Serial T2-Weighted Thoracic and Abdominal Lymphatic Imaging in Fontan Patients—New Insights into Dynamics of Lymphatic Abnormalities after Total Cavopulmonary Connection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethics Approval
2.3. Data Collection and Analysis
2.4. Magnetic Resonance Imaging Protocol after Total Cavopulmonary Connection
2.5. MRI Analysis
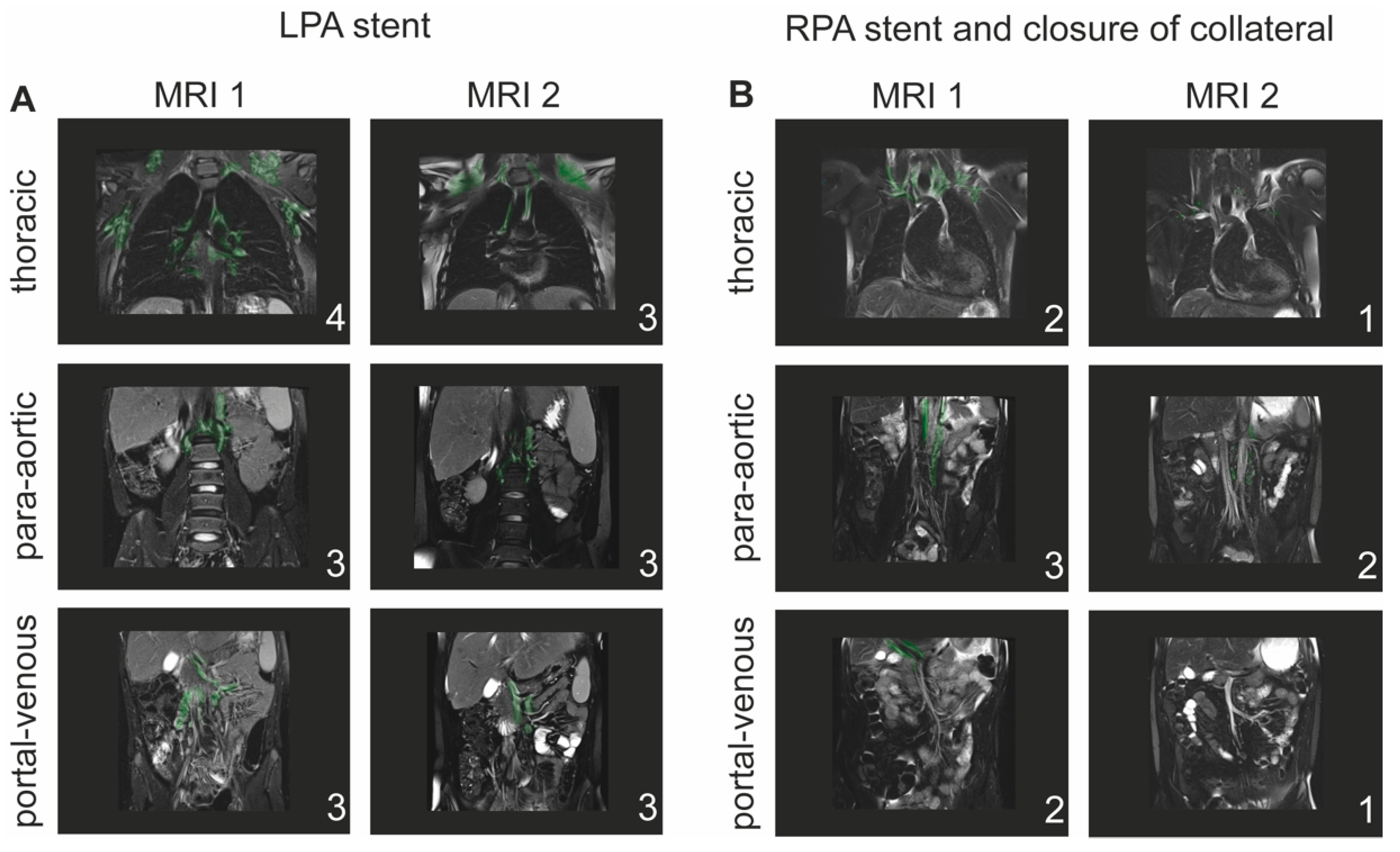
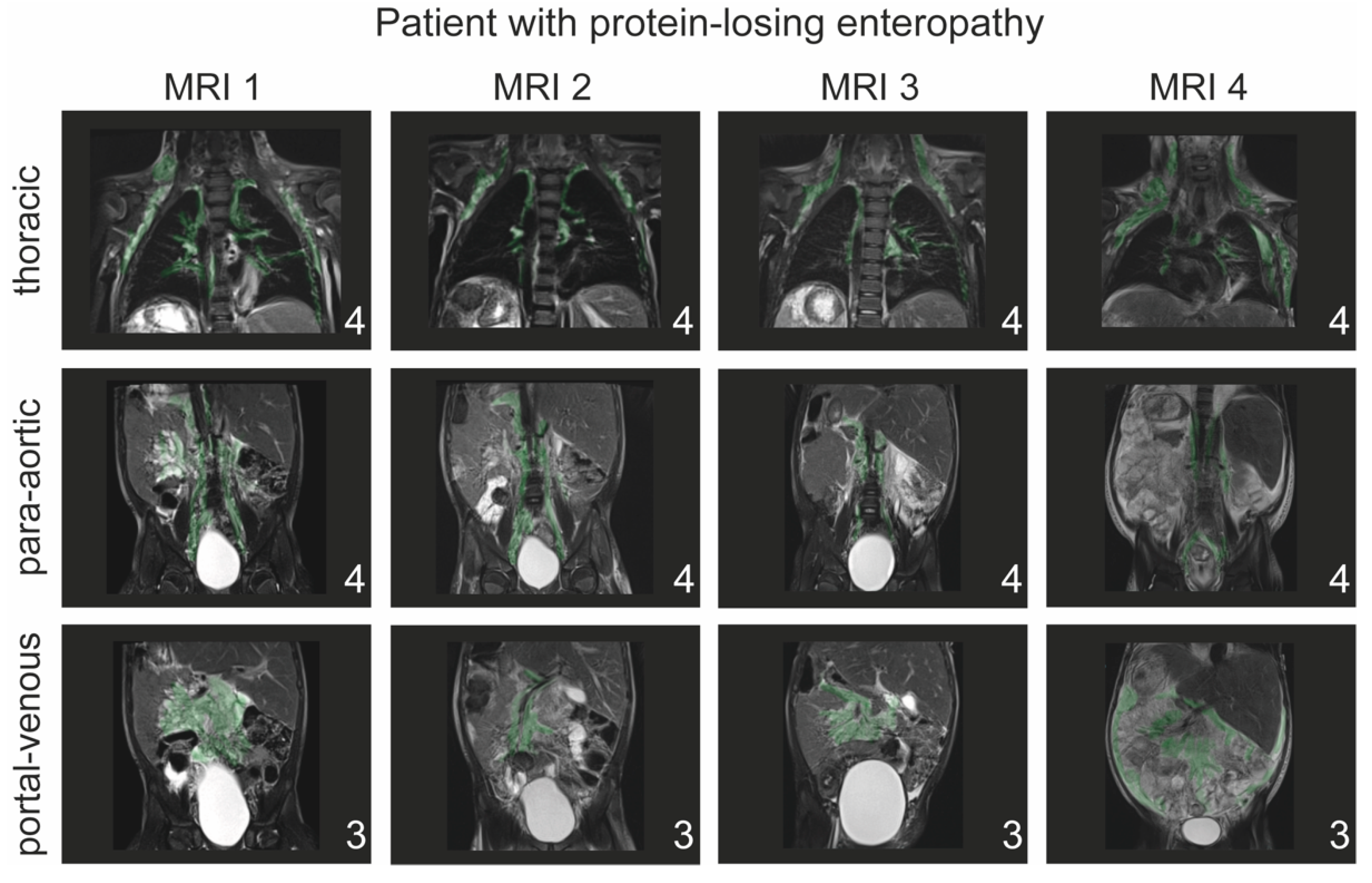
2.6. T2-Weighted Imaging and Classification of Lymphatic Abnormalities
- -
- Type 1: little or no presumed lymphatic channels within the supraclavicular region and mediastinum;
- -
- Type 2: abnormal increased lymphatic channels within the supraclavicular region without extension into the mediastinum;
- -
- Type 3: abnormal supraclavicular lymphatics with extension into the mediastinum;
- -
- Type 4: abnormal supraclavicular lymphatic channels with extension both into the mediastinum and in the interstitial pattern into the lung parenchyma.
- 1.
- Para-aortic lymphatic abnormalities:
- -
- Type 1: little or no para-aortic lymphatic abnormalities;
- -
- Type 2: para-aortic lymphatic abnormalities not following the renal arteries;
- -
- Type 3: para-aortic lymphatic abnormalities following the renal arteries;
- -
- Type 4: para-aortic lymphatic abnormalities following the renal arteries with extension to the inguinal region.
- 2.
- Portal-venous lymphatic abnormalities:
- -
- Type 1: little or no portal-venous lymphatic abnormalities;
- -
- Type 2: portal-venous lymphatic abnormalities not following the mesenteric veins;
- -
- Type 3: portal-venous lymphatic abnormalities following the mesenteric veins.
2.7. Statistics
3. Results
3.1. Patient Demographics and History
3.2. Changes in Lymphatic Abnormalities after Total Cavopulmonary Connection
3.3. Influence of Hemodynamics and Catheter Interventions on Lymphatic Abnormalities
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gewillig, M.; Brown, S.C. The Fontan circulation after 45 years: Update in physiology. Heart 2016, 102, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.M.; Griffis, H.M.; Glatz, A.C.; Rome, J.J.; Smith, C.L.; Gillespie, M.J.; Whitehead, K.K.; O’Byrne, M.L.; Biko, D.M.; Ravishankar, C.; et al. Prevalence and Cause of Early Fontan Complications: Does the Lymphatic Circulation Play a Role? J. Am. Heart Assoc. 2020, 9, e015318. [Google Scholar] [CrossRef] [PubMed]
- Rychik, J.; Dodds, K.M.; Goldberg, D.; Glatz, A.C.; Fogel, M.; Rossano, J.; Chen, J.; Pinto, E.; Ravishankar, C.; Rand, E.; et al. Protein Losing Enteropathy After Fontan Operation: Glimpses of Clarity through the Lifting Fog. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, S.; Weise, A.; Cesnjevar, R.; Rompel, O.; Rüffer, A.; Schöber, M.; Moosmann, J.; Glöckler, M. Association of Lymphatic Abnormalities with Early Complications after Fontan Operation. Thorac. Cardiovasc. Surg. 2021, 69, e1–e9. [Google Scholar] [CrossRef] [PubMed]
- Biko, D.M.; DeWitt, A.G.; Pinto, E.M.; Morrison, R.E.; Johnstone, J.A.; Griffis, H.; O’Byrne, M.L.; Fogel, M.A.; Harris, M.A.; Partington, S.L.; et al. MRI Evaluation of Lymphatic Abnormalities in the Neck and Thorax after Fontan Surgery: Relationship with Outcome. Radiology 2019, 291, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Itkin, M.; Piccoli, D.A.; Nadolski, G.; Rychik, J.; DeWitt, A.; Pinto, E.; Rome, J.; Dori, Y. Protein-Losing Enteropathy in Patients With Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 2929–2937. [Google Scholar] [CrossRef] [PubMed]
- Dori, Y.; Keller, M.S.; Fogel, M.A.; Rome, J.J.; Whitehead, K.K.; Harris, M.A.; Itkin, M. MRI of lymphatic abnormalities after functional single-ventricle palliation surgery. AJR Am. J. Roentgenol. 2014, 203, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Rompel, O.; Janka, R.; May, M.S.; Glöckler, M.; Cesnjevar, R.; Dittrich, S.; Lell, M.M.; Uder, M.; Hammon, M. Cardiac MRI in Children and Adolescents Who Have Undergone Surgical Repair of Right-Sided Congenital Heart Disease: Automated Left Ventricular Volumes and Function Analysis and Effects of Different Manual Adjustments. Rofo 2015, 187, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- Schroeder, C.; Moosmann, J.; Cesnjevar, R.; Purbojo, A.; Rompel, O.; Dittrich, S. A classification of abdominal lymphatic perfusion patterns after Fontan surgery. Eur. J. Cardio-Thorac. Surg. 2022, ezac103. [Google Scholar] [CrossRef]
- Chung, C.; Iwakiri, Y. The lymphatic vascular system in liver diseases: Its role in ascites formation. Clin. Mol. Hepatol. 2013, 19, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Biko, D.M.; Smith, C.L.; Otero, H.J.; Saul, D.; White, A.M.; DeWitt, A.; Glatz, A.C.; Piccoli, D.A.; Mamula, P.; Rome, J.J.; et al. Intrahepatic dynamic contrast MR lymphangiography: Initial experience with a new technique for the assessment of liver lymphatics. Eur. Radiol. 2019, 29, 5190–5196. [Google Scholar] [CrossRef]
- Tanaka, M.; Iwakiri, Y. Lymphatics in the liver. Curr. Opin. Immunol. 2018, 53, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Schleiger, A.; Salzmann, M.; Kramer, P.; Danne, F.; Schubert, S.; Bassir, C.; Müller, T.; Müller, H.P.; Berger, F.; Ovroutski, S. Severity of Fontan-Associated Liver Disease Correlates with Fontan Hemodynamics. Pediatr. Cardiol. 2020, 41, 736–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, C.L.; Liu, M.; Saravanan, M.; Dewitt, A.G.; Biko, D.M.; Pinto, E.M.; Escobar, F.A.; Krishnamurthy, G.; Brownell, J.N.; Mamula, P.; et al. Liver lymphatic anatomy and role in systemic lymphatic disease. Eur. Radiol. 2022, 32, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Robciuc, M.R.; Karaman, S.; Makinen, T.; Alitalo, K. Lymphatic System in Cardiovascular Medicine. Circ. Res. 2016, 118, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Lemley, B.A.; Biko, D.M.; Dewitt, A.G.; Glatz, A.C.; Goldberg, D.J.; Saravanan, M.; O’Byrne, M.L.; Pinto, E.; Ravishankar, C.; Rome, J.J.; et al. Intrahepatic Dynamic Contrast-Enhanced Magnetic Resonance Lymphangiography: Potential Imaging Signature for Protein-Losing Enteropathy in Congenital Heart Disease. J. Am. Heart Assoc. 2021, 10, e021542. [Google Scholar] [CrossRef] [PubMed]
- Savla, J.J.; Itkin, M.; Rossano, J.W.; Dori, Y. Post-Operative Chylothorax in Patients With Congenital Heart Disease. J. Am. Coll. Cardiol. 2017, 69, 2410–2422. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Suarez, K.I.; Tierradentro-Garcia, L.O.; Smith, C.L.; Krishnamurthy, G.; Escobar, F.A.; Otero, H.J.; Rapp, J.B.; Dori, Y.; Biko, D.M. Dynamic contrast-enhanced magnetic resonance lymphangiography. Pediatr. Radiol. 2022, 52, 285–294. [Google Scholar] [CrossRef] [PubMed]



| Demographics | |
|---|---|
| Sex | |
| Male (n) | 19 (57.6%) |
| Female (n) | 14 (42.4%) |
| Age at milestones | |
| Age at first surgery (days) | 9 (15) |
| Age at Glenn prodecure (months) | 6 (8) |
| Age TCPC (years) | 3.3 (9) |
| Age 1st MRI (years) | 4 (6) |
| Time between TCPC and 1st MRI (months) | 7.5 (33) |
| Age at 2nd MRI (years) | 10 (8) |
| Age at 3rd MRI (years) n = 11 | 10 (9) |
| Age at 4th MRI (years) n = 3 Age at last follow-up (years) | 11 (6) 13.5 (8) |
| Systemic left ventricle (n) | 15 (45.4%) |
| Systemic left ventricle (n) | 16 (48.5%) |
| Single ventricle with both components (n) | 2 (6.1%) |
| Fenestration (n) | 2 (6.1%) |
| Conduit size (mm) | 18 (2) |
| Syndromes | |
| Trisomy 21 | 3 (9.1%) |
| DiGeorge syndrome (22q11.2) | 1 (3.0%) |
| Cardiac malformations (n) | |
| Hypoplastic left heart syndrome | 6 (18.2%) |
| Unbalanced atrioventricular septal defect | 4 (12.1%) |
| Double-inlet left ventricle | 4 (12.1%) |
| Double-outlet left ventricle | 4 (12.1%) |
| Pulmonary atresia with intact ventricular septum | 3 (9.1%) |
| Hypoplastic or interrupted aortic arch with LV hypoplasia | 3 (9.1%) |
| Tricuspid atresia | 6 (18.2%) |
| Congenitally corrected transposition of great arteries | 2 (6.1%) |
| Criss-cross heart | 1 (3.0%) |
| Early complications (n) | 12 (36.4%) |
| Death (n) | 5 (15.2%) |
| Death causes and age at death (years) | |
| Postoperative death after conduit change | 9 |
| Portal vein thrombosis | 15 |
| Cerebral haemorrhage | 9 |
| Thromboembolic event | 7 |
| Acute transplant rejection | 23 |
| Indication for 2nd MRI | Changes in Lymph Classification | Numbers of Patients (n) |
|---|---|---|
| Clinical indication (n = 12, 36.6%) | ||
| Symptoms of protein-losing enteropathy, new onset or worsening of oedema, pleural effusion, or ascites (n = 7, 21.2%) | Stable | 1 (14.3%) |
| Improved | 3 (42.9%) | |
| Worsening | 2 (28.6%) | |
| Variation | 1 (14.3%) | |
| Cyanosis and reduced exercise capacity (n = 4, 12.2%) | Stable | 3 (75.0%) |
| Variation | 1 (25.0%) | |
| SVT (n = 1, 3%) | Variation | 1 (100%) |
| Morphologic/hemodynamic question (n = 2, 6.1%) | Stable | 1 (50.0%) |
| Variation | 1 (50.0%) | |
| Regular follow-up (n = 19, 57.6%) | Stable | 4 (21.1%) |
| Improved | 6 (31.6%) | |
| Worsening | 6 (31.6%) | |
| Variation | 2 (10.5%) |
| Lymphatic Classification | Total n = 33 | Early Complication n = 12 | No Complication n = 21 | |
|---|---|---|---|---|
| MRI 1 | Thoracic | |||
| Type 1 | - | - | - | |
| Type 2 | 4 | - | 4 (19%) | |
| Type 3 | 17 | 6 (50%) | 11 (52%) | |
| Type 4 | 12 | 6 (50%) | 6 (28%) | |
| Para-aortic | ||||
| Type 1 | 1 | 1 (8%) | - | |
| Type 2 | 2 | - | 2 (10%) | |
| Type 3 | 19 | 5 (42%) | 14 (67%) | |
| Type 4 | 10 | 6 (50%) | 5 (23%) | |
| Portal-venous | ||||
| Type 1 | 2 | 1 (8%) | 3 (14%) | |
| Type 2 | 9 | 3 (24%) | 6 (28%) | |
| Type 3 | 20 | 8 (68%) | 12 (57%) | |
| MRI 2 | Thoracic | |||
| Type 1 | 1 | - | 1 (5%) | |
| Type 2 | 3 | - | 3 (14%) | |
| Type 3 | 19 | 7 (58%) | 12 (57%) | |
| Type 4 | 9 | 4 (41%) | 4 (19%) | |
| Para-aortic | ||||
| Type 1 | - | - | - | |
| Type 2 | 2 | 1 (8%) | 1 (5%) | |
| Type 3 | 23 | 8 (68%) | 16 (76%) | |
| Type 4 | 7 | 3 (24%) | 4 (19%) | |
| Portal-venous | ||||
| Type 1 | 5 | 2 (16%) | 3 (14%) | |
| Type 2 | 6 | 1 (8%) | 6 (28%) | |
| Type 3 | 21 | 9 (75%) | 12 (57%) |
| 1st MRI | 2nd MRI | 3rd MRI | 4th MRI | |
|---|---|---|---|---|
| n = 33 | n = 33 | n = 11 | n = 3 | |
| Indication for MRI | ||||
| Routine/follow-up | 26 (78.8%) | 19 (57.6%) | 6 (54.5%) | 3 (100%) |
| Clinical indication | 6 (18.2%) | 12 (36.4%) | 5 (45.5%) | |
| Morphologic question | 1 (3.0%) | 2 (6.1%) | ||
| Body weight (kg) | 16.8 (9.2) | 37 (21.1) | 31 (23.8) | 39.6 (16.6) |
| End-diastolic volume (mL/m2 BSA) | 76.5 (32.6) | 79 (21) | 77 (19) | 68 (13.5) |
| End-systolic volume (mL/m2 BSA) | 35.5 (23) | 39.5 (21) | 40 (9) | 31 (4.7) |
| Ventricular ejection fraction (%) | 50.5 (11) | 49.5 (17) | 53 (13) | 56 (5.5) |
| Relevant AV-valve regurgitation (n) | ||||
| Mild | 10 (30.3%) | 13 (39.4%) | 3 (9.1%) | 3 (9.1%) |
| Moderate | 2 (6.1%) | 4 (12.1%) | ||
| Severe | 1 (3.0%) | |||
| Oxygen saturation (%) | 95 (3) | 95 (4.8) | 95 (2.5) | 97 (3) |
| Timing of Cardiac Catheterization and Hemodynamic Assessment | Patients (n) | Pressure (mmHg) | Lymphatic Abnormalities | ||
|---|---|---|---|---|---|
| Thoracic | Para-Aortic | Portal-Venous | |||
| Cardiac catheterization pre-Fontan | |||||
| TPG | 27 (81.8%) | 4.5 (2.75) | 0.318 * | 0.425 * | 0.706 * |
| SVC | 11 (3) | 0.909 * | 0.273 * | 0.532 * | |
| Cardiac catheterization between TCPC and MRI 1 | 11 | ||||
| SVC | (33.3%) | 16 (3.5) | 0.626 * | 0.149 * | 0.435 * |
| Cardiac catheterization between MRI 1 and 2 | |||||
| SVC | 10 | 12 (4) | 0.994 # | 0.097 # | 0.056 # |
| TPG | (30.0%) | 5 (3) | 0.242 # | 0.587 # | 0.999 # |
| Cardiac catheterization between MRI 2 and 3 | 7 | ||||
| SVC | (21.2%) | 14 (3.5) | 0.999 ** | 0.500 ** | 0.500 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moosmann, J.; Schroeder, C.; Rompel, O.; Purbojo, A.; Dittrich, S. Serial T2-Weighted Thoracic and Abdominal Lymphatic Imaging in Fontan Patients—New Insights into Dynamics of Lymphatic Abnormalities after Total Cavopulmonary Connection. J. Cardiovasc. Dev. Dis. 2022, 9, 138. https://doi.org/10.3390/jcdd9050138
Moosmann J, Schroeder C, Rompel O, Purbojo A, Dittrich S. Serial T2-Weighted Thoracic and Abdominal Lymphatic Imaging in Fontan Patients—New Insights into Dynamics of Lymphatic Abnormalities after Total Cavopulmonary Connection. Journal of Cardiovascular Development and Disease. 2022; 9(5):138. https://doi.org/10.3390/jcdd9050138
Chicago/Turabian StyleMoosmann, Julia, Christian Schroeder, Oliver Rompel, Ariawan Purbojo, and Sven Dittrich. 2022. "Serial T2-Weighted Thoracic and Abdominal Lymphatic Imaging in Fontan Patients—New Insights into Dynamics of Lymphatic Abnormalities after Total Cavopulmonary Connection" Journal of Cardiovascular Development and Disease 9, no. 5: 138. https://doi.org/10.3390/jcdd9050138
APA StyleMoosmann, J., Schroeder, C., Rompel, O., Purbojo, A., & Dittrich, S. (2022). Serial T2-Weighted Thoracic and Abdominal Lymphatic Imaging in Fontan Patients—New Insights into Dynamics of Lymphatic Abnormalities after Total Cavopulmonary Connection. Journal of Cardiovascular Development and Disease, 9(5), 138. https://doi.org/10.3390/jcdd9050138






