Chronic Kidney Disease with Mild and Mild to Moderate Reduction in Renal Function and Long-Term Recurrences of Atrial Fibrillation after Pulmonary Vein Cryoballoon Ablation
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design and Patient Population
2.2. Follow-Up and Data Collection
2.3. Research Objectives
2.4. Statistical Analyses
3. Results
3.1. Patient Population
3.2. Procedural Data
3.3. AF Recurrences after the Blanking Period
3.4. Predictors of AF Recurrences after the Blanking Period
3.5. Early Recurrence of AF (During the Blanking Period)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boriani, G.; Vitolo, M.; Diemberger, I.; Proietti, M.; Valenti, A.C.; Malavasi, V.L.; Lip, G.Y.H. Optimizing indices of atrial fibrillation susceptibility and burden to evaluate atrial fibrillation severity, risk and outcomes. Cardiovasc. Res. 2021, 117, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Steffel, J.; Collins, R.; Antz, M.; Cornu, P.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; Rowell, N.; et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace 2021, 23, 1612–1676. [Google Scholar] [CrossRef]
- Boriani, G.; Savelieva, I.; Dan, G.-A.; Deharo, J.C.; Ferro, C.; Israel, C.W.; Lane, D.A.; La Manna, G.; Morton, J.; Mitjans, A.M.; et al. Chronic kidney disease in patients with cardiac rhythm disturbances or implantable electrical devices: Clinical significance and implications for decision making-a position paper of the European Heart Rhythm Association endorsed by the Heart Rhythm Society and the Asia Pacific Heart Rhythm Society. Europace 2015, 17, 1169–1196. [Google Scholar] [CrossRef]
- Li, S.T.; Jiang, C.; He, L.; Li, Q.F.; Ding, Z.; Wu, J.H.; Hu, R.; Lv, Q.; Li, X.; Jia, C.-Q.; et al. Chronic kidney disease and risks of adverse clinical events in patients with atrial fibrillation. J. Geriatr. Cardiol. 2021, 18, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Goudis, C.; Daios, S.; Korantzopoulos, P.; Liu, T. Does CHA2DS2-VASc score predict mortality in chronic kidney disease? Internal and emergency medicine. Intern. Emerg. Med. 2021, 16, 1737–1742. [Google Scholar] [CrossRef]
- Levey, A.S.; Eckardt, K.-U.; Dorman, N.M.; Christiansen, S.L.; Cheung, M.; Jadoul, M.; Winkelmayer, W.C. Nomenclature for kidney function and disease-executive summary and glossary from a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Eur. Heart J. 2020, 41, 4592–4598. [Google Scholar] [CrossRef]
- Khan, M.S.; Bakris, G.L.; Packer, M.; Shahid, I.; Anker, S.D.; Fonarow, G.C.; Wanner, C.; Weir, M.R.; Zannad, F.; Butler, J. Kidney function assessment and endpoint ascertainment in clinical trials. Eur. Heart J. 2022, 43, 1379–1400. [Google Scholar] [CrossRef]
- Soraci, L.; Corica, F.; Corsonello, A.; Remelli, F.; Abete, P.; Bellelli, G.; Bo, M.; Cherubini, A.; Di Bari, M.; Maggio, M.; et al. Prognostic interplay of kidney function with sarcopenia, anemia, disability and cognitive impairment. The GLISTEN study. Eur. J. Intern. Med. 2021, 93, 57–63. [Google Scholar] [CrossRef]
- de Jong, Y.; Fu, E.L.; van Diepen, M.; Trevisan, M.; Szummer, K.; Dekker, F.W.; Carrero, J.J.; Ocak, G. Validation of risk scores for ischaemic stroke in atrial fibrillation across the spectrum of kidney function. Eur. Heart J. 2021, 42, 1476–1485. [Google Scholar] [CrossRef]
- Sgura, F.A.; Arrotti, S.; Magnavacchi, P.; Monopoli, D.; Gabbieri, D.; Banchelli, F.; Tondi, S.; Denegri, A.; D’Amico, R.; Guiducci, V.; et al. Kidney dysfunction and short term all-cause mortality after transcatheter aortic valve implantation. Eur. J. Intern. Med. 2020, 81, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Imberti, J.F.; Ding, W.Y.; Kotalczyk, A.; Zhang, J.; Boriani, G.; Lip, G.; Andrade, J.; Gupta, D. Catheter ablation as first-line treatment for paroxysmal atrial fibrillation: A systematic review and meta-analysis. Heart 2021, 107, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Moltrasio, M.; Iacopino, S.; Arena, G.; Pieragnoli, P.; Molon, G.; Manfrin, M.; Verlato, R.; Ottaviano, L.; Rovaris, G.; Catanzariti, D.; et al. First-line therapy: Insights from a real-world analysis of cryoablation in patients with atrial fibrillation. J. Cardiovasc. Med. 2021, 22, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Fortuni, F.; Casula, M.; Sanzo, A.; Angelini, F.; Cornara, S.; Somaschini, A.; Mugnai, G.; Rordorf, R.; De Ferrari, G.M. Meta-Analysis Comparing Cryoballoon Versus Radiofrequency as First Ablation Procedure for Atrial Fibrillation. Am. J. Cardiol. 2020, 125, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, T.; Nitta, J.; Inaba, O.; Sato, A.; Inamura, Y.; Kato, N.; Murata, K.; Ikenouchi, T.; Kono, T.; Nitta, G.; et al. Cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation in hemodialysis patients. Heart Vessel. 2020, 35, 1709–1716. [Google Scholar] [CrossRef]
- Tondo, C.; Iacopino, S.; Pieragnoli, P.; Molon, G.; Verlato, R.; Curnis, A.; Landolina, M.; Allocca, G.; Arena, G.; Fassini, G.; et al. Pulmonary vein isolation cryoablation for patients with persistent and long-standing persistent atrial fibrillation: Clinical outcomes from the real-world multicenter observational project. Heart Rhythm 2018, 15, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Stabile, G.; Bertaglia, E.; Guerra, F.; Palmisano, P.; Berisso, M.Z.; Soldati, E.; Bisignani, G.; Forleo, G.B.; Zanotto, G.; Landolina, M.; et al. Organization and procedures in contemporary catheter ablation centres: Data from the 2018 Italian Catheter Ablation Registry. J. Cardiovasc. Med. 2021, 22, 631–636. [Google Scholar] [CrossRef]
- Guenancia, C.; Hammache, N.; Docq, C.; Benali, K.; Hooks, D.; Echivard, M.; Pace, N.; Magnin-Poull, I.; de Chillou, C.; Sellal, J.-M. Efficacy and Safety of Second and Third-Generation Laser Balloon for Paroxysmal Atrial Fibrillation Ablation Compared to Radiofrequency Ablation: A Matched-Cohort. J. Cardiovasc. Dev. Dis. 2021, 8, 183. [Google Scholar] [CrossRef]
- Palmisano, P.; Ziacchi, M.; Angeletti, A.; Guerra, F.; Forleo, G.B.; Bertini, M.; Notarstefano, P.; Accogli, M.; Lavalle, C.; Bisignani, G.; et al. The Practice of Deep Sedation in Electrophysiology and Cardiac Pacing Laboratories: Results of an Italian Survey Promoted by the AIAC (Italian Association of Arrhythmology and Cardiac Pacing). J. Clin. Med. 2021, 10, 5035. [Google Scholar] [CrossRef]
- Deng, H.; Shantsila, A.; Xue, Y.; Bai, Y.; Guo, P.; Potpara, T.S.; Zhan, X.; Fang, X.; Liao, H.; Wu, S.; et al. Renal function and outcomes after catheter ablation of patients with atrial fibrillation: The Guangzhou atrial fibrillation ablation registry. Arch. Cardiovasc. Dis. 2019, 112, 420–429. [Google Scholar] [CrossRef]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.-H.; McAnulty, J.H., Jr.; Zheng, Z.-J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Huisman, M.V.; Teutsch, C.; Marler, S.; França, L.R.; Lu, S.; Lip, G.Y. Influence of BMI and geographical region on prescription of oral anticoagulants in newly diagnosed atrial fibrillation: The GLORIA-AF Registry Program. Eur. J. Intern. Med. 2020, 80, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Goto, S. Safety of antithrombotic therapy in East Asian patients. Intern. Emerg. Med. 2021, 16, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.G.; Healey, J.S.; Raina, P.; Connolly, S.J.; Ibrahim, Q.; Gupta, R.; Avezum, A.; Dans, A.L.; Lopez-Jaramillo, P.; Yeates, K.; et al. Global variations in the prevalence, treatment, and impact of atrial fibrillation in a multi-national cohort of 153 152 middle-aged individuals. Cardiovasc. Res. 2020, 117, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Vitolo, M.; Lip, G.Y.H. Understanding the global burden of atrial fibrillation and regional variations: We need improvement. Cardiovasc. Res. 2020, 117, 1420–1422. [Google Scholar] [CrossRef]
- Boriani, G.; Maniadakis, N.; Auricchio, A.; Müller-Riemenschneider, F.; Fattore, G.; Leyva, F.; Mantovani, L.G.; Siebert, M.; Willich, S.N.; Vardas, P.; et al. Health technology assessment in interventional electrophysiology and device therapy: A position paper of the European Heart Rhythm Association. Eur. Heart J. 2013, 34, 1869–1874. [Google Scholar] [CrossRef][Green Version]
- Murray, M.I.; Arnold, A.; Younis, M.; Varghese, S.; Zeiher, A.M. Cryoballoon versus radiofrequency ablation for paroxysmal atrial fibrillation: A meta-analysis of randomized controlled trials. Clin. Res. Cardiol. 2018, 107, 658–669. [Google Scholar] [CrossRef]
- Maltoni, S.; Negro, A.; Camerlingo, M.D.; Pecoraro, V.; Sassone, B.; Biffi, M.; Boriani, G. Comparison of cryoballoon and radiofrequency ablation techniques for atrial fibrillation: A meta-analysis. J. Cardiovasc. Med. 2018, 19, 725–738. [Google Scholar] [CrossRef]
- Boriani, G.; Imberti, J.F.; Valenti, A.C.; Malavasi, V.L.; Vitolo, M. Managing atrial fibrillation: The need for an individualized approach even in the emergency department. Intern. Emerg. Med. 2019, 15, 9–12. [Google Scholar] [CrossRef]
- Cappato, R. Implementation of Guidelines on Atrial Fibrillation Management in the Global Arena: So Many Actors on Stage! Eur. J. Intern. Med. 2021, 86, 22–24. [Google Scholar] [CrossRef]
- Deb, B.; Ganesan, P.; Feng, R.; Narayan, S.M. Identifying Atrial Fibrillation Mechanisms for Personalized Medicine. J. Clin. Med. 2021, 10, 5679. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Vitolo, M.; Harrison, S.L.; Lane, D.A.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; Dan, G.-A.; Boriani, G.; et al. Impact of clinical phenotypes on management and outcomes in European atrial fibrillation patients: A report from the ESC-EHRA EURObservational Research Programme in AF (EORP-AF) General Long-Term Registry. BMC Med. 2021, 19, 1–17. [Google Scholar] [CrossRef]
- Romiti, G.F.; Corica, B.; Pipitone, E.; Vitolo, M.; Raparelli, V.; Basili, S.; Boriani, G.; Harari, S.; Lip, G.Y.H.; Proietti, M.; et al. Prevalence, management and impact of chronic obstructive pulmonary disease in atrial fibrillation: A systematic review and meta-analysis of 4,200,000 patients. Eur. Heart J. 2021, 42, 3541–3554. [Google Scholar] [CrossRef] [PubMed]
- Diemberger, I.; Genovesi, S.; Massaro, G.; Reggiani, M.L.B.; Frisoni, J.; Gorlato, G.; Mauro, E.; Padeletti, M.; Vincenti, A.; Boriani, G. Meta-analysis of Clinical Outcomes of Electrical Cardioversion and Catheter Ablation in Patients with Atrial Fibrillation and Chronic Kidney Disease. Curr. Pharm. Des. 2018, 24, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Prasitlumkum, N.; Chokesuwattanaskul, R.; Kaewput, W.; Thongprayoon, C.; Tokavanich, N.; Bathini, T.; Boonpheng, B.; Vallabhajosyula, S.; Cheungpasitporn, W.; Jongnarangsin, K. Temporal trends and in-hospital complications of catheter ablation for atrial fibrillation among patients with moderate and advanced chronic kidney diseases: 2005–2018. J. Cardiovasc. Electrophysiol. 2022, 33, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Laroche, C.; Diemberger, I.; Popescu, M.I.; Rasmussen, L.H.; Petrescu, L.; Crijns, H.J.G.M.; Tavazzi, L.; Maggioni, A.P.; Lip, G.Y.H. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes. Sci. Rep. 2016, 6, 30271. [Google Scholar] [CrossRef]
- Malavasi, V.L.; Pettorelli, D.; Fantecchi, E.; Zoccali, C.; Laronga, G.; Trenti, T.; Lip, G.Y.H.; Boriani, G. Variations in clinical management of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation according to different equations for estimating renal function: Post hoc analysis of a prospective cohort. Intern. Emerg. Med. 2018, 13, 1059–1067. [Google Scholar] [CrossRef]
- Zou, L.-X.; Sun, L.; Nicholas, S.B.; Lu, Y.; Sinha, S.; Hua, R. Comparison of bias and accuracy using cystatin C and creatinine in CKD-EPI equations for GFR estimation. Eur. J. Intern. Med. 2020, 80, 29–34. [Google Scholar] [CrossRef]
- Huang, J.; Wang, X.; Hao, C.; Yang, W.; Zhang, W.; Liu, J.; Qu, H. Cystatin C and/or creatinine-based estimated glomerular filtration rate for prediction of vancomycin clearance in long-stay critically ill patients with persistent inflammation, immunosuppression and catabolism syndrome (PICS): A population pharmacokinetics analysis. Internal and emergency medicine. Intern. Emerg. Med. 2021, 16, 1883–1893. [Google Scholar] [CrossRef]
- Boriani, G.; Vitolo, M.; Lane, D.A.; Potpara, T.S.; Lip, G.Y. Beyond the 2020 guidelines on atrial fibrillation of the European society of cardiology. Eur. J. Intern. Med. 2021, 86, 1–11. [Google Scholar] [CrossRef]
- Malavasi, V.L.; Vitolo, M.; Colella, J.; Montagnolo, F.; Mantovani, M.; Proietti, M.; Potpara, T.S.; Lip, G.Y.H.; Boriani, G. Rhythm- or rate-control strategies according to 4S-AF characterization scheme and long-term outcomes in atrial fibrillation patients: The FAMo (Fibrillazione Atriale in Modena) cohort. Intern. Emerg. Med. 2021, 1–12, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Potpara, T.S.; Lip, G.Y.H.; Blomstrom-Lundqvist, C.; Boriani, G.; Van Gelder, I.C.; Heidbuchel, H.; Hindricks, G.; Camm, A.J. The 4S-AF Scheme (Stroke Risk; Symptoms; Severity of Burden; Substrate): A Novel Approach to In-Depth Characterization (Rather than Classification) of Atrial Fibrillation. Thromb. Haemost. 2020, 121, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Lip, G.Y.H.; Laroche, C.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.; Dan, G.-A.; Kalarus, Z.; Tavazzi, L.; et al. Relation of outcomes to ABC (Atrial Fibrillation Better Care) pathway adherent care in European patients with atrial fibrillation: An analysis from the ESC-EHRA EORP Atrial Fibrillation General Long-Term (AFGen LT) Registry. Europace 2020, 23, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Romiti, G.F.; Pastori, D.; Rivera-Caravaca, J.M.; Ding, W.Y.; Gue, Y.X.; Menichelli, D.; Gumprecht, J.; Kozieł, M.; Yang, P.-S.; Guo, Y.; et al. Adherence to the ‘Atrial Fibrillation Better Care’ Pathway in Patients with Atrial Fibrillation: Impact on Clinical Outcomes-A Systematic Review and Meta-Analysis of 285,000 Patients. Thromb. Haemost. 2021, 122, 406–414. [Google Scholar] [CrossRef]
- Goette, A.; Lendeckel, U. Atrial Cardiomyopathy: Pathophysiology and Clinical Consequences. Cells 2021, 10, 2605. [Google Scholar] [CrossRef]
- Malavasi, V.L.; Fantecchi, E.; Tordoni, V.; Melara, L.; Barbieri, A.; Vitolo, M.; Lip, G.Y.H.; Boriani, G. Atrial fibrillation pattern and factors affecting the progression to permanent atrial fibrillation. Internal and emergency medicine. Intern. Emerg. Med. 2020, 16, 1131–1140. [Google Scholar] [CrossRef]
- Guo, F.; Li, C.; Yang, L.; Chen, C.; Chen, Y.; Ni, J.; Fu, R.; Jiao, Y.; Meng, Y. Impact of left atrial geometric remodeling on late atrial fibrillation recurrence after catheter ablation. J. Cardiovasc. Med. 2021, 22, 909–916. [Google Scholar] [CrossRef]
- Boriani, G.; Imberti, J.F.; Vitolo, M. The challenge to improve knowledge on the interplay between subclinical atrial fibrillation, atrial cardiomyopathy, and atrial remodeling. J. Cardiovasc. Electrophysiol. 2021, 32, 1364–1366. [Google Scholar] [CrossRef]
- Andrade, J.G.; Wells, G.A.; Deyell, M.W.; Bennett, M.; Essebag, V.; Champagne, J.; Roux, J.-F.; Yung, D.; Skanes, A.; Khaykin, Y.; et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 305–315. [Google Scholar] [CrossRef]
- Blaauw, Y.; Mulder, B.; Rienstra, M. Is catheter ablation of atrial fibrillation as first-line treatment ready for prime time? Heart 2021, 107, 1605–1606. [Google Scholar] [CrossRef]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; Van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Rillig, A.; Magnussen, C.; Ozga, A.-K.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.; Eckardt, L.; Elvan, A.; et al. Early Rhythm Control Therapy in Patients With Atrial Fibrillation and Heart Failure. Circulation 2021, 144, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Vitolo, M.; Harrison, S.L.; Lane, D.A.; Fauchier, L.; Marin, F.; Nabauer, M.; Potpara, T.S.; Dan, G.-A.; Boriani, G.; et al. Real-world applicability and impact of early rhythm control for European patients with atrial fibrillation: A report from the ESC-EHRA EORP-AF Long-Term General Registry. Clin. Res. Cardiol. 2021, 111, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Laroche, C.; Diemberger, I.; Fantecchi, E.; Popescu, M.I.; Rasmussen, L.H.; Sinagra, G.; Petrescu, L.; Tavazzi, L.; Maggioni, A.P.; et al. Asymptomatic atrial fibrillation: Clinical correlates, management, and outcomes in the EORP-AF Pilot General Registry. Am. J. Med. 2014, 128, 509–518.e2. [Google Scholar] [CrossRef]
- Sgreccia, D.; Manicardi, M.; Malavasi, V.L.; Vitolo, M.; Valenti, A.C.; Proietti, M.; Lip, G.Y.H.; Boriani, G. Comparing Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation: A Systematic Review and Meta-Analysis of 81,462 Patients. J. Clin. Med. 2021, 10, 3979. [Google Scholar] [CrossRef]
- Vitolo, M.; Imberti, J.F.; Maisano, A.; Albini, A.; Bonini, N.; Valenti, A.C.; Malavasi, V.L.; Proietti, M.; Healey, J.S.; Lip, G.Y.; et al. Device-detected atrial high rate episodes and the risk of stroke/thrombo-embolism and atrial fibrillation incidence: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 92, 100–106. [Google Scholar] [CrossRef]
- Kiliszek, M.; Uziębło-Życzkowska, B.; Gorczyca, I.; Maciorowska, M.; Jelonek, O.; Wożakowska-Kapłon, B.; Wójcik, M.; Błaszczyk, R.; Gawałko, M.; Kapłon-Cieślicka, A.; et al. Symptomatic and Asymptomatic Patients in the Polish Atrial Fibrillation (POL-AF) Registry. J. Clin. Med. 2021, 10, 1091. [Google Scholar] [CrossRef]
- Stabile, G.; Iacopino, S.; Verlato, R.; Arena, G.; Pieragnoli, P.; Molon, G.; Manfrin, M.; Rovaris, G.; Curnis, A.; Bertaglia, E.; et al. Predictive role of early recurrence of atrial fibrillation after cryoballoon ablation. Europace 2020, 22, 1798–1804. [Google Scholar] [CrossRef]
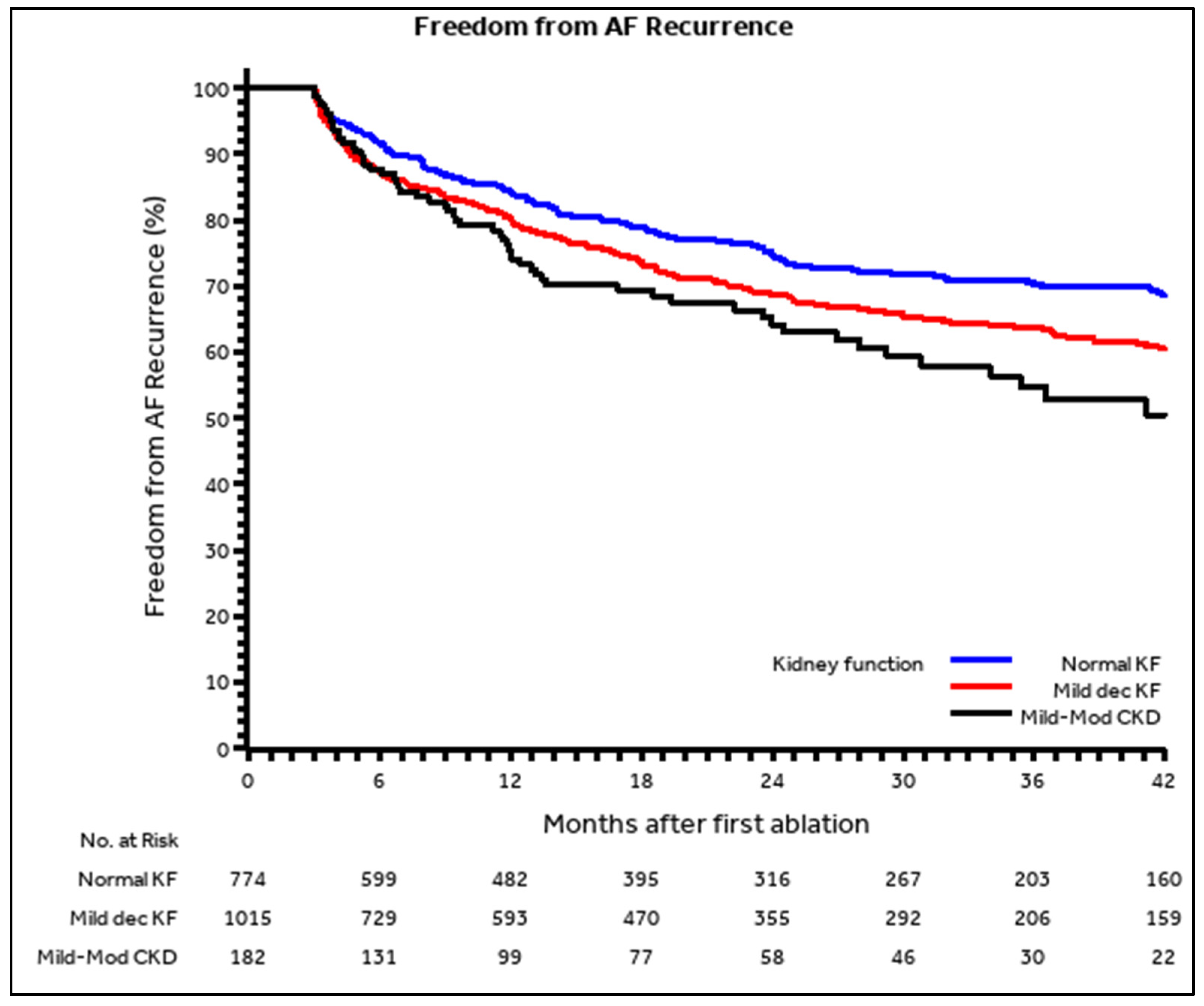
| Total n = 1971 | Normal Kidney Function (eGFR: ≥90 mL/min/1.73 m2) n = 774 | Mildly Decreased Kidney Function (eGFR: 60–89 mL/min/1.73 m2) n = 1015 | Mild to Moderate CKD (eGFR: 30–59 mL/min/1.73 m2) n = 182 | p-Value | |
|---|---|---|---|---|---|
| Age at first ablation (years), mean ± SD | 60.0 ± 10.4 | 54.6 ± 10.5 | 62.7 ± 8.7 | 68.2 ± 6.7 | <0.001 1,2 |
| Female sex, n (%) | 572 (29.0%) | 185 (23.9%) | 305 (30.0%) | 82 (45.1%) | <0.001 1,2 |
| BMI, mean ± SD | 27.1 ± 4.2 | 27.1 ± 4.4 | 27.2 ± 3.9 | 27.4 ± 4.8 | 0.768 |
| Paroxysmal, n (%) | 1450 (73.6%) | 608 (78.6%) | 710 (70.0%) | 132 (72.5%) | 0.001 |
| Months from first AF diagnosis, median (Q1–Q3) | 26 (12–60) | 24 (12–60) | 26 (12–60) | 36 (12–96) | 0.386 |
| Previous therapy using >2 AADs, n (%) | 723 (41.5%) | 266 (38.8%) | 372 (41.8%) | 85 (51.2%) | 0.014 1,2 |
| Diabetes, n (%) | 114 (6.2%) | 40 (5.5%) | 64 (6.7%) | 10 (5.9%) | 0.551 |
| Hypertension, n (%) | 1030 (52.5%) | 324 (42.0%) | 582 (57.6%) | 124 (68.1%) | <0.001 1,2 |
| History of stroke or TIA, n (%) | 85 (4.4%) | 27 (3.5%) | 45 (4.5%) | 13 (7.2%) | 0.090 |
| No underlying heart disease, n (%) | 1525 (77.9%) | 634 (82.4%) | 768 (76.3%) | 123 (67.6%) | <0.001 1,2 |
| CHA2DS2VASc ≥1 (male) or ≥2 (female), n (%) | 1261 (69.8%) | 393(54.6%) | 713 (77.3%) | 155 (93.9%) | <0.001 1,2 |
| Left atrial diameter (mm), mean ± SD | 41.8 ± 6.1 | 40.9 ± 6.2 | 42.3 ± 6.0 | 42.8 ± 6.1 | <0.001 1 |
| Left ventricular ejection fraction (%),mean ± SD | 58.7 ± 6.8 | 59.4 ± 6.2 | 58.5 ± 6.9 | 56.9 ± 8.5 | <0.001 1 |
| Total n = 1971 | Normal Kidney Function (eGFR: ≥90 mL/min/1.73 m2) n = 774 | Mildly Decreased Kidney Function (eGFR: 60–89 mL/min/1.73 m2) n = 1015 | Mild to Moderate CKD (eGFR: 30–59 mL/min/1.73 m2) n = 182 | p-Value | |
|---|---|---|---|---|---|
| Procedural characteristics | |||||
| Procedure duration (min), mean ± SD | 107.3 ± 46.8 | 105.2 ± 45.2 | 108.0 ± 47.5 | 112.3 ± 49.5 | 0.142 |
| Fluoroscopy duration (min), mean ± SD | 28.8 ± 16.4 | 29.4 ± 17.5 | 28.4 ± 15.5 | 28.8 ± 17.0 | 0.666 |
| Ablation time (min), mean ± SD | 28.4 ± 55.0 | 26.2 ± 58.6 | 28.4 ± 38.8 | 38.8 ± 99.8 | 0.0041 |
| Effective PVI, n (%) | 1956 (99.2%) | 769 (99.4%) | 1007 (99.2%) | 180 (98.9%) | 0.519 |
| Pre-ablation sinus rhythm, n (%) | 1415 (74.1%) | 587 (78.2%) | 709 (72.2%) | 119 (67.6%) | 0.0021 |
| Cardioversion, n (%) | 493 (25.0%) | 172 (22.2%) | 266 (26.2%) | 55 (30.2%) | 0.0371 |
| Post-ablation sinus rhythm, n (%) | 1841 (97.2%) | 726 (97.4%) | 950 (97.2%) | 165 (95.9%) | 0.517 |
| Acute procedural complications | |||||
| Patients with at least one complication, n (%) | 73 (3.7%) | 27 (3.5%) | 41 (4.0%) | 5 (2.7%) | 0.641 |
| Transient Diaphragmatic Paralysis, n (%) | 41 (2.1%) | 15 (1.9%) | 24 (2.4%) | 2 (1.1%) | 0.594 |
| Permanent Diaphragmatic Paralysis, n (%) | 2 (0.1%) | 1 (0.1%) | 1 (0.1%) | 0 (0.0%) | 1.000 |
| Pericardial effusion, n (%) | 7 (0.4%) | 3 (0.4%) | 3 (0.3%) | 1 (0.5%) | 0.620 |
| Cardiac Tamponade, n (%) | 4 (0.2%) | 1 (0.1%) | 2 (0.2%) | 1 (0.5%) | 0.415 |
| AV Fistula, n (%) | 4 (0.2%) | 2 (0.3%) | 1 (0.1%) | 1 (0.5%) | 0.300 |
| Femoral pseudo-aneurism, n (%) | 2 (0.1%) | 1 (0.1%) | 1 (0.1%) | 0 (0.0%) | 1.000 |
| Stroke, n (%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ---- |
| TIA, n (%) | 2 (0.1%) | 1 (0.1%) | 1 (0.1%) | 0 (0.0%) | 1.000 |
| Hematoma, n (%) | 5 (0.3%) | 2 (0.3%)) | 3 (0.3%) | 0 (0.0%) | 1.000 |
| Other minor complications, n (%) | 7 (0.4%) | 2 (0.3%) | 5 (0.5%) | 0 (0.0%) | 0.851 |
| Univariate Analysis | ||
| HR (95% CI) | p-Value | |
| Mild to moderate CKD (eGFR 30–59 mL/min/1.73 m2) vs. eGFR 60 mL/min/1.73 m2 or higher | 1.40 (1.06–1.83) | 0.017 |
| Female gender | 1.04 (0.86–1.26) | 0.674 |
| Age at first ablation (years) ≥ 65 | 1.12 (0.94–1.34) | 0.201 |
| Paroxysmal AF | 0.76 (0.63–0.92) | 0.004 |
| Months from the first episode of atrial arrhythmia > 12 months | 1.27 (1.03–1.58) | 0.025 |
| Number of tested AAD ≥ 2 | 1.26 (1.05–1.51) | 0.014 |
| Underlying heart disease | 1.08 (0.88–1.33) | 0.466 |
| Hypertension | 1.09 (0.91–1.30) | 0.349 |
| CHA2DS2VASc ≥ 1 (male) or ≥2 (female) | 1.09 (0.90–1.33) | 0.371 |
| LVEF (%, continuous) | 0.99 (0.98–1.01) | 0.265 |
| Multivariable Analysis | ||
| Mild to moderate CKD (eGFR 30–59) vs. eGFR 60 mL/min/1.73 m2 or higher | 1.38 (1.02–1.86) | 0.037 |
| Female gender | 0.99 (0.81–1.22) | 0.957 |
| Age at first ablation (years) ≥ 65 | 1.05 (0.86–1.27) | 0.626 |
| Paroxysmal AF | 0.78 (0.64–0.96) | 0.019 |
| Months from the first episode of atrial arrhythmia > 12 months | 1.27 (1.03–1.57) | 0.026 |
| Univariate Analysis | ||
| HR (95% CI) | p-value | |
| Mild to moderate CKD (eGFR 30–59 mL/min/1.73 m2) vs. eGFR 60 mL/min/1.73 m2 or higher | 1.54 (0.94–2.50) | 0.084 |
| Female gender | 0.94 (0.66–1.35) | 0.755 |
| Age at first ablation (years) ≥ 65 | 0.95 (0.68–1.32) | 0.746 |
| Paroxysmal AF | 0.50 (0.36–0.69) | <0.001 |
| Months from first episode of atrial arrhythmia > 12 months | 1.37 (0.92–2.02) | 0.120 |
| Number of tested AAD ≥ 2 | 1.59 (1.14–2.22) | 0.007 |
| Underlying heart disease | 1.28 (0.89–1.86) | 0.185 |
| Hypertension | 0.79 (0.58–1.10) | 0.163 |
| CHA2DS2VASc ≥ 1 (male) or ≥2 (female) | 0.92 (0.64–1.32) | 0.660 |
| LVEF (%, continuous) | 0.98 (0.96–1.00) | 0.112 |
| Multivariable analysis | ||
| Mild to moderate CKD (eGFR 30–59) vs. eGFR 60 mL/min/1.73 m2 or higher | 1.56 (0.92–2.63) | 0.099 |
| Female gender | 0.90 (0.61–1.32) | 0.581 |
| Age at first ablation (years) ≥ 65 | 0.86 (0.59–1.23) | 0.401 |
| Paroxysmal AF | 0.48 (0.34–0.68) | <0.001 |
| Months from first episode of atrial arrhythmia > 12 months | 1.56 (1.11–2.19) | 0.011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boriani, G.; Iacopino, S.; Arena, G.; Pieragnoli, P.; Verlato, R.; Manfrin, M.; Molon, G.; Rovaris, G.; Curnis, A.; Perego, G.B.; et al. Chronic Kidney Disease with Mild and Mild to Moderate Reduction in Renal Function and Long-Term Recurrences of Atrial Fibrillation after Pulmonary Vein Cryoballoon Ablation. J. Cardiovasc. Dev. Dis. 2022, 9, 126. https://doi.org/10.3390/jcdd9050126
Boriani G, Iacopino S, Arena G, Pieragnoli P, Verlato R, Manfrin M, Molon G, Rovaris G, Curnis A, Perego GB, et al. Chronic Kidney Disease with Mild and Mild to Moderate Reduction in Renal Function and Long-Term Recurrences of Atrial Fibrillation after Pulmonary Vein Cryoballoon Ablation. Journal of Cardiovascular Development and Disease. 2022; 9(5):126. https://doi.org/10.3390/jcdd9050126
Chicago/Turabian StyleBoriani, Giuseppe, Saverio Iacopino, Giuseppe Arena, Paolo Pieragnoli, Roberto Verlato, Massimiliano Manfrin, Giulio Molon, Giovanni Rovaris, Antonio Curnis, Giovanni Battista Perego, and et al. 2022. "Chronic Kidney Disease with Mild and Mild to Moderate Reduction in Renal Function and Long-Term Recurrences of Atrial Fibrillation after Pulmonary Vein Cryoballoon Ablation" Journal of Cardiovascular Development and Disease 9, no. 5: 126. https://doi.org/10.3390/jcdd9050126
APA StyleBoriani, G., Iacopino, S., Arena, G., Pieragnoli, P., Verlato, R., Manfrin, M., Molon, G., Rovaris, G., Curnis, A., Perego, G. B., Dello Russo, A., Landolina, M., Vitolo, M., Tondo, C., & on behalf of the 1STOP ClinicalService Investigators. (2022). Chronic Kidney Disease with Mild and Mild to Moderate Reduction in Renal Function and Long-Term Recurrences of Atrial Fibrillation after Pulmonary Vein Cryoballoon Ablation. Journal of Cardiovascular Development and Disease, 9(5), 126. https://doi.org/10.3390/jcdd9050126










