents can provoke the illness [2].
Moreover, it is well known that the immune system plays a key role in the pathogenesis of the disease [3], but the mechanisms of interaction between etiologic agents and pathogenesis are not completely understood. As a consequence, myocarditis can resolve without sequelae or evolve into inflammatory cardiomyopathy [4], but to date there are no risk factors predicting poor outcome or chronic disease.
Finally, evidence in therapy remains weak and controversial.
In this review, we summarize the milestones in diagnosis and provide an overview of the therapy of myocarditis in children.
2. Definition of Myocarditis and Diagnostic Criteria
As stated by the WHO/International Society and Federation of Cardiology (WHO/ISFC), myocarditis is an inflammatory disease of the myocardium diagnosed by endomyocardial biopsy (EMB) using established histological, immunological and immunohistochemical criteria [5].
In 1987, Aretz et al. performed the diagnosis of myocarditis on Dallas criteria (Table 1 and Figure 1), on the basis of the presence of inflammatory infiltrate and myocyte necrosis in EMB samples [6]. Even at present, the EMB remains the gold standard for proven myocarditis, despite its invasiveness and the possibility of false-negative results due to sampling errors [2].

Table 1.
The Dallas Criteria.
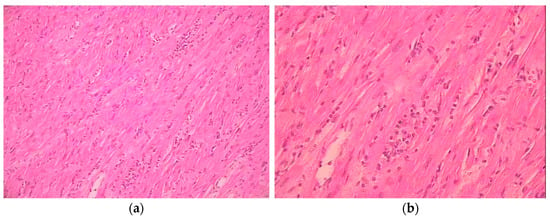
Figure 1.
Myocardial histological sample showing lymphocytic infiltrate and myocyte necrosis. (a) ×10; (b) ×40.
In 1991, Gagliardi et al. [7] demonstrated the role of cardiac MRI to confirm myocarditis in pediatric age, and evidence has increased in the subsequent years supporting the importance of the technique also during the follow-up [8]. However, formal criteria (i.e., Lake Louise criteria) were published 18 years later, in 2009 [9] and revised in 2018 [10]. Although many studies were developed in adults, they seem to maintain comparable sensitivity also in children [11], but only single center studies are available in the pediatric population.
Notably, myocarditis can sometimes evolve to inflammatory cardiomyopathy, that is, myocarditis in association with cardiac dysfunction following an autoimmune, infectious or idiopathic pathway [12], eventually ending in dilated cardiomyopathy [1,13].
3. Epidemiology
The incidence rate of acute myocarditis in children is reported to be 0.9 per 100,000 children per year in the US, with a rising trend from 2007 to 2016 [14], and up to 1.95 per 100,000 children/year in one national European study [15].
In children, myocarditis has a bimodal distribution with peaks in those younger than 2 years and in teenagers between 13 and 18 years old [16]. Potential risk factors in children with myocarditis include males, Caucasian background, and low socio-economic status. [14]
In-hospital mortality significantly declined over time, being recorded at 6.1% ± 1.3% in 2016 [14].
4. Causes
Myocarditis can be caused by several different agents. The most prevalent etiology is infectious (i.e., viral), but many other non-infectious agents can be causative [2].
4.1. Viral Myocarditis
Viruses are the most relevant cause of myocarditis in children. The first report of viral isolation in humans was written in 1969 when a Coxsackie virus group B was isolated in the heart of a 15-year-old boy with myocarditis during necroscopy [17], but other viral causative agents were subsequently reported.
Historically, the most frequently encountered agents were Enterovirus (namely Coxsackieviruses B) and adenovirus [18], but Parvovirus B19 [19] and human herpes virus 6 are also emerging [18].
Myocarditis secondary to cytomegalovirus can be life-threating in immunosuppressed patients [20].
In the current pandemic era, SARS-CoV-2 virus is emerging as a possible cause of myocardial injury, and accumulating evidence suggests that in children it can cause a multisystemic inflammatory syndrome with cardiac involvement, especially in patients with congenital heart diseases [21].
Certain recent studies advise caution in addressing viral etiopathogenesis to viral causes due to potential survival of these viruses in different tissues even weeks or months after initial contact [22]. Further studies are needed to derive conclusions.
4.2. Infectious Non-Viral Causes
Trypanosoma cruzi can cause myocarditis in the form of Chagas disease with major distribution in South America, but increasingly described in different parts of the world. More rarely, bacteria such as M. tuberculosis, Mycoplasma spp. and Borrelia spp., parasitic infestations and T. gondii can induce the illness [20].
4.3. Noninfectious Etiology
In children, noninfectious agents causing myocarditis are rare events. Autoimmune myocarditis is suspected when EMB shows inflammatory response but fails to detect infective agents and other causes are unlikely. The heart can be primarily involved or affected in the context of systemic autoimmune diseases as rheumatic fever or systemic lupus erythematosus [13,23]. More rarely, other causative agents encompass hypersensitivity reactions, medications (sulfa-drugs and anthracyclines) and toxins [2,20].
Recently, myocarditis after mRNA vaccine against SARS-CoV2 has been reported in adolescents [24]. According to a recent US Survey Study in children from 12 to 18 years old, vaccination-related adverse events occurred in 1/1000 recipients, and myocarditis represented 4.3% of the reported events (0.0043% of all adverse events). Notably, most cases were mild or moderate and resolved favorably [25], as previously reported also in adults [26].
5. Pathogenesis
The immune response plays a pivotal role in the genesis and perpetration of myocardial damage, as demonstrated in animal studies [27,28]. However, the interaction of specific viruses (or other causative agents) with the immune system and the role of autoimmunity in the pathogenesis of myocarditis and inflammatory cardiomyopathy are not fully understood [4].
Myocarditis represents the acute phase of the myocardial inflammation and can be viral, post-infectious, immune-mediated or primarily an organ-specific autoimmune condition [13].
5.1. Viral Myocarditis
Focusing on viral myocarditis, the pathogenesis of myocardial damage can vary depending on the pathogen. In virus-induced active myocarditis, cardiotropic and vasculotropic viruses, such as adenovirus or enterovirus, directly injure cardiac myocytes or vessels, leading to inflammatory reactions. In virus-associated myocarditis, the pathogen can be detected in tissue samples, but its role in the myocardial damage is not clear as the infection could be latent, such as in herpesviruses and Parvovirus B19. Other viruses can provoke a cytokine storm or activate the cellular immune response through molecular mimicry [4].
Once the virus infects the heart, the immune system initially responds according to the innate pathway (acute phase, day 1 to day 7). The innate immune system encompasses mast cells, natural killer (NK) cells, dendritic cells, neutrophils, eosinophils, basophils and monocytes, but their activation is influenced by the type of pathogen and the molecules expressed by the damaged cardiac myocytes. In particular, monocytes infiltrate myocardium and differentiate into macrophages that produce pro-inflammatory cytokines and activate the adaptive immune response. The innate immune response is, therefore, crucial to overcome viral infection, but it can also cause excessive myocardial disruption and dysfunction, especially when exaggerated or prolonged [29,30].
The switch to the adaptive mechanisms of immunity marks the beginning of the subacute phase that usually resolves the infection within 4 weeks (often in 14 days) and in which the major effectors are the T cells [4]. However, if these mechanisms fail to eliminate the pathogen or if the immune response persists, inflammation becomes chronic and can induce the production of cardiac autoantibodies and autoimmune reaction [31,32,33,34].
In this setting, genetic and external factors interact differently in the pathogenesis of myocarditis [35,36], leading to different clinical pictures and outcomes.
5.2. Autoimmune Pathway
In some cases, the immune response is inappropriately raised compared to the need of the organism (hypersensitivity) or is triggered by the loss of self-tolerance (autoimmune post-injury reactions) [33]; in others, myocytes may expose self antigens or neoantigens that can be attacked by autoreactive antibodies. In summary, autoimmune inflammatory cardiomyopathy can be primarily autoimmune or represent the final stage of a pathogenetic pathway in which the causative agents (mainly viruses) interact differently in genetically predisposed patients [13].
As in other autoimmune diseases, at least two of the Rose–Witebsky criteria should be satisfied to diagnose autoimmune illnesses [37]. Referring to myocarditis, it is well known that the inflammatory infiltrate along with an abnormal HLA expression in the myocardium can be detected, without proving the presence of infectious agents or known inflammatory causes [38,39]. Circulating cardiac autoantibodies can be detected in up to 60% of patients with inflammatory cardiomyopathy and in their relatives also many years before the clinical picture becomes manifest [40,41]. Moreover, autoantibody and/or autoreactive lymphocytes have also been detected in myocardial tissue samples of affected individuals [3]. Many classes of autoantibodies have been discovered; they are directed against several autoantigens, mainly α- and β-myosin heavy chain, and some of them can directly damage the myocardium [42,43]. On the other hand, the presence of autoantibodies is important to guide the subsequent immune-modulating treatment [44]. The efficacy of immunosuppressive therapy is still controversial, but recent evidence is encouraging [45,46] (see also Therapy).
6. Signs and Symptoms
Another challenging point in the management of pediatric myocarditis is the clinical presentation. Even if the majority of patients complain of symptoms, these can vary widely, ranging from unapparent infections or unspecific symptoms to acute heart failure refractory to therapy, arrhythmias, angina-like presentation and sudden cardiac death [16,47,48].
Fever at presentation can be present in 58% of patients with biopsy-confirmed myocarditis (versus 15% of patients with dilated cardiomyopathy, p = 0.002) [49].
In a recently published multicentric cohort study, chest pain and respiratory distress appeared to be more frequent in patients with mildly depressed to normal ventricular function, while gastrointestinal and unspecific symptoms were prevalent in patients with moderately to severely depressed ventricular function. Dyspnea and viral prodromes were equally prevalent in the two groups [16].
The median length of stay in stable hospitalized patients has been reported as 6.1 days. In a recently published German prospective multicentric registry on pediatric myocarditis, factors associated with major cardiovascular adverse events were fulminant presentation, monocytes as inflammatory infiltrate, persistence inflammation and younger age [46].
6.1. Heart Failure
According to a recent retrospective, serial cross-sectional study, hospitalization for heart failure related to pediatric myocarditis has remained stable over time (27%) [14].
In one study, the incidence of adverse events including death and heart transplantation was 13%. Risk factors at logistic regression analysis included younger age, female sex and higher left ventricular end-diastolic diameter (LVEDd) z-score at time of presentation [16], but data on predictors of negative outcome are not uniform [50].
Fulminant myocarditis is the clinical manifestation of a rapidly evolving heart failure, as a result of widespread inflammation of the myocardium [51]. In a single-center retrospective study investigating children with fulminant myocarditis, risk factors for cardiac arrest or MCS seemed to be higher peak BNP levels and inotropic scores [52].
6.2. Arrhythmias
Arrhythmias are frequent in children with myocarditis. Among rhythm disturbances, tachyarrhythmias are more than twice as common as bradyarrhythmias (13% versus 6.4%) [14]. Ventricular tachycardia is the most frequently encountered rhythm disturbance in hospitalized patients [14], but ventricular tachycardia, ventricular fibrillation, supraventricular tachycardias, and atrial fibrillation or flutter have also been reported [53].
In contrast, complete heart block is the most common bradyarrhythmia in children [54], and its detection with ECG should raise suspicion of myocarditis [14].
6.3. Chest Pain and Angina-Like Presentation
Chest pain is rarely due to cardiac pathology in children; nonetheless, it is a well described symptom during myocarditis and pericarditis [55]. It can be associated with ECG changes, an increase in troponin level and abnormalities of regional or global wall motion, mimicking myocardial infarction [56].
6.4. Sudden Death
According to data in the literature [57], myocarditis accounts for 8% of the sudden deaths of known cardiovascular cause in young competitive athletes (mean age 19 ± 6 years). In ~75% of cases, the affected individuals were males, and ~50% had one feature among the following: viral prodrome, syncope, unspecific symptoms, chest pain or palpitation [58].
7. Diagnosis in the Clinical Setting
In the absence of EMB or CMR, the diagnosis of myocarditis in the clinical setting can only be suspected by the merger of different data including medical history, signs, symptoms, biomarkers, electrocardiogram (ECG) and echocardiographic features, as none of these is specific or pathognomonic for myocarditis. Major milestones in the diagnosis of myocarditis in children are shown in Figure 2, Panel a.
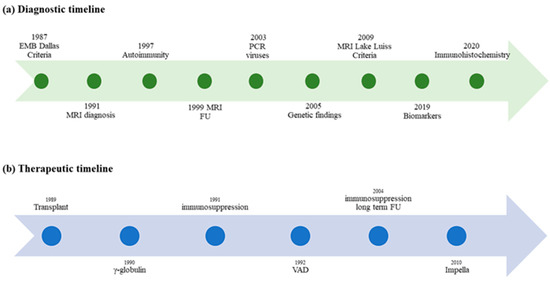
Figure 2.
Timelines of milestones in diagnosis and therapy of myocarditis in children. (a) Milestones of diagnosis in pediatric myocarditis over time; (b) Evidence of therapy in children with myocarditis: the therapeutic approach is not supported by specific pediatric clinical trials.
7.1. Electrocardiography
All patients with suspected myocarditis should be investigated with a standard electrocardiogram (ECG). As almost any other first-line investigation in the field, an ECG is neither sensitive nor specific for the diagnosis [1]; however, it can be useful to add suspicion together with a reasonable clinical presentation, keeping in mind that coronary ischemic disease is very rare in the pediatric population.
In children, an ECG can detect sinus tachycardia, nonspecific repolarization abnormalities, diffuse concave ST-segment elevation, low-voltage QRS complexes in the limb leads [59] and arrhythmias [53].
7.2. Echocardiography
Echocardiography is another first-line investigation that should be performed on the suspicion of myocarditis. As with electrocardiography, it lacks sensitivity and specificity, but it can be useful to exclude primary valvular or congenital heart diseases and to monitor the patient over time, thanks to its availability, the possibility of real-time assessment and significant tolerability even in children [1,2].
Among others, echocardiography can detect variable degrees of left or right systolic impairment or left ventricle dilatation, regional wall motion abnormalities, thickened myocardium typically disproportioned compared to ventricular enlargement due to wall edema, pericardial effusion, intracardiac thrombosis, and secondary valvular regurgitation [60].
More recently, tissue Doppler for evaluation of diastolic function [61] and myocardial strain [62] has shown correlation with EBM o CMR findings and ultimately with outcomes [63].
7.3. Laboratory Findings and Biomarkers
Common inflammatory biomarkers include erythrocyte sedimentation rate and PCR. They can be elevated in myocarditis as in other inflammatory illnesses, including pericarditis [1].
Traditional cardiac biomarkers encompass troponins, B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP). Troponin levels rise in cases of myocardial injury and can be elevated in acute myocarditis [64]. BNP and NT-proBNP increase in cases of myocardial dysfunction, can discriminate cardiac from non-cardiac causes of dyspnea [65], and peak BNP seem to be a risk factor for poor outcome in children with fulminant myocarditis [52].
Although all of the above-mentioned molecules are unspecific for the diagnosis, they can be useful to support or suspect possible myocarditis, especially in the acute setting. In contrast to the adult population, in fact, other causes of myocardial injury and dysfunction such as coronary diseases are very rare in children. Importantly, these biomarkers are of more value when normal biomarkers do not rule out myocarditis [1].
7.4. Endomyocardial Biopsy (EMB)
EMB represents the gold standard for the diagnosis of proven myocarditis following the Dallas criteria listed in Table 1 [6]. The development of immunohistochemistry allowed physicians to differentiate the type of inflammatory infiltrate according to their clusters of differentiation (CDs) and to detect HLA expression. The distinction of leukocytes (CD45+), T lymphocytes (CD3+) and their subtypes (CD4+ or CD8+) and macrophages (CD68+) led to higher detection rates in the diagnosis of myocarditis, improving the sensitivity and specificity of the EMB [66]. According to the Working Group on Myocardial and Pericardial Diseases of the European Society of Cardiology, an inflammatory infiltrate should be defined if ≥4 leucocytes/mm2 are detected in the tissue sample. Up to 4 monocytes/mm2 can be found in the specimen along with ≥7 T lymphocytes (CD3+)/mm2 [1].
Furthermore, IHC examination can also detect the presence of autoantibodies within the damaged myocardium [42,43].
Notably, once the myocarditis is proven or suspected, the viral origin can be confirmed by PCR in myocardial tissue. In 1987, a hybridization in situ technique was used to identify enteroviral RNA in myocardial cells, using molecularly cloned coxsackievirus B3 cDNA as a diagnostic probe [67]. Since then, the PCR technique has been widely used to detect viral genome in EMB samples [18]. This technique can diagnose the specific pathogen, improving the value of the EMB [68]. Contrarily, viral serology of peripheral blood and PCR of peripheral samples (i.e., urine, blood or stool) poorly correlate with the final diagnosis [1]. Good concordance between myocardial and blood PCRs can be a reasonable element in contributing to diagnostic criteria.
Importantly, results from EMB showing negative viral PCR in association with positivity of cardiac autoantibodies indicate an immune-mediated illness and are the basis for a safe immunosuppressive therapy [13].
The two major concerns regarding EMB are the invasiveness and the rate of false-negative results. The complication rate can vary and is higher in children with suspected cardiomyopathy than in heart transplant recipients already on inotropic support [69,70]. However, it is generally a feasible procedure even in very small infants, and in large multicenter studies, the incidence rates of major adverse events related to myocardial biopsy in children with suspected cardiomyopathy in three recent cohorts were 13.2% [71], 5% [70] and 2.6% [46], respectively.
Giant Cell Myocarditis (GCM)
EMB plays a major role in cases of rapidly progressive heart failure refractory to medical treatment [1]. The paradigm of such presentation is represented by giant cell myocarditis, also known as Fiedler myocarditis, a distinct and rare type of myocardial inflammation whose first clinical manifestation is frequently cardiogenic shock. Clustered macrophages (i.e., giant cells) and lymphocytes with subsequent heart muscle cell destruction are the histological basis for the myocardial infiltrate.
The first, and for many years unique, case of pediatric giant cell myocarditis was reported in 1955 by Goldberg, who described the case of an infant that died at 6 weeks after birth [72]. GCM is in fact rare in children, and it usually affects young and middle-aged adults and presents rapidly with progressive heart failure with or without electrical instability [73].
The etiology is still not completely understood, but it seems to be mediated by T lymphocyte dysregulation. Its association with multisystemic conditions including autoimmune diseases (inflammatory bowel disease, rheumatoid arthritis, systemic lupus erythematosus, autoimmune thyroiditis, etc.) in 20% of cases or malignant thymoma and lymphoma supports the hypothesis related to T lymphocyte involvement [74,75,76].
The differential diagnosis includes other types of fulminant myocarditis such as lymphocytic myocarditis, eosinophilic myocarditis and granulomatous diseases. Among these, lymphocytic myocarditis is prevalent, while eosinophilic or giant cell fulminant myocarditis are rarer but have to be considered in the differential diagnosis, and EMB is the tool for the right diagnosis [77].
In GCM, an early and appropriate diagnosis is crucial [76] due to poorer prognosis in the absence of combined immunosuppressive therapy and / or MCS (on the basis of clinical presentation) [73]. Prompt MCS is fundamental in fulminant cases to improve survival, preserving multiorgan function as a bridge to recovery or heart transplantation [78].
7.5. Cardiovascular Magnetic Resonance (CMR)
The importance of CMR for the diagnosis and follow-up of myocarditis in children has been noted since the early 1990s [7,8]. However, the diagnostic criteria are not specific for the pediatric population and are derived from adult cohorts.
According to the original Lake Louise Criteria published in 2009 [9], the diagnosis of myocarditis is probable if at least two of the following three criteria are satisfied:
- (1)
- Regional high T2 intensity signal or high T2 intensity signal ratio suggestive for myocardial edema;
- (2)
- Increased early gadolinium enhancement (EGE) ratio, suggestive for hyperemia and/or capillary leak;
- (3)
- Presence of late gadolinium enhancement (LGE) of non-ischemic pattern, a sign of non-ischemic necrosis.
The accuracy, sensitivity and specificity of these criteria were 78%, 67% and 91%, respectively [79].
Unfortunately, after the acute phase, the intensity signals of both T2 and EGE become more homogeneous, making subacute inflammation (more) difficult to detect.
In the subsequent years, myocardial mapping of T1, T2, and extracellular volume has emerged as a tool for tissue characterization, since the relaxation time is more significant than the signal intensity [80].
Following these technical enhancements, the Lake Louise Criteria were revised in 2018 [10]. Typical features of myocarditis at CMR are shown in Figure 3.
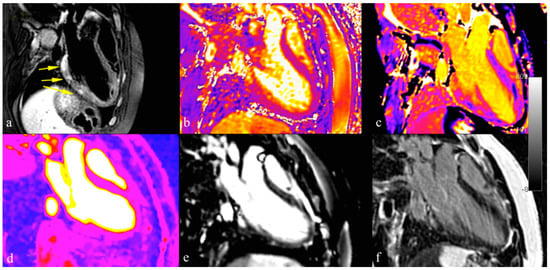
Figure 3.
Different techniques for myocarditis assessment with cardiovascular magnetic resonance imaging: T2 STIR sequences reveal areas of myocardial edema in acute setting (panel a); T1 and T2 mapping techniques improved detection of myocardial edema and inflammation (panel b,c); perfusion imaging and early enhancement can be used to detect hyperemia condition (panel d,e); late gadolinium enhancement sequences confirmed acute myocardial inflammatory damage and assessment of myocardial fibrosis (panel f).
Myocardial inflammation leads to an increase in T1, T2 relaxation time and extracellular volume (ECV). T2 mapping can differentiate acute inflammation from other forms of inflammation; native T1 mapping is less specific for active myocarditis and should be used when negative, to rule out myocarditis. ECV is a marker of fibrosis and inflammation. Importantly, it can also show changes that are undetectable with LGE sequences, pointing out diffuse myocardial inflammation in patients with negative LGE [81].
In a recent meta-analysis, Pan et al. [82] suggest that adding the new parameters to the standard Lake Louise Criteria can be significantly useful in improving sensitivity in diagnosis and management of acute myocarditis [83].
Even if the original and the revised Lake Louise Criteria have been validated only in the adult population, they seem to maintain their value also in the pediatric population [46]. However specific issues including sedation and younger age are related to a lower image quality [84].
8. Therapy
Giving the gap in understanding entirely the mechanisms of pathogenesis of myocardial damage, it is of no surprise that data on therapy are inconclusive. Treatment in children recalls that of adults, taking into account that viral pathogenesis and an acute clinical picture are more frequent in the pediatric population compared to adults. Milestones of therapy in myocarditis are shown in Figure 2b.
8.1. Symptoms-Based Therapy
Symptoms-based therapy should be the aim in handling pediatric myocarditis in the acute phase. Arrythmias can occur and should be resolved as soon as possible.
According to a recent retrospective, serial cross-sectional study, hospitalization for heart failure related to myocarditis has remained stable at 27% over time [14].
In the post-acute phase, oral HF treatment should be started in children with reduced ejection fraction. Contrarily, the benefit of such therapy in patients with preserved ejection fraction is under investigation [2]. Butts et al. reported 55% of children with heart failure therapy after hospital discharge [16].
8.2. Circulatory Support
In case of cardiogenic shock and low cardiac output, the cardiac pump has to be supported by inotropic therapy (milrinone over epinephrine or dopamine). In refractory cases, pediatric MCS can be considered lifesaving [85,86,87].
Apart from ECMO, advanced strategies for selected children affected by myocarditis could include heart transplantation or a durable left ventricle assist device (LVAD). ECMO support is necessary in 6.7% ± 0.9% and VAD in 1.4% ± 0.3% of the children [14]. Importantly, weaning from MCS is observed in a higher percent of myocarditis pediatric patients [46] compared to those affected by dilated cardiomyopathy and congenital heart diseases [88]. However, there are not enough data to derived solid conclusions [89].
In the subset of children with myocarditis, LVAD can be implanted as a bridge to recovery or heart transplantation. The development of new-generation devices and improvements in the management of major complications (bleeding, infection, cerebrovascular events and right ventricular failure) have allowed better outcomes in children with advanced heart failure.
After MCS application, further management can be divided into different settings. Most cases recover with or without sequelae [12,90]. In the absence of recovery, children must be referred for heart transplantation whenever eligible for the procedure [91].
8.3. Immunosuppressive Therapy (IT)
Immunosuppressive therapy encompasses corticosteroids alone or in combination with corticosteroid-sparing agents.
Corticosteroids have been studied in myocarditis of rheumatic origin since 1950, but the results of trials in adults were inconclusive [92,93]. In children, only limited studies with prednisone in combination with azathioprine or cyclosporine [45,94] or case series [95] are available.
However, results seem to highlight the efficacy of IT and are substantiated by an encouraging recent metanalysis [96].
In conclusion, the results of trials in adults were inconclusive, but subsequent investigations supported the effectiveness of corticosteroids in the autoimmune phase of the disease if no active viral infection is present [2,3].
8.4. Intravenous Immunoglobulin (IVIG)
The use of IVIG has been reported in the treatment of pediatric myocarditis since 1990 [97], even if its pharmacodynamics are poorly understood. IVIG has anti-inflammatory, anti-viral, and immunomodulatory properties [98].
In the study by Drucker and colleagues, IVIG administration in children with myocarditis was associated with an improved recovery of left ventricular function and with a tendency to better survival during the first year after presentation [97], but again, the results are not uniform [99].
8.5. Antivirals
Antiviral medications have been used since the 1980s [27,100,101]. A recent statement of the AHA on pediatric myocarditis advises the use of antiviral agents for myocarditis in case of active infection (even with no need for documentation of viral persistence within the myocardium) [2].
9. Follow-Up
After the acute phase, pediatric myocarditis can resolve without sequelae in a reasonable percentage of patients. A long follow-up study carried out on children with biopsy-proven myocarditis reported 83% of survival after a follow-up of 13 years [45]. According to a recent prospective study on children with biopsy-proven myocarditis, with a median follow-up time of 11 years, mortality was comparable in acute, chronic and healed myocarditis, ranging from 6% to 9% [46].
During the follow-up period, clinical assessment, ECG and echocardiography should be performed regularly [102,103,104].
At long-term follow up, CMR can be a useful tool to detect fibrosis and persistence of myocarditis despite a normal EF on transthoracic echocardiogram [105,106,107,108].
The role of EMB during follow-up is controversial. However, it might be considered in selected cases when LV dysfunction and increased serum inflammatory biomarkers or PCR are persistent [2].
10. Conclusions
Myocarditis is a tricky disease to diagnose and manage. EMB remains the gold standard for proven myocarditis, whose etiology can be further investigated through immunohistochemistry and PCR on myocardial samples.
Nowadays, MRI has an important role in the diagnostic flow-chart and during follow-up also in children.
The etiology is heterogeneous (mostly viral in children), the clinical manifestations are broad-ranging, and knowledge of the pathogenetic mechanism(s) for single patients is needed to tailor the therapy and improve the outcome of these patients.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef] [PubMed]
- Law, Y.M.; Lal, A.K.; Chen, S.; Čiháková, D.; Cooper, L.T., Jr.; Deshpande, S.; Godown, J.; Grosse-Wortmann, L.; Robinson, J.D.; Towbin, J.A. Diagnosis and Management of Myocarditis in Children: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e123–e135. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Malipiero, G.; Marcolongo, R.; Iliceto, S. Myocarditis: A Clinical Overview. Curr. Cardiol. Rep. 2017, 19, 63. [Google Scholar] [CrossRef] [PubMed]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.P.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Richardson, P.; McKenna, R.W.; Bristow, M.; Maisch, B.; Mautner, B.; O'Connell, J.; Olsen, E.; Thiene, G.; Goodwin, J.; Gyarfas, I.; et al. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of Cardiomyopathies. Circulation 1996, 93, 841–842. [Google Scholar] [CrossRef]
- Aretz, H.T.; Billingham, M.E.; Edwards, W.D.; Factor, S.M.; Fallon, J.T.; Fenoglio, J.J. Jr.; Olsen, E.G.; Schoen, F.J. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1987, 1, 3–14. [Google Scholar]
- Gagliardi, M.G.; Bevilacqua, M.; Di Renzi, P.; Picardo, S.; Passariello, R.; Marcelletti, C. Usefulness of magnetic resonance imaging for diagnosis of acute myocarditis in infants and children, and comparison with endomyocardial biopsy. Am. J. Cardiol. 1991, 68, 1089–1091. [Google Scholar] [CrossRef]
- Gagliardi, M.G.; Polletta, B.; Di Renzi, P. MRI for the diagnosis and follow-up of myocarditis. Circulation 1999, 99, 458–459. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Sechtem, U.; Schulz-Menger, J.; Holmvang, G.; Alakija, P.; Cooper, L.T.; White, J.A.; Abdel-Aty, H.; Gutberlet, M.; Prasad, S.; et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J. Am. Coll. Cardiol. 2009, 53, 1475–1487. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef]
- Cornicelli, M.D.; Rigsby, C.K.; Rychlik, K.; Pahl, E.; Robinson, J.D. Diagnostic performance of cardiovascular magnetic resonance native T1 and T2 mapping in pediatric patients with acute myocarditis. J. Cardiovasc. Magn. Reson. 2019, 21, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliardi, M.G.; Bevilacqua, M.; Squitieri, C.; Boldrini, R.; Di Julio, D.P.; Marcelletti, C. Dilated cardiomyopathy caused by acute myocarditis in pediatric patients: Evolution of myocardial damage in a group of potential heart transplant candidates. J. Heart Lung Transpl. 1993, 12, S224–S229. [Google Scholar]
- Caforio, A.L.P.; Marcolongo, R.; Jahns, R.; Fu, M.; Felix, S.B.; Iliceto, S. Immune-mediated and autoimmune myocarditis: Clinical presentation, diagnosis and management. Heart Fail. Rev. 2013, 18, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Vasudeva, R.; Bhatt, P.; Lilje, C.; Desai, P.; Amponsah, J.; Umscheid, J.; Parmar, N.; Bhatt, N.; Adupa, R.; Pagad, S.; et al. Trends in Acute Myocarditis Related Pediatric Hospitalizations in the United States, 2007–2016. Am. J. Cardiol. 2021, 149, 95–102. [Google Scholar] [CrossRef]
- Arola, A.; Pikkarainen, E.; Sipilä, J.O.T.; Pykäri, J.; Rautava, P.; Kytö, V. Occurrence and Features of Childhood Myocarditis: A Nationwide Study in Finland. J. Am. Heart Assoc. 2017, 6, e005306. [Google Scholar] [CrossRef] [Green Version]
- Butts, R.J.; Boyle, G.J.; Deshpande, S.R.; Gambetta, K.; Knecht, K.R.; Prada-Ruiz, C.A.; Richmond, M.E.; West, S.C.; Lal, A.K. Characteristics of Clinically Diagnosed Pediatric Myocarditis in a Contemporary Multi-Center Cohort. Pediatr. Cardiol. 2017, 38, 1175–1182. [Google Scholar] [CrossRef]
- Longson, M.; Cole, F.M.; Davies, D. Isolation of a Coxsackie virus group B, type 5, from the heart of a fatal case of myocarditis in an adult. J. Clin. Pathol. 1969, 22, 654–658. [Google Scholar] [CrossRef] [Green Version]
- Bowles, N.E.; Ni, J.; Kearney, D.L.; Pauschinger, M.; Schultheiss, H.P.; McCarthy, R.; Hare, J.; Bricker, J.T.; Bowles, K.R.; Towbin, J.A. Detection of viruses in myocardial tissues by polymerase chain reaction: Evidence of adenovirus as a common cause of myocarditis in children and adults. J. Am. Coll. Cardiol. 2003, 42, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Gagliardi, M.G.; Fierabracci, A.; Pilati, M.; Chinali, M.; Bassano, C.; Saura, F.; Giovannoni, I.; Francalanci, P. The impact of specific viruses on clinical outcome in children presenting with acute heart failure. Int. J. Mol. Sci. 2016, 17, 486. [Google Scholar] [CrossRef]
- Foerster, S.R.; Canter, C.E. Contemporary etiology, outcomes, and therapy in pediatric myocarditis. Prog. Pediatr. Cardiol. 2011, 31, 123–128. [Google Scholar] [CrossRef]
- Sanna, G.; Serrau, G.; Bassareo, P.P.; Neroni, P.; Fanos, V.; Marcialis, M.A. Children’s heart and COVID-19: Up-to-date evidence in the form of a systematic review. Eur. J. Pediatr. 2020, 179, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Santonja, C.; Santos-Briz, A.; Palmedo, G.; Kutzner, H.; Requena, L. Detection of human parvovirus B19 DNA in 22% of 1815 cutaneous biopsies of a wide variety of dermatological conditions suggests viral persistence after primary infection and casts doubts on its pathogenic significance. Br. J. Dermatol. 2017, 177, 1060–1065. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Calabrese, F.; Angelini, A.; Tona, F.; Vinci, A.; Bottaro, S.; Ramondo, A.; Carturan, E.; Iliceto, S.; Thiene, G.; et al. A prospective study of biopsy-proven myocarditis: Prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur. Heart J. 2007, 28, 1326–1333. [Google Scholar] [CrossRef] [Green Version]
- Dionne, A.; Sperotto, F.; Chamberlain, S.; Baker, A.L.; Powell, A.J.; Prakash, A.; Castellanos, D.A.; Saleeb, S.F.; De Ferranti, S.D.; Newburger, J.W.; et al. Association of Myocarditis with BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021, 6, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Kohli, U.; Desai, L.; Chowdhury, D.; Harahsheh, A.S.; Yonts, A.B.; Ansong, A.; Sabati, A.; Nguyen, H.H.; Hussain, T.; Khan, D.; et al. mRNA Coronavirus Disease 2019 Vaccine-Associated Myopericarditis in Adolescents: A Survey Study. J. Pediatr. 2022, 243, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Barda, N.; Hoss, S.; Richter, I.; Wiessman, M.; Aviv, Y.; Grinberg, T.; Auster, O.; Dagan, N.; Balicer, R.D.; et al. Myocarditis after COVID-19 Vaccination in a Large Health Care Organization. N. Engl. J. Med. 2021, 385, 2132–2139. [Google Scholar] [CrossRef] [PubMed]
- Matsumori, A.; Wang, H.; Abelmann, W.H.; Crumpacker, C.S. Treatment of viral myocarditis with ribavirin in an animal preparation. Circulation 1985, 71, 834–839. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Matsumori, A. Treatment of coxsackievirus B3 myocarditis by immunoactive peptide in an animal model. Clin. Immunol. Immunopathol. 1992, 65, 65–69. [Google Scholar] [CrossRef]
- Huang, C.H.; Vallejo, J.G.; Kollias, G.; Mann, D.L. Role of the Innate Immune System in Acute Viral Myocarditis. Basic Res. Cardiol. 2009, 104, 228. [Google Scholar] [CrossRef]
- Heymans, S.; Eriksson, U.; Lehtonen, J.; Cooper, L.T. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 2348–2364. [Google Scholar] [CrossRef]
- Liu, P.; Aitken, K.; Kong, Y.Y.; Opavsky, M.A.; Martino, T.; Dawood, F.; Wen, W.H.; Kozeiradzki, I.; Bachmaier, K.; Straus, D.; et al. The tyrosine kinase p56lck is essential in coxsackievirus B3-mediated heart disease. Nat. Med. 2000, 6, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Anzai, A.; Mindur, J.E.; Halle, L.; Sano, S.; Choi, J.L.; He, S.; McAlpine, C.S.; Chan, C.T.; Kahles, F.; Valet, C.; et al. Self-reactive CD4 + IL-3 + T cells amplify autoimmune inflammation in myocarditis by inciting monocyte chemotaxis. J. Exp. Med. 2019, 216, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.R. Myocarditis: Infection versus autoimmunity. J. Clin. Immunol. 2009, 29, 730–737. [Google Scholar] [CrossRef]
- Klingel, K.; Hohenadl, C.; Canu, A.; Albrecht, M.; Seemann, M.; Mall, G.; Kandolf, R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: Quantitative analysis of virus replication, tissue damage, and inflammation. Proc. Natl. Acad. Sci. USA 1992, 89, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caforio, A.L.P.; Iliceto, S. Genetically determined myocarditis: Clinical presentation and immunological characteristics. Curr. Opin. Cardiol. 2008, 23, 219–226. [Google Scholar] [CrossRef]
- Campuzano, O.; Fernández-Falgueras, A.; Sarquella-Brugada, G.; Sanchez, O.; Cesar, S.; Mademont, I.; Allegue, C.; Mates, J.; Pérez-Serra, A.; Coll, M.; et al. A Genetically Vulnerable Myocardium May Predispose to Myocarditis. J. Am. Coll. Cardiol. 2015, 66, 2913–2914. [Google Scholar] [CrossRef] [Green Version]
- Rose, N.R.; Bona, C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunol. Today 1993, 14, 426–430. [Google Scholar] [CrossRef]
- Mahon, N.G.; Madden, B.P.; Caforio, A.L.P.; Elliott, P.M.; Haven, A.J.; Keogh, B.E.; Davies, M.J.; McKenna, W.J. Immunohistologic evidence of myocardial disease in apparently healthy relatives of patients with dilated cardiomyopathy. J. Am. Coll. Cardiol. 2002, 39, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Kindermann, I.; Kindermann, M.; Kandolf, R.; Klingel, K.; Bültmann, B.; Müller, T.; Lindinger, A.; Böhm, M. Predictors of outcome in patients with suspected myocarditis. Circulation 2008, 118, 639–648. [Google Scholar] [CrossRef] [Green Version]
- Caforio, A.L.; Keeling, P.J.; Zachara, E.; Mestroni, L.; Camerini, F.; Mann, J.M.; Bottazzo, G.F.; McKenna, W.J. Evidence from family studies for autoimmunity in dilated cardiomyopathy. Lancet 1994, 344, 773–777. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Marcolongo, R.; Basso, C.; Iliceto, S. Clinical presentation and diagnosis of myocarditis. Heart 2015, 101, 1332–1344. [Google Scholar] [CrossRef] [PubMed]
- Caforio, A.L.P.; Angelini, A.; Blank, M.; Shani, A.; Kivity, S.; Goddard, G.; Doria, A.; Schiavo, A.; Testolina, M.; Bottaro, S.; et al. Passive transfer of affinity-purified anti-heart autoantibodies (AHA) from sera of patients with myocarditis induces experimental myocarditis in mice. Int. J. Cardiol. 2015, 179, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Becker, B.F.; Schultheiss, H.P. Antibodies to the ADP/ATP carrier, an autoantigen in myocarditis and dilated cardiomyopathy, penetrate into myocardial cells and disturb energy metabolism in vivo. Circ. Res. 1989, 64, 179–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caforio, A.L.; Goldman, J.H.; Haven, A.J.; Baig, K.M.; Libera, L.D.; McKenna, W.J. Circulating cardiac-specific autoantibodies as markers of autoimmunity in clinical and biopsy-proven myocarditis. The Myocarditis Treatment Trial Investigators. Eur. Heart J. 1997, 18, 270–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gagliardi, M.G.; Bevilacqua, M.; Bassano, C.; Leonardi, B.; Boldrini, R.; Camassei, F.D.; Fierabracci, A.; Ugazio, A.G.; Bottazzo, G.F. Long term follow up of children with myocarditis treated by immunosuppression and of children with dilated cardiomyopathy. Heart 2004, 90, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Seidel, F.; Opgen-Rhein, B.; Rentzsch, A.; Boehne, M.; Wannenmacher, B.; Boecker, D.; Reineker, K.; Grafmann, M.; Wiegand, G.; Hecht, T.; et al. Clinical characteristics and outcome of biopsy-proven myocarditis in children—Results of the German prospective multicentre registry “MYKKE”. Int. J. Cardiol. 2022, 357, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Pickardt, T.; Fischer, M.; Opgen-Rhein, B.; Papakostas, K.; Böcker, D.; Jakob, A.; Khalil, M.; Mueller, G.C.; Schmidt, F.; et al. Toward evidence-based diagnosis of myocarditis in children and adolescents: Rationale, design, and first baseline data of MYKKE, a multicenter registry and study platform. Am. Heart J. 2017, 187, 133–144. [Google Scholar] [CrossRef]
- Durani, Y.; Egan, M.; Baffa, J.; Selbst, S.M.; Nager, A.L. Pediatric myocarditis: Presenting clinical characteristics. Am. J. Emerg. Med. 2009, 27, 942–947. [Google Scholar] [CrossRef]
- Suthar, D.; Dodd, D.A.; Godown, J. Identifying Non-Invasive Tools to Distinguish Acute Myocarditis from Dilated Cardiomyopathy in Children. Pediatr. Cardiol. 2018, 39, 1134–1138. [Google Scholar] [CrossRef]
- Fairweather, D.L.; Cooper, L.T.; Blauwet, L.A. Sex and Gender Differences in Myocarditis and Dilated Cardiomyopathy. Curr. Probl. Cardiol. 2013, 38, 7–46. [Google Scholar] [CrossRef] [Green Version]
- Kociol, R.D.; Cooper, L.T.; Fang, J.C.; Moslehi, J.J.; Pang, P.S.; Sabe, M.A.; Shah, R.V.; Sims, D.B.; Thiene, G.; Vardeny, O. Recognition and Initial Management of Fulminant Myocarditis: A Scientific Statement from the American Heart Association. Circulation 2020, 141, E69–E92. [Google Scholar] [CrossRef] [PubMed]
- Casadonte, J.R.; Mazwi, M.L.; Gambetta, K.E.; Palac, H.L.; McBride, M.E.; Eltayeb, O.M.; Monge, M.C.; Backer, C.L.; Costello, J.M. Risk Factors for Cardiac Arrest or Mechanical Circulatory Support in Children with Fulminant Myocarditis. Pediatr. Cardiol. 2017, 38, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, R.; Sumitomo, N.; Komori, A.; Abe, Y.; Nakamura, T.; Fukuhara, J.; Matsumura, M.; Miyashita, M.; Kanamaru, H.; Ayusawa, M.; et al. The follow-up evaluation of electrocardiogram and arrhythmias in children with fulminant myocarditis. Circ. J. 2011, 75, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canter, C.E.; Simpson, K.P. Diagnosis and treatment of myocarditis in children in the current era. Circulation 2014, 129, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindal, A.; Singhi, S. Acute chest pain. Indian J. Pediatr. 2011, 78, 1262–1267. [Google Scholar] [CrossRef]
- Angelini, A.; Calzolari, V.; Calabrese, F.; Boffa, G.M.; Maddalena, F.; Chioin, R.; Thiene, G. Myocarditis mimicking acute myocardial infarction: Role of endomyocardial biopsy in the differential diagnosis. Heart 2000, 84, 245–250. [Google Scholar] [CrossRef] [Green Version]
- Maron, B.J.; Doerer, J.J.; Haas, T.S.; Tierney, D.M.; Mueller, F.O. Sudden deaths in young competitive athletes: Analysis of 1866 deaths in the United States, 1980–2006. Circulation 2009, 119, 1085–1092. [Google Scholar] [CrossRef]
- Harris, K.M.; Mackey-Bojack, S.; Bennett, M.; Nwaudo, D.; Duncanson, E.; Maron, B.J. Sudden Unexpected Death Due to Myocarditis in Young People, Including Athletes. Am. J. Cardiol. 2021, 143, 131–134. [Google Scholar] [CrossRef]
- Jhamnani, S.; Fuisz, A.; Lindsay, J. The spectrum of electrocardiographic manifestations of acute myocarditis: An expanded understanding. J. Electrocardiol. 2014, 47, 941–947. [Google Scholar] [CrossRef]
- Skouri, H.N.; Dec, G.W.; Friedrich, M.G.; Cooper, L.T. Noninvasive imaging in myocarditis. J. Am. Coll. Cardiol. 2006, 48, 2085–2093. [Google Scholar] [CrossRef] [Green Version]
- Khoo, N.S.; Smallhorn, J.F.; Atallah, J.; Kaneko, S.; MacKie, A.S.; Paterson, I. Altered left ventricular tissue velocities, deformation and twist in children and young adults with acute myocarditis and normal ejection fraction. J. Am. Soc. Echocardiogr. 2012, 25, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Wisotzkey, B.L.; Soriano, B.D.; Albers, E.L.; Ferguson, M.; Buddhe, S. Diagnostic role of strain imaging in atypical myocarditis by echocardiography and cardiac MRI. Pediatr. Radiol. 2018, 48, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Gursu, H.A.; Cetin, I.I.; Azak, E.; Kibar, A.E.; Surucu, M.; Orgun, A.; Pamuk, U. The assessment of treatment outcomes in patients with acute viral myocarditis by speckle tracking and tissue Doppler methods. Echocardiography 2019, 36, 1666–1674. [Google Scholar] [CrossRef] [PubMed]
- Lauer, B.; Niederau, C.; Kühl, U.; Schannwell, M.; Pauschinger, M.; Strauer, B.E.; Schultheiss, H.P. Cardiac troponin T in patients with clinically suspected myocarditis. J. Am. Coll. Cardiol. 1997, 30, 1354–1359. [Google Scholar] [CrossRef] [Green Version]
- Januzzi, J.L.; Camargo, C.A.; Anwaruddin, S.; Baggish, A.L.; Chen, A.A.; Krauser, D.G.; Tung, R.; Cameron, R.; Nagurney, J.T.; Chae, C.U.; et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am. J. Cardiol. 2005, 95, 948–954. [Google Scholar] [CrossRef]
- Katzmann, J.L.; Schlattmann, P.; Rigopoulos, A.G.; Noutsias, E.; Bigalke, B.; Pauschinger, M.; Tschope, C.; Sedding, D.; Schulze, P.C.; Noutsias, M. Meta-analysis on the immunohistological detection of inflammatory cardiomyopathy in endomyocardial biopsies. Heart Fail. Rev. 2020, 25, 277–294. [Google Scholar] [CrossRef]
- Kandolf, R.; Ameis, D.; Kirschner, P.; Canu, A.; Hofschneider, P.H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: An approach to the diagnosis of viral heart disease. Proc. Natl. Acad. Sci. USA 1987, 84, 6272–6276. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.B.; Webber, S.; Fricker, F.J.; Jaffe, R.; Demmler, G.; Kearney, D.; Zhang, Y.H.; Bodurtha, J.; Gelb, B.; Ni, J.; et al. Acute myocarditis. Rapid diagnosis by PCR in children. Circulation 1994, 90, 330–339. [Google Scholar] [CrossRef] [Green Version]
- Pophal, S.G.; Sigfusson, G.; Booth, K.L.; Bacanu, S.A.; Webber, S.A.; Ettedgui, J.A.; Neches, W.H.; Park, S.C. Complications of endomyocardial biopsy in children. J. Am. Coll. Cardiol. 1999, 34, 2105–2110. [Google Scholar] [CrossRef] [Green Version]
- Mills, K.I.; Vincent, J.A.; Zuckerman, W.A.; Hoffman, T.M.; Canter, C.E.; Marshall, A.C.; Blume, E.D.; Bergersen, L.; Daly, K.P. Is Endomyocardial Biopsy a Safe and Useful Procedure in Children with Suspected Cardiomyopathy? Pediatr. Cardiol. 2016, 37, 1200–1210. [Google Scholar] [CrossRef]
- Brighenti, M.; Donti, A.; Giulia Gagliardi, M.; Maschietto, N.; Marini, D.; Lombardi, M.; Vairo, U.; Agnoletti, G.; Milanesi, O.; Pongiglione, G.; et al. Endomyocardial biopsy safety and clinical yield in pediatric myocarditis: An Italian perspective. Catheter. Cardiovasc. Interv. 2016, 87, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, G.M. Myocarditis of giant-cell type in an infant. Am. J. Clin. Pathol. 1955, 25, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.T.; Berry, G.J.; Shabetai, R. Idiopathic giant-cell myocarditis—natural history and treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. N. Engl. J. Med. 1997, 336, 1860–1866. [Google Scholar] [CrossRef]
- Kittleson, M.M.; Minhas, K.M.; Irizarry, R.A.; Ye, S.Q.; Edness, G.; Breton, E.; Conte, J.V.; Tomaselli, G.; Garcia, J.G.N.; Hare, J.M. Gene expression in giant cell myocarditis: Altered expression of immune response genes. Int. J. Cardiol. 2005, 102, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Rosenstein, E.D.; Zucker, M.J.; Kramer, N. Giant cell myocarditis: Most fatal of autoimmune diseases. Semin. Arthritis Rheum. 2000, 30, 1–16. [Google Scholar] [CrossRef]
- Xu, J.; Brooks, E.G. Giant Cell Myocarditis: A Brief Review. Arch. Pathol. Lab. Med. 2016, 140, 1429–1434. [Google Scholar] [CrossRef] [Green Version]
- Hang, W.; Chen, C.; Seubert, J.M.; Wang, D.W. Fulminant myocarditis: A comprehensive review from etiology to treatments and outcomes. Signal Transduct. Target Ther. 2020, 5, 287. [Google Scholar] [CrossRef]
- Brailovsky, Y.; Masoumi, A.; Bijou, R.; Oliveros, E.; Sayer, G.; Takeda, K.; Uriel, N. Fulminant Giant Cell Myocarditis Requiring Bridge with Mechanical Circulatory Support to Heart Transplantation. JACC Case Rep. 2022, 4, 265–270. [Google Scholar] [CrossRef]
- Friedrich, M.G. Cardiovascular Magnetic Resonance for Myocardial Inflammation: Lake Louise Versus Mapping? Circ. Cardiovasc. Imaging 2018, 11, e008010. [Google Scholar] [CrossRef] [Green Version]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef] [Green Version]
- Radunski, U.K.; Lund, G.K.; Stehning, C.; Schnackenburg, B.; Bohnen, S.; Adam, G.; Blankenberg, S.; Muellerleile, K. CMR in patients with severe myocarditis: Diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc. Imaging 2014, 7, 667–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.A.; Lee, Y.J.; Salerno, M. Diagnostic performance of extracellular volume, native T1, and T2 mapping versus Lake Louise criteria by cardiac magnetic resonance for detection of acute myocarditis a meta-analysis. Circ. Cardiovasc. Imaging 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luetkens, J.A.; Faron, A.; Isaak, A.; Dabir, D.; Kuetting, D.; Feisst, A.; Schmeel, F.C.; Sprinkart, A.M.; Thomas, D. Comparison of Original and 2018 Lake Louise Criteria for Diagnosis of Acute Myocarditis: Results of a Validation Cohort. Radiol. Cardiothorac. Imaging 2019, 1, e190010. [Google Scholar] [CrossRef]
- Pitak, B.; Opgen-Rhein, B.; Schubert, S.; Reineker, K.; Wiegand, G.; Boecker, D.; Rentzsch, A.; Ruf, B.; Özcan, S.; Wannenmacher, B.; et al. Cardiovascular magnetic resonance in children with suspected myocarditis: Current practice and applicability of adult protocols. Cardiol. Young 2022, 1–9. [Google Scholar] [CrossRef]
- Pennington, D.G.; Swartz, M.T. Circulatory support in infants and children. Ann. Thorac. Surg. 1993, 55, 233–237. [Google Scholar] [CrossRef]
- Ghelani, S.J.; Spaeder, M.C.; Pastor, W.; Spurney, C.F.; Klugman, D. Demographics, trends, and outcomes in pediatric acute myocarditis in the United States, 2006 to 2011. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Dimas, V.V.; Morray, B.H.; Kim, D.W.; Almond, C.S.; Shahanavaz, S.; Tume, S.C.; Peng, L.F.; McElhinney, D.B.; Justino, H. A multicenter study of the impella device for mechanical support of the systemic circulation in pediatric and adolescent patients. Catheter. Cardiovasc. Interv. 2017, 90, 124–129. [Google Scholar] [CrossRef]
- Miera, O.; Germann, M.; Cho, M.Y.; Photiadis, J.; Walter, E.M.D.; Hetzer, R.; Berger, F.; Schmitt, K.R.L. Bridge to recovery in children on ventricular assist devices-protocol, predictors of recovery, and long-term follow-up. J. Heart Lung Transplant. 2018, 37, 1459–1466. [Google Scholar] [CrossRef]
- de By, T.M.M.H.; Schweiger, M.; Waheed, H.; Berger, F.; Hübler, M.; Özbaran, M.; Maruszewski, B.; Napoleone, C.P.; Loforte, A.; Meyns, B.; et al. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS): First EUROMACS Paediatric (Paedi-EUROMACS) report. Eur. J. Cardiothorac. Surg. 2018, 54, 800–808. [Google Scholar] [CrossRef]
- Foerster, S.R.; Canter, C.E.; Cinar, A.; Sleeper, L.A.; Webber, S.A.; Pahl, E.; Kantor, P.F.; Alvarez, J.A.; Colan, S.D.; Jefferies, J.L.; et al. Ventricular remodeling and survival are more favorable for myocarditis than for idiopathic dilated cardiomyopathy in childhood: An outcomes study from the Pediatric Cardiomyopathy Registry. Circ. Heart Fail. 2010, 3, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Gajarski, R.J.; Towbin, J.A. Recent advances in the etiology, diagnosis, and treatment of myocarditis and cardiomyopathies in children. Curr. Opin. Pediatr. 1995, 7, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.W.; O’Connell, J.B.; Herskowitz, A.; Rose, N.R.; McManus, B.M.; Billingham, M.E.; Moon, T.E. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N. Engl. J. Med. 1995, 333, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Hufnagel, G.; Pankuweit, S.; Richter, A.; Schönian, U.; Maisch, B. The European Study of Epidemiology and Treatment of Cardiac Inflammatory Diseases (ESETCID). First epidemiological results. Herz 2000, 25, 279–285. [Google Scholar] [CrossRef]
- Chan, K.Y.; Iwahara, M.; Benson, L.N.; Wilson, G.J.; Freedom, R.M. Immunosuppressive therapy in the management of acute myocarditis in children: A clinical trial. J. Am. Coll. Cardiol. 1991, 17, 458–460. [Google Scholar] [CrossRef]
- Marcolongo, R.; Rizzo, S.; Cerutti, A.; Reffo, E.; Castaldi, B.; Baritussio, A.; Basso, C.; Di Salvo, G.; Caforio, A.L.P. The multiple faces of autoimmune/immune-mediated myocarditis in children: A biopsy-proven case series treated with immunosuppressive therapy. ESC Heart Fail. 2021, 8, 1604. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Li, X.; Li, D. Immunosuppressive Treatment for Myocarditis in the Pediatric Population: A Meta-Analysis. Front. Pediatr. 2019, 7, 430. [Google Scholar] [CrossRef] [PubMed]
- Drucker, N.A.; Colan, S.D.; Lewis, A.B.; Beiser, A.S.; Wessel, D.L.; Takahashi, M.; Baker, A.L.; Perez-Atayde, A.R.; Newburger, J.W. Gamma-globulin treatment of acute myocarditis in the pediatric population. Circulation 1994, 89, 252–257. [Google Scholar] [CrossRef] [Green Version]
- Basta, M.; Branch, D.R. 7th International Immunoglobulin Conference: Mechanisms of Action. Clin. Exp. Immunol. 2014, 178, 87. [Google Scholar] [CrossRef] [Green Version]
- Lin, M.S.; Tseng, Y.H.; Chen, M.Y.; Chung, C.M.; Tsai, M.H.; Wang, P.C.; Chang, J.J.; Chen, T.H.; Lin, Y.S. In-hospital and post-discharge outcomes of pediatric acute myocarditis underwent after high-dose steroid or intravenous immunoglobulin therapy. BMC Cardiovasc. Disord. 2019, 19, 10. [Google Scholar] [CrossRef]
- Ettedgui, J.A.; Ladusans, E.; Bamford, M. Complete heart block as a complication of varicella. Int. J. Cardiol. 1987, 14, 362–365. [Google Scholar] [CrossRef]
- Shabtai, M.; Luft, B.; Waltzer, W.C.; Anaise, D.; Rapaport, F.T. Massive cytomegalovirus pneumonia and myocarditis in a renal transplant recipient: Successful treatment with DHPG. Transplant. Proc. 1988, 20, 562–563. [Google Scholar] [PubMed]
- Dasgupta, S.; Iannucci, G.; Mao, C.; Clabby, M.; Oster, M.E. Myocarditis in the pediatric population: A review. Congenit. Heart Dis. 2019, 14, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Nardi, P.; Pellegrino, A.; Scafuri, A.; Olevano, C.; Bassano, C.; Zeitani, J.; Chiariello, L. Survival and durability of mitral valve repair surgery for degenerative mitral valve disease. J. Card. Surg. 2011, 26, 360–366. [Google Scholar] [CrossRef]
- Gentili, F.; Cafiero, G.; Perrone, M.A.; Bianco, M.; Salvati, A.; Giordano, U.; Kikina, S.S.; Guccione, P.; De Zorzi, A.; Galletti, L.; et al. The Effects of Physical Inactivity and Exercise at Home in Young Patients with Congenital Heart Disease during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2021, 18, 10065. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, P.; Piazza, I. Myocarditis in the pediatric population: CMR image appraisal of myocardial inflammation. Int. J. Cardiol. 2021, 329, 249–250. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.G.; Crea, F.; Polletta, B.; Bassano, C.; La Vigna, G.; Ballerini, L.; Ragonese, P. Coronary microvascular endothelial dysfunction in transplanted children. Eur. Heart J. 2001, 22, 254–260. [Google Scholar] [CrossRef]
- Leonardi, B.; Secinaro, A.; Calvieri, C.; Perrone, M.A.; Gimigliano, F.; Muscogiuri, G.; Carotti, A.; Drago, F. The role of 3D imaging in the follow-up of patients with repaired tetralogy of Fallot. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1698–1709. [Google Scholar] [CrossRef]
- Leonardi, B.; Gentili, F.; Perrone, M.A.; Sollazzo, F.; Cocomello, L.; Kikina, S.S.; Wald, R.M.; Palmieri, V.; Secinaro, A.; Gagliardi, M.G.; et al. Cardiopulmonary Exercise Testing in Repaired Tetralogy of Fallot: Multiparametric Overview and Correlation with Cardiac Magnetic Resonance and Physical Activity Level. J. Cardiovasc. Dev. Dis. 2022, 9, 26. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).