Left Ventricular Noncompaction Is Associated with Valvular Regurgitation and a Variety of Arrhythmias
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Clinical Data
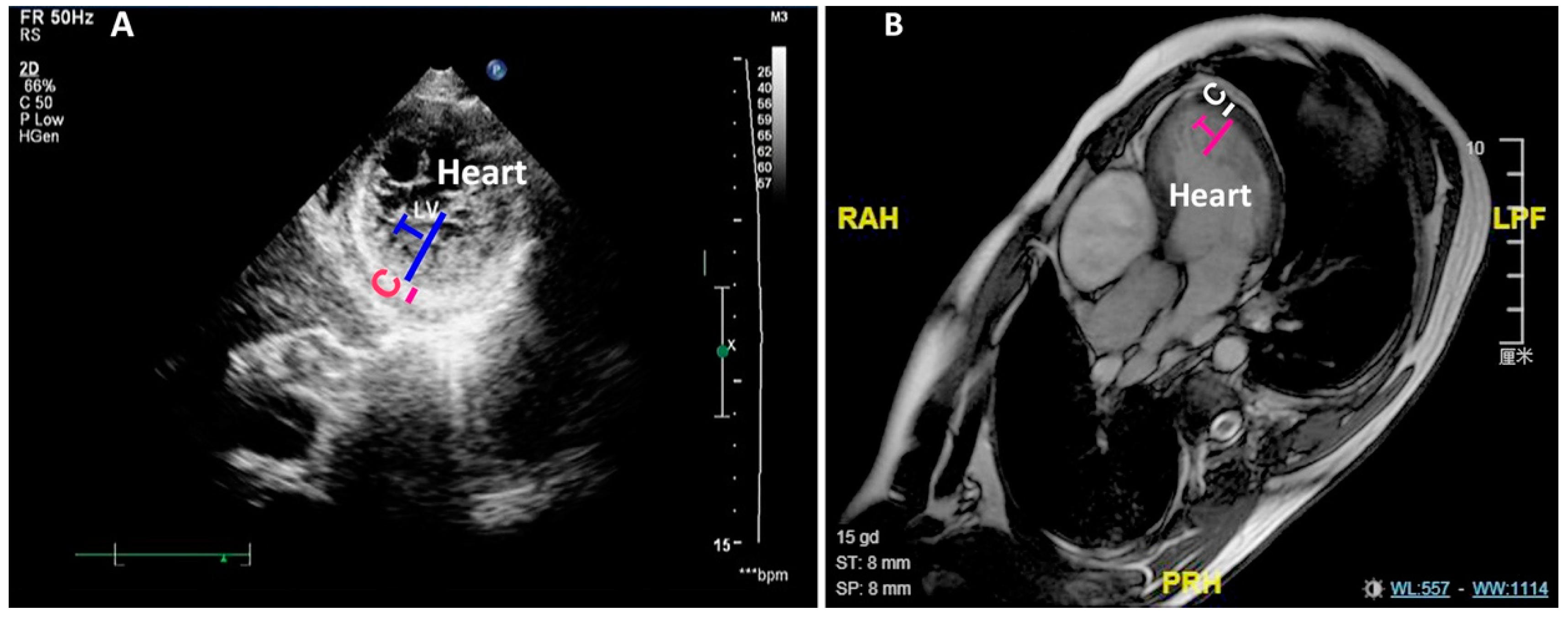
2.2. Diagnosis of LVNC and RVNC
2.3. Ventricular Systolic Funciton
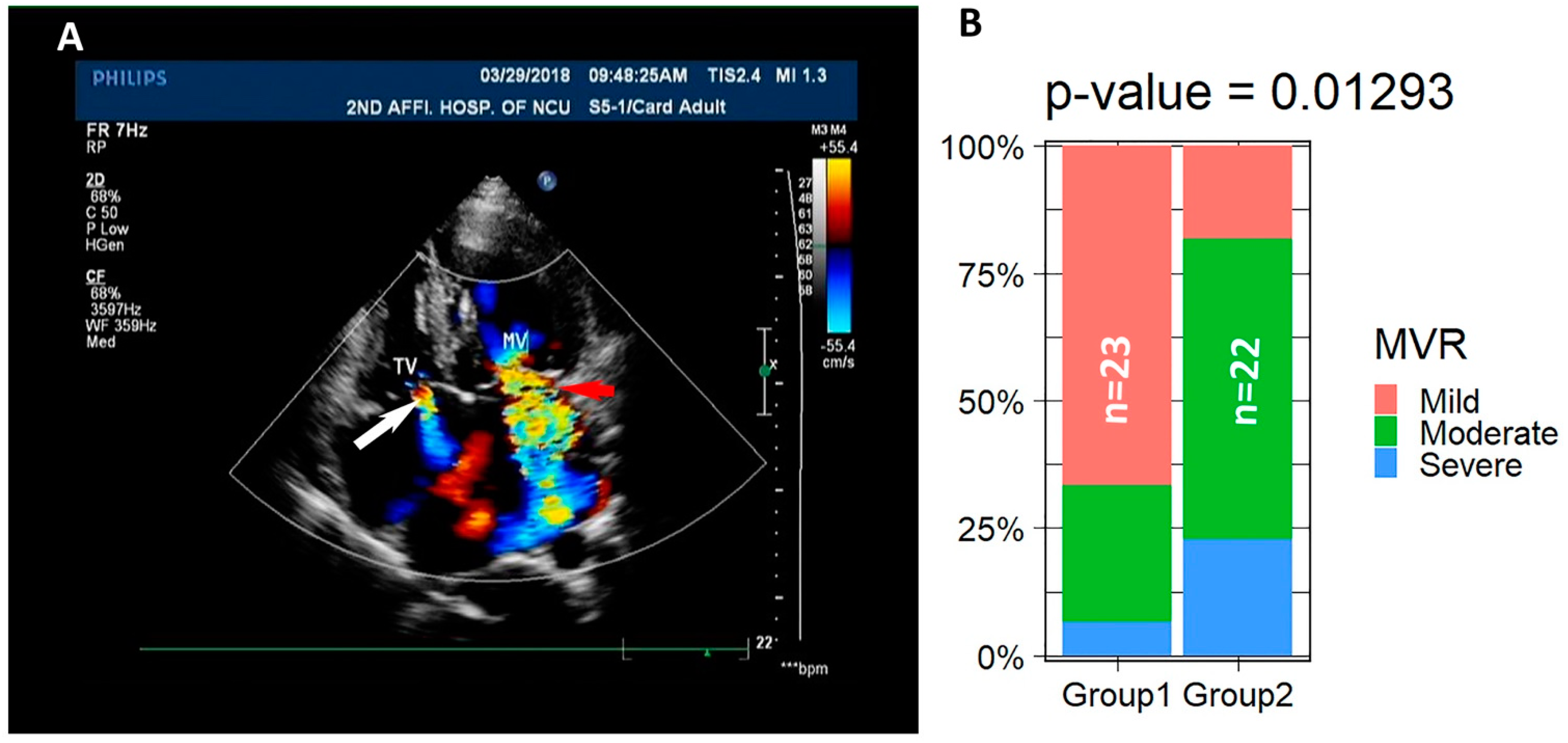
2.4. Vavular Regurditation
2.5. Statistics
2.6. Statement
3. Results, Figures and Tables
3.1. Clinical Symptoms of LVNC Patients
3.2. LVNC Patients Associate with Valvular Regurgitation
3.3. The LVNC Patients Display Reduced Contractility and Thickness Ratio of Trabecular Layer to Compact Layer Negatively Correlates with Cardiac Contractility
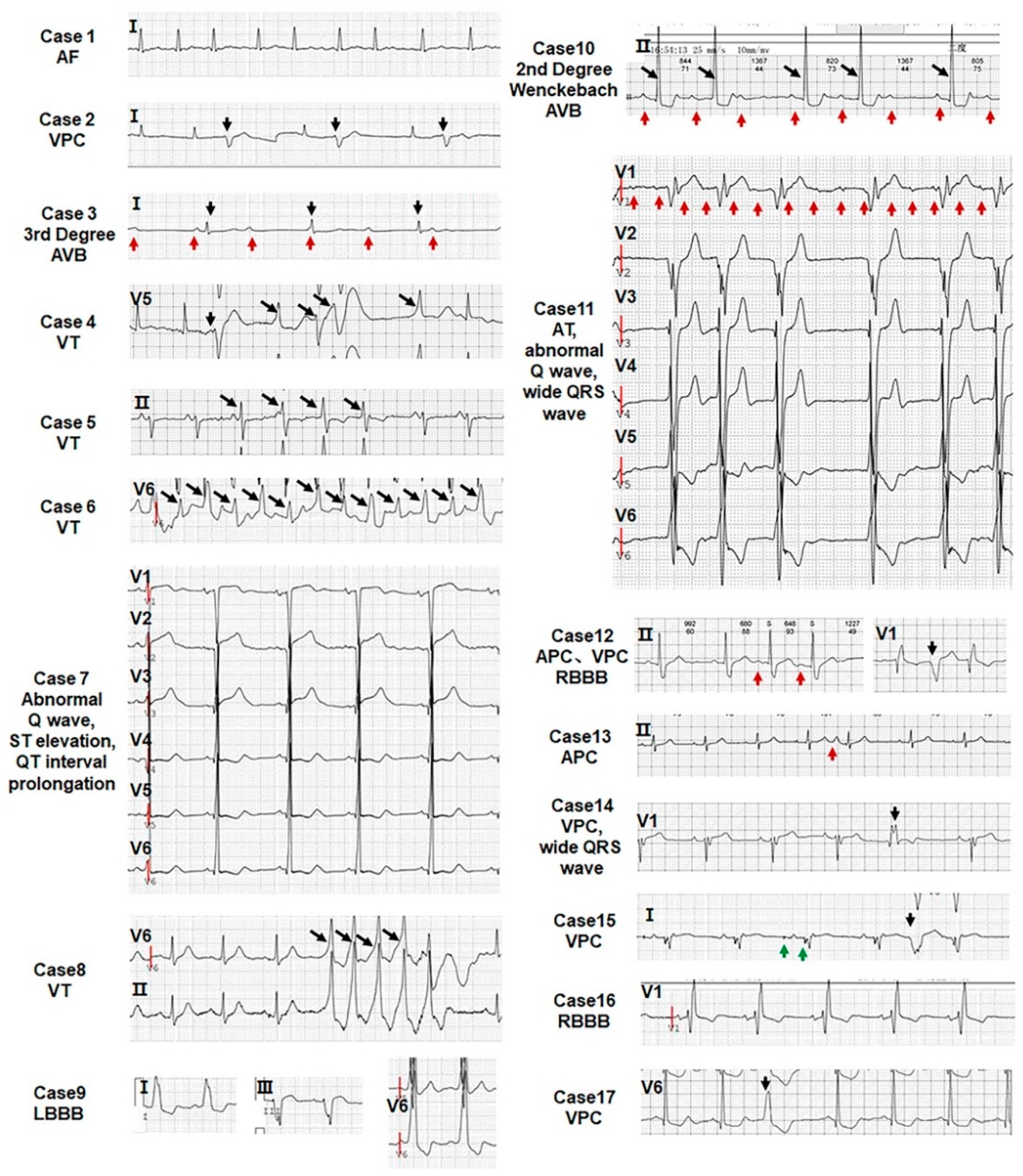
3.4. All LVNC Patients Are Associated with Arrhythmias
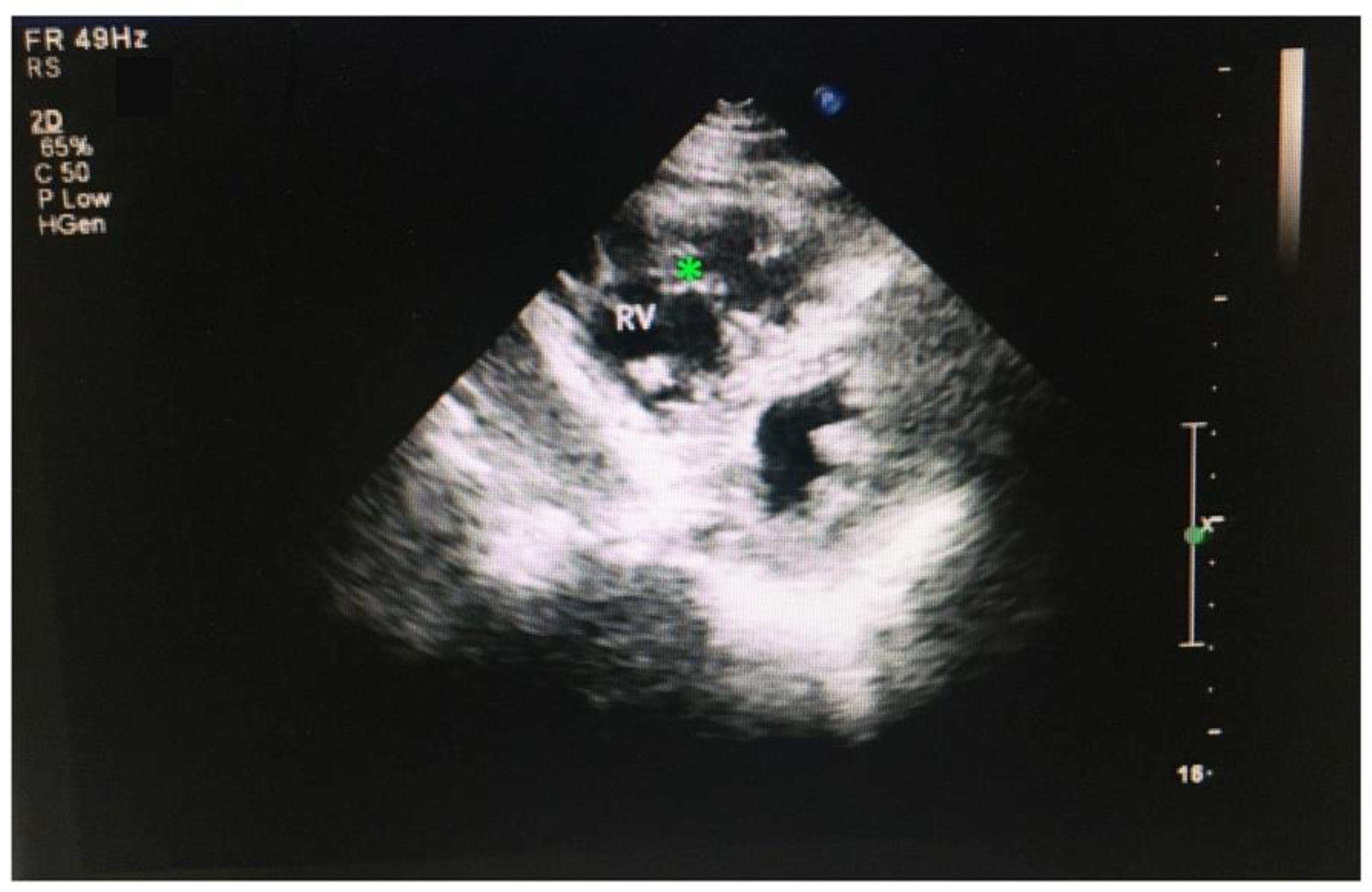
3.5. RVNC Does Not Associate with Reduced Contractility
4. Discussion
4.1. Congenital LVNC or Acquired LVNC
4.2. Reduced Contractility Is a Cause or a Consequence of LVNC
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pignatelli, R.H.; McMahon, C.J.; Dreyer, W.J.; Denfield, S.W.; Price, J.; Belmont, J.W.; Craigen, W.J.; Wu, J.; El Said, H.; Bezold, L.I.; et al. Clinical characterization of left ventricular noncompaction in children: A relatively common form of cardiomyopathy. Circulation 2003, 108, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A.; Jefferies, J.L. Cardiomyopathies Due to Left Ventricular Noncompaction, Mitochondrial and Storage Diseases, and Inborn Errors of Metabolism. Circ. Res. 2017, 121, 838–854. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Towbin, J.A.; Thiene, G.; Antzelevitch, C.; Corrado, D.; Arnett, D.; Moss, A.J.; Seidman, C.E.; Young, J.B.; American Heart, A.; et al. Contemporary definitions and classification of the cardiomyopathies: An American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006, 113, 1807–1816. [Google Scholar] [CrossRef]
- Feldt, R.H.; Rahimtoola, S.H.; Davis, G.D.; Swan, H.J.; Titus, J.L. Anomalous ventricular myocardial patterns in a child with complex congenital heart disease. Am. J. Cardiol. 1969, 23, 732–734. [Google Scholar] [CrossRef]
- Stahli, B.E.; Gebhard, C.; Biaggi, P.; Klaassen, S.; Valsangiacomo Buechel, E.; Attenhofer Jost, C.H.; Jenni, R.; Tanner, F.C.; Greutmann, M. Left ventricular non-compaction: Prevalence in congenital heart disease. Int. J. Cardiol. 2013, 167, 2477–2481. [Google Scholar] [CrossRef]
- Finsterer, J.; Stollberger, C.; Towbin, J.A. Left ventricular noncompaction cardiomyopathy: Cardiac, neuromuscular, and genetic factors. Nat. Rev.. Cardiol. 2017, 14, 224–237. [Google Scholar] [CrossRef]
- Camuglia, A.C.; Younger, J.F.; McGaughran, J.; Lo, A.; Atherton, J.J. Cardiac myosin-binding protein C gene mutation expressed as hypertrophic cardiomyopathy and left ventricular noncompaction within two families: Insights from cardiac magnetic resonance in clinical screening: Camuglia MYBPC3 gene mutation and MRI. Int. J. Cardiol. 2013, 168, 2950–2952. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Shou, W. Potential Common Pathogenic Pathways for the Left Ventricular Noncompaction Cardiomyopathy (LVNC). Pediatr. Cardiol. 2018, 39, 1099–1106. [Google Scholar] [CrossRef]
- Arbustini, E.; Weidemann, F.; Hall, J.L. Left ventricular noncompaction: A distinct cardiomyopathy or a trait shared by different cardiac diseases? J. Am. Coll. Cardiol. 2014, 64, 1840–1850. [Google Scholar] [CrossRef]
- Samsa, L.A.; Yang, B.; Liu, J. Embryonic cardiac chamber maturation: Trabeculation, conduction, and cardiomyocyte proliferation. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 157–168. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Qu, X.; Chang, C.P.; Shou, W. Molecular mechanism of ventricular trabeculation/compaction and the pathogenesis of the left ventricular noncompaction cardiomyopathy (LVNC). Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.; Kelly, R.G.; Miquerol, L. Defects in Trabecular Development Contribute to Left Ventricular Noncompaction. Pediatr. Cardiol. 2019, 40, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Guo, H.; Li, J.; Myint, T.; Pittman, W.; Yang, L.; Zhong, W.; Schwartz, R.J.; Schwarz, J.J.; Singer, H.A.; et al. Numb family proteins are essential for cardiac morphogenesis and progenitor differentiation. Development 2014, 141, 281–295. [Google Scholar] [CrossRef]
- Sedmera, D.; Thomas, P.S. Trabeculation in the embryonic heart. Bioessays 1996, 18, 607. [Google Scholar] [CrossRef] [PubMed]
- Jenni, R.; Rojas, J.; Oechslin, E. Isolated noncompaction of the myocardium. N. Engl. J. Med. 1999, 340, 966–967. [Google Scholar] [CrossRef]
- Weiford, B.C.; Subbarao, V.D.; Mulhern, K.M. Noncompaction of the ventricular myocardium. Circulation 2004, 109, 2965–2971. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.; Nguyen, T.H.M.; Sicard, P.; Buttigieg, E.; Tran, T.T.; Kober, F.; Varlet, I.; Sturny, R.; Costa, M.W.; Harvey, R.P.; et al. Deletion of Nkx2-5 in trabecular myocardium reveals the developmental origins of pathological heterogeneity associated with ventricular non-compaction cardiomyopathy. PLoS Genet. 2018, 14, e1007502. [Google Scholar] [CrossRef]
- Towbin, J.A.; Lorts, A.; Jefferies, J.L. Left ventricular non-compaction cardiomyopathy. Lancet 2015, 386, 813–825. [Google Scholar] [CrossRef]
- Parent, J.J.; Towbin, J.A.; Jefferies, J.L. Left ventricular noncompaction in a family with lamin A/C gene mutation. Tex. Heart Inst. J. 2015, 42, 73–76. [Google Scholar] [CrossRef]
- Jefferies, J.L.; Wilkinson, J.D.; Sleeper, L.A.; Colan, S.D.; Lu, M.; Pahl, E.; Kantor, P.F.; Everitt, M.D.; Webber, S.A.; Kaufman, B.D.; et al. Cardiomyopathy Phenotypes and Outcomes for Children With Left Ventricular Myocardial Noncompaction: Results From the Pediatric Cardiomyopathy Registry. J. Card. Fail 2015, 21, 877–884. [Google Scholar] [CrossRef]
- Ikeda, U.; Minamisawa, M.; Koyama, J. Isolated left ventricular non-compaction cardiomyopathy in adults. J. Cardiol. 2015, 65, 91–97. [Google Scholar] [CrossRef]
- Shieh, J.T.; Jefferies, J.L.; Chin, A.J. Disorders of left ventricular trabeculation/compaction or right ventricular wall formation. Am. J. Med. Genet. C Semin. Med. Genet. 2013, 163, 141–143. [Google Scholar] [CrossRef][Green Version]
- Karkucinska-Wieckowska, A.; Trubicka, J.; Werner, B.; Kokoszynska, K.; Pajdowska, M.; Pronicki, M.; Czarnowska, E.; Lebiedzinska, M.; Sykut-Cegielska, J.; Ziolkowska, L.; et al. Left ventricular noncompaction (LVNC) and low mitochondrial membrane potential are specific for Barth syndrome. J. Inherit. Metab. Dis. 2013, 36, 929–937. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, W.; Sun, X.; Yoshimoto, M.; Chen, Z.; Zhu, W.; Liu, J.; Shen, Y.; Yong, W.; Li, D.; et al. Fkbp1a controls ventricular myocardium trabeculation and compaction by regulating endocardial Notch1 activity. Development 2013, 140, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bucker, S.; Jungblut, B.; Bottger, T.; Cinnamon, Y.; Tchorz, J.; Muller, M.; Bettler, B.; Harvey, R.; Sun, Q.Y.; et al. Inhibition of Notch2 by Numb/Numblike controls myocardial compaction in the heart. Cardiovasc. Res. 2012, 96, 276–285. [Google Scholar] [CrossRef]
- Ryan, T.D.; Ware, S.M.; Lucky, A.W.; Towbin, J.A.; Jefferies, J.L.; Hinton, R.B. Left ventricular noncompaction cardiomyopathy and aortopathy in a patient with recessive dystrophic epidermolysis bullosa. Circ. Heart Fail. 2012, 5, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A. Left ventricular noncompaction: A new form of heart failure. Heart Fail. Clin. 2010, 6, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, R.A.; Anderson, R.H.; Elliott, P.M. Isolated left ventricular non-compaction: The case for abnormal myocardial development. Cardiol. Young 2007, 17, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Stollberger, C.; Finsterer, J. Left ventricular hypertrabeculation/noncompaction. J. Am. Soc. Echocardiogr. 2004, 17, 91–100. [Google Scholar] [CrossRef]
- Rasouli, S.J.; Stainier, D.Y.R. Regulation of cardiomyocyte behavior in zebrafish trabeculation by Neuregulin 2a signaling. Nat. Commun. 2017, 8, 15281. [Google Scholar] [CrossRef]
- Passer, D.; van de Vrugt, A.; Atmanli, A.; Domian, I.J. Atypical Protein Kinase C-Dependent Polarized Cell Division Is Required for Myocardial Trabeculation. Cell Rep. 2016, 14, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Amilburu, V.; Rasouli, S.J.; Staudt, D.W.; Nakajima, H.; Chiba, A.; Mochizuki, N.; Stainier, D.Y.R. In Vivo Visualization of Cardiomyocyte Apicobasal Polarity Reveals Epithelial to Mesenchymal-like Transition during Cardiac Trabeculation. Cell Rep. 2016, 17, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.V.; Fukuda, R.; Augustine, S.M.; Maischein, H.M.; Stainier, D.Y. N-cadherin relocalization during cardiac trabeculation. Proc. Natl. Acad. Sci. USA 2016, 113, 7569–7574. [Google Scholar] [CrossRef]
- Liu, J.; Bressan, M.; Hassel, D.; Huisken, J.; Staudt, D.; Kikuchi, K.; Poss, K.D.; Mikawa, T.; Stainier, D.Y. A dual role for ErbB2 signaling in cardiac trabeculation. Development 2010, 137, 3867–3875. [Google Scholar] [CrossRef]
- Finsterer, J.; Stollberger, C.; Wegmann, R.; Janssen, L.A. Acquired left ventricular hypertrabeculation/noncompaction in myotonic dystrophy type 1. Int. J. Cardiol. 2009, 137, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Wu, M. Mechanisms of Trabecular Formation and Specification During Cardiogenesis. Pediatr Cardiol. 2018, 39, 1082–1089. [Google Scholar] [CrossRef]
- Li, J.; Miao, L.; Shieh, D.; Spiotto, E.; Li, J.; Zhou, B.; Paul, A.; Schwartz, R.J.; Firulli, A.B.; Singer, H.A.; et al. Single-Cell Lineage Tracing Reveals that Oriented Cell Division Contributes to Trabecular Morphogenesis and Regional Specification. Cell Rep. 2016, 15, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, J. Numb family proteins: Novel players in cardiac morphogenesis and cardiac progenitor cell differentiation. Biomol. Concepts 2015, 6, 137–148. [Google Scholar] [CrossRef]
- Miao, L.; Li, J.; Li, J.; Tian, X.; Lu, Y.; Hu, S.; Shieh, D.; Kanai, R.; Zhou, B.Y.; Zhou, B.; et al. Notch signaling regulates Hey2 expression in a spatiotemporal dependent manner during cardiac morphogenesis and trabecular specification. Sci. Rep. 2018, 8, 2678. [Google Scholar] [CrossRef]
- Jimenez-Amilburu, V.; Stainier, D.Y.R. The transmembrane protein Crb2a regulates cardiomyocyte apicobasal polarity and adhesion in zebrafish. Development 2019, 146, dev171207. [Google Scholar] [CrossRef]
- Miao, L.; Li, J.; Li, J.; Lu, Y.; Shieh, D.; Mazurkiewicz, J.E.; Barroso, M.; Schwarz, J.J.; Xin, H.B.; Singer, H.A.; et al. Cardiomyocyte orientation modulated by the Numb family proteins-N-cadherin axis is essential for ventricular wall morphogenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 15560–15569. [Google Scholar] [CrossRef] [PubMed]
- Del Monte-Nieto, G.; Ramialison, M.; Adam, A.A.S.; Wu, B.; Aharonov, A.; D’Uva, G.; Bourke, L.M.; Pitulescu, M.E.; Chen, H.; de la Pompa, J.L.; et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nature 2018, 557, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Harmelink, C.; Baldwin, H.S. Tie2 regulates endocardial sprouting and myocardial trabeculation. JCI Insight 2019, 5, e96002. [Google Scholar] [CrossRef]
- Rhee, S.; Chung, J.I.; King, D.A.; D’Amato, G.; Paik, D.T.; Duan, A.; Chang, A.; Nagelberg, D.; Sharma, B.; Jeong, Y.; et al. Endothelial deletion of Ino80 disrupts coronary angiogenesis and causes congenital heart disease. Nat. Commun. 2018, 9, 368. [Google Scholar] [CrossRef]
- Sandireddy, R.; Cibi, D.M.; Gupta, P.; Singh, A.; Tee, N.; Uemura, A.; Epstein, J.A.; Singh, M.K. Semaphorin 3E/PlexinD1 signaling is required for cardiac ventricular compaction. JCI Insight 2019, 4, e125908. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Li, Y.; He, L.; Zhang, H.; Huang, X.; Liu, Q.; Pu, W.; Zhang, L.; Li, Y.; Zhao, H.; et al. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat. Commun. 2017, 8, 87. [Google Scholar] [CrossRef]
- Shemisa, K.; Li, J.; Tam, M.; Barcena, J. Left ventricular noncompaction cardiomyopathy. Cardiovasc Diagn Ther. 2013, 3, 170–175. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Stollberger, C.; Finsterer, J.; Blazek, G. Left ventricular hypertrabeculation/noncompaction and association with additional cardiac abnormalities and neuromuscular disorders. Am. J. Cardiol. 2002, 90, 899–902. [Google Scholar] [CrossRef]
- Oechslin, E.; Klaassen, S. Left Ventricular Noncompaction: Phenotype in an Integrated Model of Cardiomyopathy? J. Am. Coll. Cardiol. 2019, 73, 1612–1615. [Google Scholar] [CrossRef] [PubMed]
- van Waning, J.I.; Caliskan, K.; Hoedemaekers, Y.M.; van Spaendonck-Zwarts, K.Y.; Baas, A.F.; Boekholdt, S.M.; van Melle, J.P.; Teske, A.J.; Asselbergs, F.W.; Backx, A.; et al. Genetics, Clinical Features, and Long-Term Outcome of Noncompaction Cardiomyopathy. J. Am. Coll. Cardiol. 2018, 71, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Xu, R.; Li, X.; Xu, H.Y.; Yang, Z.G.; Wang, Y.N.; Fan, H.M.; Guo, Y.K. The mitral regurgitation effects of cardiac structure and function in left ventricular noncompaction. Sci. Rep. 2021, 11, 4616. [Google Scholar] [CrossRef] [PubMed]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef]
- Kolokotronis, K.; Kuhnisch, J.; Klopocki, E.; Dartsch, J.; Rost, S.; Huculak, C.; Mearini, G.; Stork, S.; Carrier, L.; Klaassen, S.; et al. Biallelic mutation in MYH7 and MYBPC3 leads to severe cardiomyopathy with left ventricular noncompaction phenotype. Hum. Mutat. 2019, 40, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Kayvanpour, E.; Sedaghat-Hamedani, F.; Gi, W.T.; Tugrul, O.F.; Amr, A.; Haas, J.; Zhu, F.; Ehlermann, P.; Uhlmann, L.; Katus, H.A.; et al. Clinical and genetic insights into non-compaction: A meta-analysis and systematic review on 7598 individuals. Clin. Res. Cardiol. 2019, 108, 1297–1308. [Google Scholar] [CrossRef]
- Steffel, J.; Duru, F. Rhythm disorders in isolated left ventricular noncompaction. Ann. Med. 2012, 44, 101–108. [Google Scholar] [CrossRef]
- Miyake, C.Y.; Kim, J.J. Arrhythmias in left ventricular noncompaction. Card. Electrophysiol Clin. 2015, 7, 319–330. [Google Scholar] [CrossRef]
- Szentpali, Z.; Szili-Torok, T.; Caliskan, K. Primary electrical disorder or primary cardiomyopathy? A case with a unique association of noncompaction cardiomyopathy and cathecolaminergic polymorphic ventricular tachycardia caused by ryanodine receptor mutation. Circulation 2013, 127, 1165–1166. [Google Scholar] [CrossRef][Green Version]
- Nakashima, K.; Kusakawa, I.; Yamamoto, T.; Hirabayashi, S.; Hosoya, R.; Shimizu, W.; Sumitomo, N. A left ventricular noncompaction in a patient with long QT syndrome caused by a KCNQ1 mutation: A case report. Heart Vessel. 2013, 28, 126–129. [Google Scholar] [CrossRef]
- Ichida, F.; Hamamichi, Y.; Miyawaki, T.; Ono, Y.; Kamiya, T.; Akagi, T.; Hamada, H.; Hirose, O.; Isobe, T.; Yamada, K.; et al. Clinical features of isolated noncompaction of the ventricular myocardium: Long-term clinical course, hemodynamic properties, and genetic background. J. Am. Coll. Coll. Cardiol. 1999, 34, 233–240. [Google Scholar] [CrossRef]
- Ritter, M.; Oechslin, E.; Sutsch, G.; Attenhofer, C.; Schneider, J.; Jenni, R. Isolated noncompaction of the myocardium in adults. Mayo Clin. Proc. 1997, 72, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Brescia, S.T.; Rossano, J.W.; Pignatelli, R.; Jefferies, J.L.; Price, J.F.; Decker, J.A.; Denfield, S.W.; Dreyer, W.J.; Smith, O.; Towbin, J.A.; et al. Mortality and sudden death in pediatric left ventricular noncompaction in a tertiary referral center. Circulation 2013, 127, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, E.N.; Attenhofer Jost, C.H.; Rojas, J.R.; Kaufmann, P.A.; Jenni, R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: A distinct cardiomyopathy with poor prognosis. J. Am. Coll. Cardiol. 2000, 36, 493–500. [Google Scholar] [CrossRef]
- Murphy, R.T.; Thaman, R.; Blanes, J.G.; Ward, D.; Sevdalis, E.; Papra, E.; Kiotsekoglou, A.; Tome, M.T.; Pellerin, D.; McKenna, W.J.; et al. Natural history and familial characteristics of isolated left ventricular non-compaction. Eur. Heart J. 2005, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Aras, D.; Tufekcioglu, O.; Ergun, K.; Ozeke, O.; Yildiz, A.; Topaloglu, S.; Deveci, B.; Sahin, O.; Kisacik, H.L.; Korkmaz, S. Clinical features of isolated ventricular noncompaction in adults long-term clinical course, echocardiographic properties, and predictors of left ventricular failure. J. Card. Fail. 2006, 12, 726–733. [Google Scholar] [CrossRef]
- Stollberger, C.; Finsterer, J. Arrhythmias and left ventricular hypertrabeculation/noncompaction. Curr. Pharm. Pharm. Des. 2010, 16, 2880–2894. [Google Scholar] [CrossRef]
- Saglam, M.; Saygin, H.; Kozan, H.; Ozturk, E.; Mutlu, H. Noncompaction of Ventricular Myocardium Involving the Right Ventricle. Korean Circ. J. 2015, 45, 439–441. [Google Scholar] [CrossRef]
- Wengrofsky, P.; Armenia, C.; Oleszak, F.; Kupferstein, E.; Rednam, C.; Mitre, C.A.; McFarlane, S.I. Left Ventricular Trabeculation and Noncompaction Cardiomyopathy: A Review. EC Clin. Exp. Anat. 2019, 2, 267–283. [Google Scholar]
- Dusek, J.; Ostadal, B.; Duskova, M. Postnatal persistence of spongy myocardium with embryonic blood supply. Arch. Pathol. 1975, 99, 312–317. [Google Scholar]
- Luxan, G.; Casanova, J.C.; Martinez-Poveda, B.; Prados, B.; D’Amato, G.; MacGrogan, D.; Gonzalez-Rajal, A.; Dobarro, D.; Torroja, C.; Martinez, F.; et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 2013, 19, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hallett, M.A.; Zhu, W.; Rubart, M.; Liu, Y.; Yang, Z.; Chen, H.; Haneline, L.S.; Chan, R.J.; Schwartz, R.J.; et al. Dishevelled-associated activator of morphogenesis 1 (Daam1) is required for heart morphogenesis. Development 2011, 138, 303–315. [Google Scholar] [CrossRef] [PubMed]
- de Soysa, T.Y.; Ranade, S.S.; Okawa, S.; Ravichandran, S.; Huang, Y.; Salunga, H.T.; Schricker, A.; Del Sol, A.; Gifford, C.A.; Srivastava, D. Single-cell analysis of cardiogenesis reveals basis for organ-level developmental defects. Nature 2019, 572, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Tizon-Marcos, H.; de la Paz Ricapito, M.; Pibarot, P.; Bertrand, O.; Bibeau, K.; Le Ven, F.; Sinha, S.; Engert, J.; Bedard, E.; Pasian, S.; et al. Characteristics of trabeculated myocardium burden in young and apparently healthy adults. Am. J. Cardiol. 2014, 114, 1094–1099. [Google Scholar] [CrossRef] [PubMed]

| Case # | Sex | Age | Arrhythmia | LVNC | T/C | EF (50–70%) | FS (>25%) | NYHA | LVd (35–55) | LVs (25–37) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 39 | AF | Y | 2/1 | 48 | 25 | II or III | 70 | 53 |
| 2 | M | 68 | VPC | Y | 5/1 | 38 | 19 | I | 52 | 42 |
| 3 | F | 27 | III°AVB | Y | 3/1 | 66 | 36 | I | 51 | 33 |
| 4 | F | 12 | VT | Y | 3/1 | 67 | 38 | I | 54 | 33 |
| 5 | M | 62 | VPC, VT | Y | 4/1 | 22 | 10 | II or III | 58 | 52 |
| 6 | M | 14 | VT | Y | 3/1 | 30 | 15 | IV | 64 | 55 |
| 7 | F | 81 | AQW, STE, QTIP | Y | 5/1 | 41 | 20 | III | 56 | 45 |
| 8 | M | 29 | VT, VPC | Y | 3/1 | 53 | 27 | I | 48 | 35 |
| 9 | F | 60 | LBBB | Y | 2/1 | 38 | 19 | II or III | 66 | 54 |
| 10 | M | 35 | Ⅱ°Ⅰ AVB | Y | 2/1 | 62 | 33 | II | 42 | 28 |
| 11 | M | 41 | AT, AQW | Y | 3/1 | 31 | 16 | III | 58 | 49 |
| 12 | M | 71 | VPC, APC, RBBB | Y | 4/1 | 36 | 18 | III | 58 | 48 |
| 13 | M | 55 | APC | RVNC | 2.5/1 | 68 | 38 | I | 47 | 29 |
| 14 | M | 31 | VPC, wide QRS wave | Y | 2.5/1 | 52 | 26 | II | 41 | 30 |
| 15 | M | 28 | SSS, VPC | Y | 3/1 | 23 | 11 | III | 64 | 56 |
| 16 | M | 59 | RBBB | Y | 5/1 | 18 | 8 | II | 60 | 55 |
| 17 | M | 57 | VPC | Y | 2.5/1 | 58 | 31 | II | 48 | 33 |
| 18 | M | 40 | SB, STE, TWC | Y | 3/1 | 42 | 21 | II or III | 58 | 45 |
| 19 | M | 61 | AF, VPC, RBBB | Y | 3.5/1 | 35 | 17 | IV | 63 | 52 |
| 20 | F | 75 | AT, VPC, VT | Y | 2.5/1 | 44 | 22 | II or III | 59 | 45 |
| 21 | M | 62 | AF, VPC, STE, ST-TWC | Y | 3.5/1 | 22 | 10 | IV | 91 | 81 |
| 22 | M | 49 | SB, AQW | RVNC LVNC | >3/1 | 40 | 22 | I | 47 | 37 |
| 23 | M | 65 | ST, LBBB, ST-TWC | Y | 3/1 | 38 | 19 | II or III | 71 | 59 |
| 24 | M | 70 | AQW, ST-TWC | Y | 5/1 | 34 | 17 | II or III | 72 | 60 |
| 25 | F | 71 | ST-TWC, | Y | 2.5/1 | 30 | 15 | II or III | 65 | 56 |
| 26 | M | 63 | VPC, AQW | Y | 5.6/1 | 38 | 12 | IV | 72 | 58 |
| 27 | M | 42 | ST-TWC | Y | 2.5/1 | 67 | 37 | I | 46 | 29 |
| 28 | M | 55 | VPC | Y | 3/1 | 45 | 23 | II | 60 | 46 |
| 29 | F | 22 | ST-TWC, Q-TIP | Y | 3/1 | 27 | 13 | II | 68 | 59 |
| 30 | F | 42 | Atrial flutter, VPC | Y | 2.5/1 | 38 | 19 | II | 62 | 50 |
| 31 | M | 61 | LBBB | Y | 2.3/1 | 16 | 17 | II or III | 86 | 79 |
| 32 | M | 32 | AQW | Y | >3/1 | 34 | 17 | II | 66 | 55 |
| 33 | M | 78 | VPC, VT | Y | >3/1 | 36 | 18 | II | 68 | 56 |
| 34 | M | 68 | AF | Y | 3.6/1 | 34 | 17 | II or III | 65 | 54 |
| 36 | M | 37 | APC | Y | 4.4/1 | 42 | 19 | II | 66 | 54 |
| 37 | M | 21 | ST, AQW, ST-TWC | Y | >3/1 | 29 | 14 | IV | 64 | 55 |
| 38 | F | 72 | LBBB | Y | 2.4/1 | 23 | 11 | II | 64 | 57 |
| 39 | F | 68 | VPC, LBBB | Y | 2.6/1 | 15 | 10 | III | 59 | 53 |
| 40 | M | 66 | AF, AVB, LBBB, VPC | Y | 2.6/1 | 33 | 19 | III | 82 | 67 |
| 41 | F | 48 | AF, ST-TWC | Y | 2.6/1 | 40 | 20 | II | 55 | 44 |
| 42 | M | 58 | AF, LBBB | Y | 5.2/1 | 20 | 9 | II | 79 | 71 |
| 43 | F | 57 | VPC | Y | 2.4/1 | 38 | 19 | II or III | 63 | 52 |
| 44 | M | 60 | AQW | Y | 5.0/1 | 31 | 15 | II | 65 | 55 |
| 45 | M | 57 | AF | Y | >2/1 | 38 | 19 | II | 70 | 57 |
| 46 | F | 67 | LBBB | Y | 3.8/1 | 37 | 19 | II | 61 | 49 |
| 47 | F | 63 | P-RIP, TWC | Y | 2.5/1 | 34 | 17 | II | 70 | 59 |
| Case # | Gender | Age | Valvular Regurgitation |
|---|---|---|---|
| 1 | M | 39 | Severe MVR, severe TVR, Moderate PR, Mild AR |
| 2 | M | 68 | Mild MVR, mild TVR, mild AR |
| 3 | F | 27 | Mild MVR, mild TVR, mild AR |
| 4 | F | 12 | Moderate MVR, mild TVR, mild AR |
| 5 | M | 62 | Severe MVR, mild TVR |
| 6 | M | 14 | Moderate MVR, mild TVR |
| 7 | F | 81 | Moderate MVR, mild TVR, mild PR |
| 8 | M | 29 | Mild TVR |
| 9 | F | 60 | Moderate MVR, mild TVR, mild AR |
| 10 | M | 35 | Mild TVR |
| 11 | M | 41 | Moderate MVR, mild TVR, mild AR |
| 12 | M | 71 | Mild MVR, mild TVR |
| 13 | M | 55 | Moderate TVR, mild PR |
| 14 | M | 31 | Moderate TVR, Ebstein’s anomaly |
| 15 | M | 28 | Mild MVR, mild TVR, mild AR |
| 16 | M | 59 | Moderate MVR, mild TVR |
| 17 | M | 57 | Mild MVR, mild TVR |
| 18 | M | 40 | Mild MVR, mild TVR |
| 19 | M | 61 | Moderate-severe MVR, moderate-severeTVR, mild AR |
| 20 | F | 75 | Moderate MVR, mild-moderate TVR, mild AR |
| 21 | M | 62 | Moderate-severe MVR, mild TVR, mild AR |
| 22 | M | 49 | Mild-moderate TVR |
| 23 | M | 65 | Moderate MVR, mild-moderate TVR |
| 24 | M | 70 | MVR prolapse, mild AR |
| 25 | F | 71 | Mild-moderate MVR, Mild-moderate AR |
| 26 | M | 63 | Mild-moderate MVR |
| 27 | M | 42 | - |
| 28 | M | 55 | Mild MVR, mild TVR |
| 29 | F | 22 | - |
| 30 | F | 42 | Mild MVR |
| 31 | M | 61 | Mild MVR |
| 32 | M | 32 | Moderate MVR, mild TVR |
| 33 | M | 78 | Mild MVR, mild TVR, mild AR |
| 34 | M | 68 | Severe MVR, mild TVR, mild AR |
| 36 | M | 37 | - |
| 37 | M | 21 | Mild MVR, mild TVR |
| 38 | F | 72 | Moderate MVR, mild TVR, mild AR, mild PR |
| 39 | F | 68 | Moderate-severe MVR, mild-moderate TVR, mild AR, mild PR |
| 40 | M | 66 | Moderate MVR, mild TVR, mild AR |
| 41 | F | 48 | Mild MVR, mild TVR |
| 42 | M | 58 | Mild MVR, mild TVR |
| 43 | F | 57 | - |
| 44 | M | 60 | Moderate MVR, mild TVR |
| 45 | M | 57 | Mild-moderate AR, mild MVR, mild TVR, mild PR |
| 46 | F | 67 | Mild-moderate MVR, mild TVR, mild AR |
| 47 | F | 63 | Moderate MVR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Miao, L.; Xia, L.; Abdelnasser, H.Y.; Zhang, F.; Lu, Y.; Nusrat, A.; Tabassum, M.; Li, J.; Wu, M. Left Ventricular Noncompaction Is Associated with Valvular Regurgitation and a Variety of Arrhythmias. J. Cardiovasc. Dev. Dis. 2022, 9, 49. https://doi.org/10.3390/jcdd9020049
Li Q, Miao L, Xia L, Abdelnasser HY, Zhang F, Lu Y, Nusrat A, Tabassum M, Li J, Wu M. Left Ventricular Noncompaction Is Associated with Valvular Regurgitation and a Variety of Arrhythmias. Journal of Cardiovascular Development and Disease. 2022; 9(2):49. https://doi.org/10.3390/jcdd9020049
Chicago/Turabian StyleLi, Qing, Lianjie Miao, Lihong Xia, Hala Y. Abdelnasser, Fang Zhang, Yangyang Lu, Anika Nusrat, Mantasha Tabassum, Juxiang Li, and Mingfu Wu. 2022. "Left Ventricular Noncompaction Is Associated with Valvular Regurgitation and a Variety of Arrhythmias" Journal of Cardiovascular Development and Disease 9, no. 2: 49. https://doi.org/10.3390/jcdd9020049
APA StyleLi, Q., Miao, L., Xia, L., Abdelnasser, H. Y., Zhang, F., Lu, Y., Nusrat, A., Tabassum, M., Li, J., & Wu, M. (2022). Left Ventricular Noncompaction Is Associated with Valvular Regurgitation and a Variety of Arrhythmias. Journal of Cardiovascular Development and Disease, 9(2), 49. https://doi.org/10.3390/jcdd9020049







