An Inverse Correlation between the Atherogenic Index of Plasma and Heart Failure: An Analysis of the National Health and Nutrition Examination Survey 2017–March 2020 Pre-Pandemic Data
Abstract
1. Introduction
2. Materials and Methods
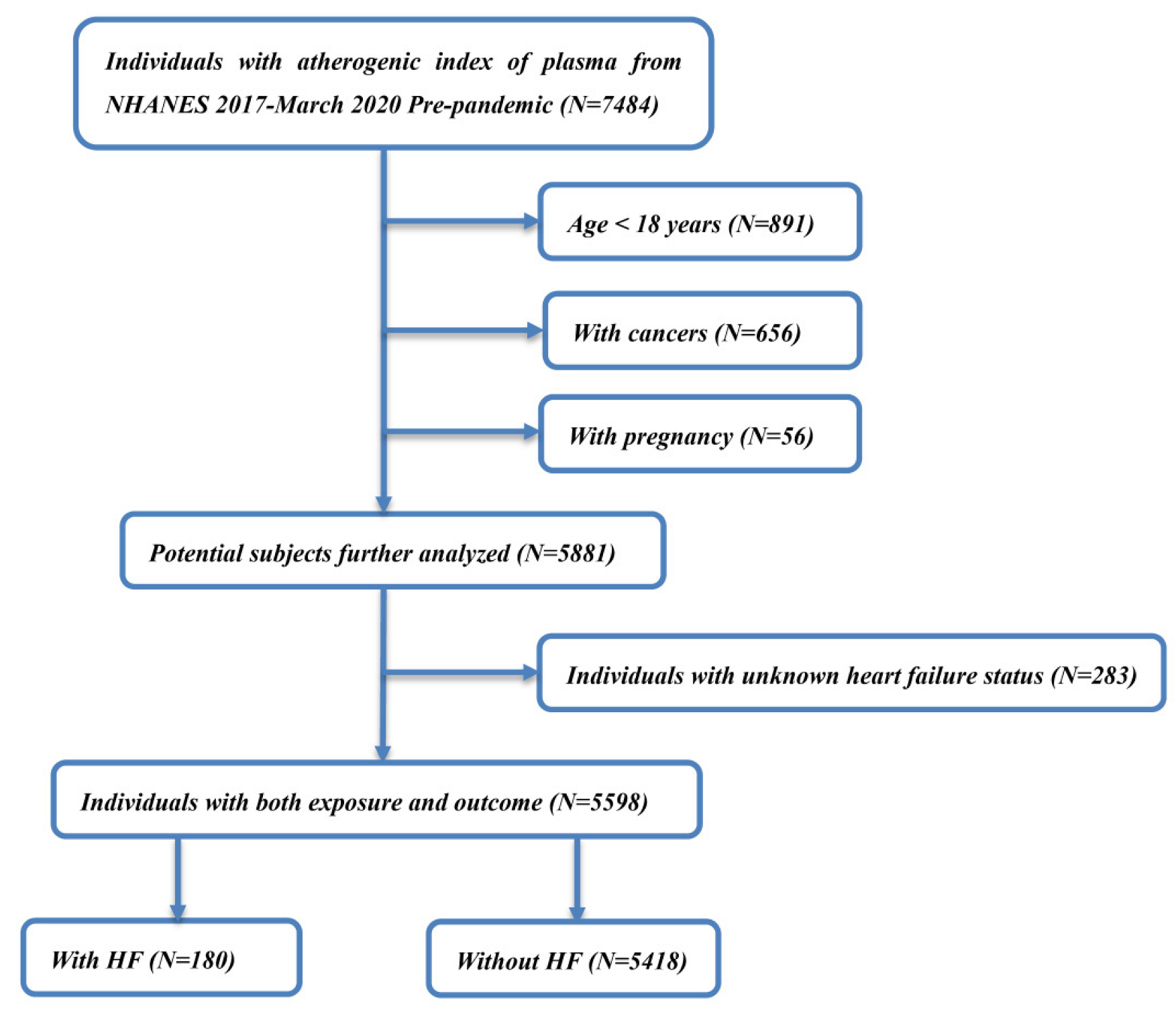
2.1. Study Population
2.2. Definitions of HF and AIP
2.3. Covariates
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Included Individuals
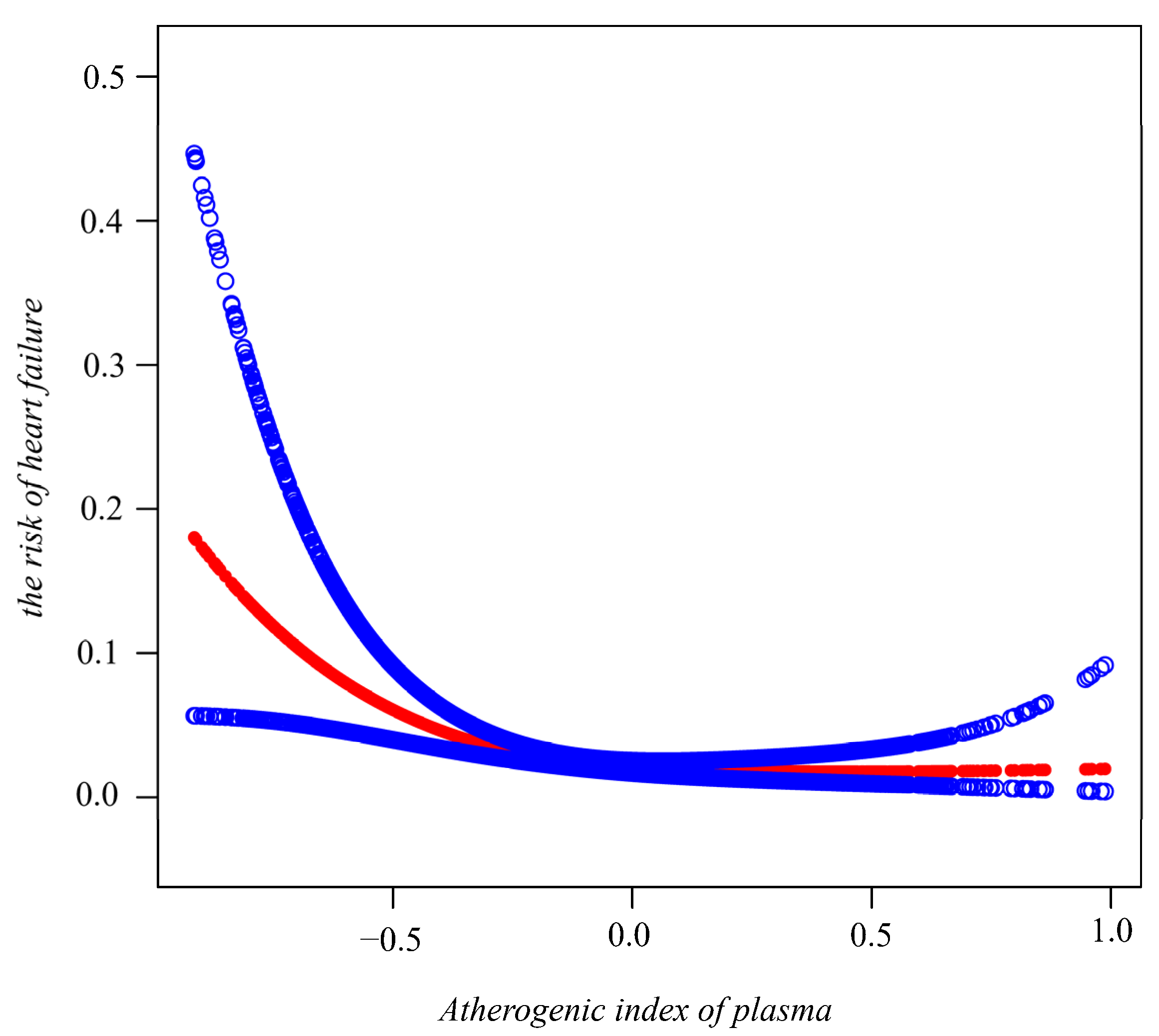
3.2. AIP and the Risk of HF
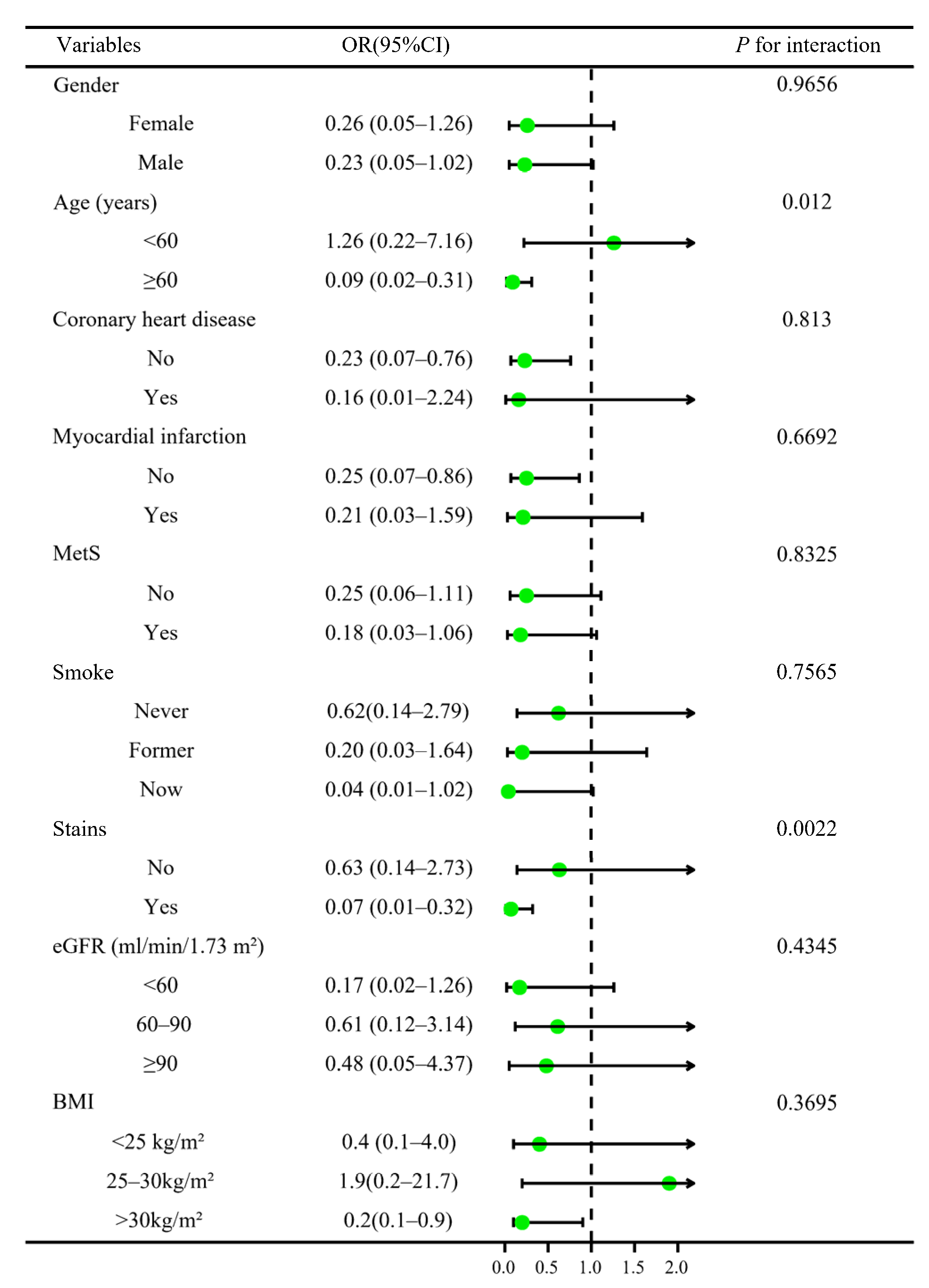
3.3. Stratified Analysis and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davison, B.; Cotter, G. Why is heart failure so important in the 21st century? Eur. J. Heart Fail. 2015, 17, 122–124. [Google Scholar] [CrossRef]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef] [PubMed]
- Clark, K.A.A.; Reinhardt, S.W.; Chouairi, F.; Miller, P.E.; Kay, B.; Fuery, M.; Guha, A.; Ahmad, T.; Desai, N.R. Trends in Heart Failure Hospitalizations in the US from 2008 to 2018. J. Card. Fail. 2022, 28, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef]
- Moran, A.E.; Forouzanfar, M.H.; Roth, G.A.; Mensah, G.A.; Ezzati, M.; Flaxman, A.; Murray, C.J.; Naghavi, M. The global burden of ischemic heart disease in 1990 and 2010: The Global Burden of Disease 2010 study. Circulation 2014, 129, 1493–1501. [Google Scholar] [CrossRef]
- Follath, F. Ischemic versus non-ischemic heart failure: Should the etiology be determined? Heart Fail Monit. 2001, 1, 122–125. [Google Scholar]
- Kalantar-Zadeh, K.; Block, G.; Horwich, T.; Fonarow, G.C. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J. Am. Coll. Cardiol. 2004, 43, 1439–1444. [Google Scholar] [CrossRef]
- Niroumand, S.; Khajedaluee, M.; Khadem-Rezaiyan, M.; Abrishami, M.; Juya, M.; Khodaee, G.; Dadgarmoghaddam, M. Atherogenic Index of Plasma (AIP): A marker of cardiovascular disease. Med. J. Islamic Repub. Iran 2015, 29, 240. [Google Scholar]
- Nwagha, U.I.; Ikekpeazu, E.J.; Ejezie, F.E.; Neboh, E.E.; Maduka, I.C. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr. Health Sci. 2010, 10, 248–252. [Google Scholar]
- Sattler, E.L.P.; Ishikawa, Y.; Trivedi-Kapoor, R.; Zhang, D.; Quyyumi, A.A.; Dunbar, S.B. Association between the Prognostic Nutritional Index and Dietary Intake in Community-Dwelling Older Adults with Heart Failure: Findings from NHANES III. Nutrients 2019, 11, 2608. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef]
- Jaddoe, V.W.; de Jonge, L.L.; Hofman, A.; Franco, O.H.; Steegers, E.A.; Gaillard, R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: Population based cohort study. BMJ 2014, 348, g14. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef]
- Dobiásová, M.; Frohlich, J. The plasma parameter log (TG/HDL-C) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, S.; Feng, X.; Yang, J.; Qiao, J.; Zhao, Y.; Shi, D.; Zhou, Y. The sensibility of the new blood lipid indicator--atherogenic index of plasma (AIP) in menopausal women with coronary artery disease. Lipids Health Dis. 2020, 19, 27. [Google Scholar] [CrossRef]
- Shin, H.R.; Song, S.; Cho, J.A.; Ly, S.Y. Atherogenic Index of Plasma and Its Association with Risk Factors of Coronary Artery Disease and Nutrient Intake in Korean Adult Men: The 2013-2014 KNHANES. Nutrients 2022, 14, 1071. [Google Scholar] [CrossRef]
- Goliasch, G.; Wiesbauer, F.; Blessberger, H.; Demyanets, S.; Wojta, J.; Huber, K.; Maurer, G.; Schillinger, M.; Speidl, W.S. Premature myocardial infarction is strongly associated with increased levels of remnant cholesterol. J. Clin. Lipidol. 2015, 9, 801–806.e801. [Google Scholar] [CrossRef]
- Kim, S.H.; Cho, Y.K.; Kim, Y.J.; Jung, C.H.; Lee, W.J.; Park, J.Y.; Huh, J.H.; Kang, J.G.; Lee, S.J.; Ihm, S.H. Association of the atherogenic index of plasma with cardiovascular risk beyond the traditional risk factors: A nationwide population-based cohort study. Cardiovasc. Diabetol. 2022, 21, 81. [Google Scholar] [CrossRef]
- Velagaleti, R.S.; Massaro, J.; Vasan, R.S.; Robins, S.J.; Kannel, W.B.; Levy, D. Relations of lipid concentrations to heart failure incidence: The Framingham Heart Study. Circulation 2009, 120, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Anber, V.; Griffin, B.A.; McConnell, M.; Packard, C.J.; Shepherd, J. Influence of plasma lipid and LDL-subfraction profile on the interaction between low density lipoprotein with human arterial wall proteoglycans. Atherosclerosis 1996, 124, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, J.; Dobiásová, M. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin. Chem. 2003, 49, 1873–1880. [Google Scholar] [CrossRef] [PubMed]
- Younis, N.N.; Soran, H.; Pemberton, P.; Charlton-Menys, V.; Elseweidy, M.M.; Durrington, P.N. Small dense LDL is more susceptible to glycation than more buoyant LDL in Type 2 diabetes. Clin. Sci. 2013, 124, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Karadağ, M.K.; Yıldırım, E. Relationship of atherogenic index of plasma and mean platelet volume with ejection fraction in ischemic and nonischemic heart failure. Biomark. Med. 2019, 13, 175–183. [Google Scholar] [CrossRef]
- Bo, M.S.; Cheah, W.L.; Lwin, S.; Moe Nwe, T.; Win, T.T.; Aung, M. Understanding the Relationship between Atherogenic Index of Plasma and Cardiovascular Disease Risk Factors among Staff of an University in Malaysia. J. Nutr. Metab. 2018, 2018, 7027624. [Google Scholar] [CrossRef]
- Zhu, X.; Yu, L.; Zhou, H.; Ma, Q.; Zhou, X.; Lei, T.; Hu, J.; Xu, W.; Yi, N.; Lei, S. Atherogenic index of plasma is a novel and better biomarker associated with obesity: A population-based cross-sectional study in China. Lipids Health Dis. 2018, 17, 37. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Q.; Zhang, Q.; Wan, Z.; Hu, L.; Xu, R.; Cheng, A.; Lv, Y.; Wang, L. Sex-Specific Association between Serum Vitamin D Status and Lipid Profiles: A Cross-Sectional Study of a Middle-Aged and Elderly Chinese Population. J. Nutr. Sci. Vitaminol. 2020, 66, 105–113. [Google Scholar] [CrossRef]
- Dobiásová, M.; Frohlich, J.; Sedová, M.; Cheung, M.C.; Brown, B.G. Cholesterol esterification and atherogenic index of plasma correlate with lipoprotein size and findings on coronary angiography. J. Lipid Res. 2011, 52, 566–571. [Google Scholar] [CrossRef]
- Wu, T.T.; Gao, Y.; Zheng, Y.Y.; Ma, Y.T.; Xie, X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 197. [Google Scholar] [CrossRef]
- Nansseu, J.R.; Moor, V.J.; Nouaga, M.E.; Zing-Awona, B.; Tchanana, G.; Ketcha, A. Atherogenic index of plasma and risk of cardiovascular disease among Cameroonian postmenopausal women. Lipids Health Dis. 2016, 15, 49. [Google Scholar] [CrossRef]
| Q1 | Q2 | Q3 | Q4 | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 43.07 (41.14, 44.99) | 45.93 (44.20, 47.65) | 47.51 (45.49, 49.53) | 48.05 (46.06, 50.04) | 0.0202 |
| BMI (kg/m2) | 25.98 (25.32, 26.65) | 28.70 (27.48, 29.93) | 31.20 (30.58, 31.81) | 32.29 (31.20, 33.38) | <0.0001 |
| Waist circumference (cm) | 90.09 (88.37, 91.81) | 97.92 (95.22, 100.62) | 104.21 (102.48, 105.94) | 107.66 (105.18, 110.14) | <0.0001 |
| eGFR (ml/min/1.73 m2) | 99.51 (95.99, 103.04) | 97.19 (94.74, 99.63) | 95.55 (93.19, 97.92) | 94.80 (92.21, 97.40) | 0.2329 |
| Glucose (mg/dl) | 100.20 (99.33, 101.08) | 104.51 (102.91, 106.10) | 109.69 (106.68, 112.71) | 123.53 (117.08, 129.98) | <0.0001 |
| HbA1c (%) | 5.35 (5.31, 5.39) | 5.50 (5.44, 5.57) | 5.72 (5.64, 5.80) | 6.03 (5.87, 6.19) | <0.0001 |
| ALT (IU/L) | 20.17 (17.21, 23.12) | 21.97 (19.88, 24.06) | 23.72 (21.67, 25.77) | 27.52 (26.14, 28.89) | 0.0022 |
| AST (IU/L) | 23.29 (20.99, 25.59) | 22.22 (20.53, 23.92) | 21.78 (20.17, 23.40) | 22.26 (21.59, 22.94) | 0.7912 |
| Total protein (g/L) | 71.09 (70.23, 71.94) | 70.72 (70.26, 71.19) | 71.08 (70.69, 71.48) | 70.80 (70.10, 71.50) | 0.4635 |
| Albumin (g/L) | 41.27 (40.69, 41.85) | 40.49 (40.06, 40.92) | 39.98 (39.55, 40.41) | 40.38 (39.82, 40.93) | 0.0054 |
| Globulin (g/L) | 29.81 (29.10, 30.52) | 30.23 (29.86, 30.61) | 31.10 (30.75, 31.45) | 30.42 (29.90, 30.95) | 0.0033 |
| Creatinine (mg/dl) | 0.84 (0.82, 0.87) | 0.86 (0.83, 0.89) | 0.87 (0.85, 0.90) | 0.90 (0.86, 0.95) | 0.0952 |
| Uric acid (mg/dl) | 4.83 (4.69, 4.97) | 5.32 (5.18, 5.47) | 5.70 (5.54, 5.86) | 5.96 (5.78, 6.14) | <0.0001 |
| Blood urea nitrogen (mg/dl) | 14.32 (13.70, 14.94) | 13.82 (13.33, 14.32) | 14.24 (13.76, 14.72) | 15.22 (14.32, 16.11) | 0.0778 |
| Triglyceride (mg/dl) | 49.28 (47.76, 50.79) | 75.86 (72.84, 78.88) | 109.00 (106.89, 111.10) | 207.61 (191.35, 223.86) | <0.0001 |
| Total cholesterol (mg/dl) | 176.11 (171.32, 180.90) | 182.52 (176.68, 188.35) | 186.87 (181.56, 192.19) | 199.78 (195.03, 204.54) | <0.0001 |
| HDL cholesterol (mg/dl) | 69.71 (67.70, 71.71) | 55.98 (54.23, 57.74) | 48.84 (47.75, 49.93) | 41.09 (40.11, 42.07) | <0.0001 |
| LDL cholesterol (mg/dl) | 96.58 (92.56, 100.61) | 111.39 (106.98, 115.80) | 116.24 (111.91, 120.56) | 119.13 (115.17, 123.09) | <0.0001 |
| hs-CRP (mg/L) | 2.31 (1.72, 2.90) | 4.26 (3.04, 5.48) | 4.26 (3.70, 4.81) | 4.51 (3.57, 5.45) | 0.0001 |
| Sex | <0.0001 | ||||
| Female | 60.76 (53.83, 67.29) | 52.89 (45.27, 60.37) | 47.27 (42.08, 52.51) | 34.72 (26.26, 44.27) | |
| Male | 39.24 (32.71, 46.17) | 47.11 (39.63, 54.73) | 52.73 (47.49, 57.92) | 65.28 (55.73, 73.74) | |
| Race/ethnicity | <0.0001 | ||||
| Mexican American | 6.84 (3.77, 12.10) | 8.79 (4.69, 15.89) | 11.53 (7.82, 16.68) | 13.25 (9.09, 18.92) | |
| Non-Hispanic Black | 17.37 (12.21, 24.11) | 14.70 (11.47, 18.63) | 9.71 (6.26, 14.76) | 5.39 (3.17, 9.02) | |
| Non-Hispanic White | 59.84 (52.59, 66.68) | 59.81 (52.22, 66.95) | 57.27 (52.48, 61.93) | 61.76 (56.49, 66.78) | |
| Others | 15.95 (11.85, 21.12) | 16.70 (11.24, 24.11) | 21.49 (16.81, 27.05) | 19.60 (15.75, 24.11) | |
| Education level | <0.0001 | ||||
| Less Than High School | 7.73 (5.14, 11.48) | 9.52 (7.11, 12.63) | 14.07 (11.02, 17.80) | 17.26 (13.33, 22.06) | |
| High school or GED | 25.05 (20.16, 30.67) | 25.37 (19.67, 32.07) | 32.66 (27.11, 38.75) | 28.94 (24.26, 34.11) | |
| Above high school | 67.22 (60.87, 72.99) | 65.11 (57.20, 72.27) | 53.26 (46.93, 59.49) | 53.80 (47.14, 60.33) | |
| Marital status | 0.0880 | ||||
| Unmarried | 44.65 (36.10, 53.53) | 38.87 (33.49, 44.53) | 34.52 (27.61, 42.14) | 35.02 (27.97, 42.79) | |
| Married | 55.35 (46.47, 63.90) | 61.13 (55.47, 66.51) | 65.48 (57.86, 72.39) | 64.98 (57.21, 72.03) | |
| Coronary heart disease | 0.0289 | ||||
| No | 97.46 (94.57, 98.83) | 98.50 (96.79, 99.31) | 97.10 (94.65, 98.45) | 94.18 (87.05, 97.50) | |
| Yes | 2.54 (1.17, 5.43) | 1.50 (0.69, 3.21) | 2.90 (1.55, 5.35) | 5.82 (2.50, 12.95) | |
| Heart attach | 0.0013 | ||||
| No | 99.31 (98.03, 99.76) | 97.01 (94.23, 98.48) | 96.20 (92.97, 97.97) | 94.37 (88.90, 97.23) | |
| Yes | 0.69 (0.24, 1.97) | 2.99 (1.52, 5.77) | 3.80 (2.03, 7.03) | 5.63 (2.77, 11.10) | |
| Angina | <0.0001 | ||||
| No | 99.56 (98.59, 99.86) | 98.79 (97.28, 99.46) | 97.60 (95.23, 98.80) | 93.86 (87.88, 96.99) | |
| Yes | 0.44 (0.14, 1.41) | 1.21 (0.54, 2.72) | 2.40 (1.20, 4.77) | 6.14 (3.01, 12.12) | |
| Stroke | 0.4363 | ||||
| No | 98.57 (96.80, 99.37) | 97.51 (95.34, 98.69) | 97.01 (94.57, 98.37) | 97.40 (95.82, 98.39) | |
| Yes | 1.43 (0.63, 3.20) | 2.49 (1.31, 4.66) | 2.99 (1.63, 5.43) | 2.60 (1.61, 4.18) | |
| MetS | <0.0001 | ||||
| No | 94.41 (90.13, 96.90) | 89.10 (84.11, 92.66) | 68.12 (60.91, 74.55) | 33.62 (23.75, 45.17) | |
| Yes | 5.59 (3.10, 9.87) | 10.90 (7.34, 15.89) | 31.88 (25.45, 39.09) | 66.38 (54.83, 76.25) | |
| Smoke | 0.0049 | ||||
| Never | 62.15 (55.96, 67.97) | 60.21 (51.53, 68.29) | 58.19 (53.14, 63.07) | 46.39 (39.26, 53.66) | |
| Former | 25.77 (19.78, 32.84) | 23.45 (17.57, 30.56) | 22.18 (16.67, 28.87) | 30.67 (25.98, 35.79) | |
| Now | 12.08 (7.96, 17.91) | 16.34 (12.81, 20.61) | 19.63 (14.04, 26.76) | 22.95 (16.96, 30.29) | |
| Diabetes | <0.0001 | ||||
| No | 96.21 (94.89, 97.20) | 93.59 (91.00, 95.47) | 83.82 (79.51, 87.37) | 72.91 (67.96, 77.36) | |
| Yes | 3.79 (2.80, 5.11) | 6.41 (4.53, 9.00) | 16.18 (12.63, 20.49) | 27.09 (22.64, 32.04) | |
| Hypertension | 0.0004 | ||||
| No | 71.77 (61.89, 79.92) | 70.89 (63.22, 77.53) | 55.24 (47.32, 62.90) | 54.14 (45.93, 62.14) | |
| Yes | 28.23 (20.08, 38.11) | 29.11 (22.47, 36.78) | 44.76 (37.10, 52.68) | 45.86 (37.86, 54.07) | |
| Statins use | <0.0001 | ||||
| No | 86.39 (80.93, 90.47) | 89.58 (85.13, 92.81) | 81.94 (78.13, 85.21) | 75.97 (69.22, 81.64) | |
| Yes | 13.61 (9.53, 19.07) | 10.42 (7.19, 14.87) | 18.06 (14.79, 21.87) | 24.03 (18.36, 30.78) | |
| Antidiabetic drugs | <0.0001 | ||||
| No | 97.10 (95.81, 98.01) | 94.91 (92.84, 96.40) | 86.35 (82.79, 89.26) | 77.71 (72.23, 82.38) | |
| Yes | 2.90 (1.99, 4.19) | 5.09 (3.60, 7.16) | 13.65 (10.74, 17.21) | 22.29 (17.62, 27.77) | |
| Antihypertensive drugs | 0.1399 | ||||
| No | 98.05 (96.25, 99.00) | 95.48 (90.46, 97.92) | 95.14 (91.78, 97.17) | 94.93 (92.20, 96.74) | |
| Yes | 1.95 (1.00, 3.75) | 4.52 (2.08, 9.54) | 4.86 (2.83, 8.22) | 5.07 (3.26, 7.80) |
| Exposure |
Non-Adjusted OR (95%CI), p-Value |
Adjust I OR (95%CI), p-Value |
Adjust II OR (95%CI), p-Value |
|---|---|---|---|
| AIP | 2.47 (1.57, 3.89) <0.0001 | 1.73 (1.00, 2.99) 0.0481 | 0.28 (0.10, 0.78) 0.0154 |
| AIP quartile | |||
| Q1 | 1(Reference) | 1(Reference) | 1(Reference) |
| Q2 | 1.18 (0.74, 1.87) 0.4937 | 0.99 (0.60, 1.62) 0.9648 | 0.38 (0.20, 0.72) 0.0033 |
| Q3 | 1.03 (0.64, 1.65) 0.9173 | 0.64 (0.39, 1.07) 0.0879 | 0.24 (0.12, 0.48) <0.0001 |
| Q4 | 2.16 (1.43, 3.27) 0.0003 | 1.41 (0.89, 2.24) 0.1433 | 0.32 (0.14, 0.74) 0.0075 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; He, L.; Xie, H.; Xie, X.; Wang, H. An Inverse Correlation between the Atherogenic Index of Plasma and Heart Failure: An Analysis of the National Health and Nutrition Examination Survey 2017–March 2020 Pre-Pandemic Data. J. Cardiovasc. Dev. Dis. 2022, 9, 412. https://doi.org/10.3390/jcdd9120412
Xue J, He L, Xie H, Xie X, Wang H. An Inverse Correlation between the Atherogenic Index of Plasma and Heart Failure: An Analysis of the National Health and Nutrition Examination Survey 2017–March 2020 Pre-Pandemic Data. Journal of Cardiovascular Development and Disease. 2022; 9(12):412. https://doi.org/10.3390/jcdd9120412
Chicago/Turabian StyleXue, Jianying, Lu He, Hang Xie, Xuegang Xie, and Haiyan Wang. 2022. "An Inverse Correlation between the Atherogenic Index of Plasma and Heart Failure: An Analysis of the National Health and Nutrition Examination Survey 2017–March 2020 Pre-Pandemic Data" Journal of Cardiovascular Development and Disease 9, no. 12: 412. https://doi.org/10.3390/jcdd9120412
APA StyleXue, J., He, L., Xie, H., Xie, X., & Wang, H. (2022). An Inverse Correlation between the Atherogenic Index of Plasma and Heart Failure: An Analysis of the National Health and Nutrition Examination Survey 2017–March 2020 Pre-Pandemic Data. Journal of Cardiovascular Development and Disease, 9(12), 412. https://doi.org/10.3390/jcdd9120412




