Diagnostic Performance of Serial High-Sensitivity Cardiac Troponin Measurements in the Emergency Setting
Abstract
:1. Introduction
2. Materials and Methods
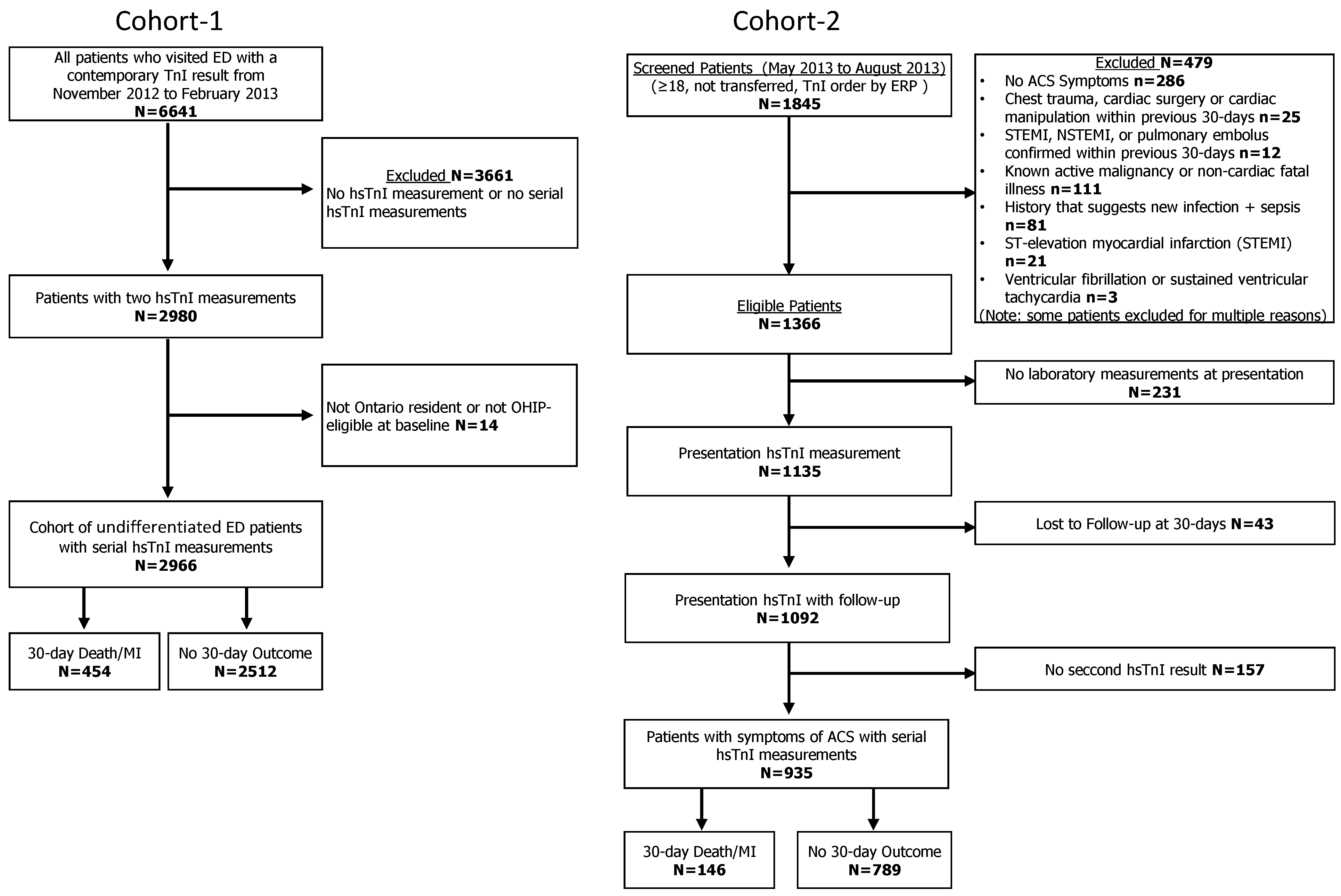
2.1. Study Design and Participants
2.2. Health Outcomes
2.3. Laboratory Testing and Algorithms Evaluated
2.4. Statistical Analyses
3. Results
4. Interpretation
4.1. Limitations
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Trial Registration
Sample Availability
References
- Wu, A.H.B.; Christenson, R.H.; Greene, D.N.; Jaffe, A.S.; Kavsak, P.A.; Ordonez-Llanos, J.; Apple, F.S. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. 2018, 64, 645–655. [Google Scholar]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef]
- Reichlin, T.; Twerenbold, R.; Wildi, K.; Gimenez, M.R.; Bergsma, N.; Haaf, P.; Druey, S.; Puelacher, C.; Moehring, B.; Freese, M.; et al. Prospective validation of a 1-hour algorithm to rule-out and rule-in acute myocardial infarction using a high-sensitivity cardiac troponin T assay. CMAJ Can. Med. Assoc. J. 2015, 187, E243–E252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, J.T.; Twerenbold, R.; Ojeda, F.; Sörensen, N.A.; Chapman, A.R.; Shah, A.S.V.; Anand, A.; Boeddinghaus, J.; Nestelberger, T.; Badertscher, P.; et al. Application of High-Sensitivity Troponin in Suspected Myocardial Infarction. N. Engl. J. Med. 2019, 380, 2529–2540. [Google Scholar] [CrossRef] [PubMed]
- Pickering, J.W.; Greenslade, J.H.; Cullen, L.; Flaws, D.; Parsonage, W.; Aldous, S.; George, P.; Worster, A.; Kavsak, P.A.; Than, M.P. Assessment of the European Society of Cardiology 0-Hour/1-Hour Algorithm to Rule-Out and Rule-In Acute Myocardial Infarction. Circulation 2016, 134, 1532–1541. [Google Scholar] [CrossRef]
- Allen, B.R.; Christenson, R.H.; Cohen, S.A.; Nowak, R.; Wilkerson, R.G.; Mumma, B.; Madsen, T.; McCord, J.; Huis In’t Veld, M.; Massoomi, M.; et al. Diagnostic Performance of High-Sensitivity Cardiac Troponin T Strategies and Clinical Variables in a Multisite US Cohort. Circulation 2021, 143, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Lambrakis, K.; Papendick, C.; French, J.K.; Quinn, S.; Blyth, A.; Seshadri, A.; Edmonds, M.J.R.; Chuang, A.; Khan, E.; Nelson, A.J.; et al. Late Outcomes of the RAPID-TnT Randomized Controlled Trial: 0/1-Hour High-Sensitivity Troponin T Protocol in Suspected ACS. Circulation 2021, 144, 113–125. [Google Scholar] [CrossRef]
- Than, M.; Herbert, M.; Flaws, D.; Cullen, L.; Hess, E.; Hollander, J.E.; Diercks, D.; Ardagh, M.W.; Kline, J.A.; Munro, Z.; et al. What is an acceptable risk of major adverse cardiac event in chest pain patients soon after discharge from the Emergency Department? A clinical survey. Int. J. Cardiol. 2013, 166, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.; Anand, A.; Sandoval, Y.; Lee, K.K.; Smith, S.W.; Adamson, P.D.; Chapman, A.R.; Langdon, T.; Sandeman, D.; Vaswani, A.; et al. High-sensitivity cardiac troponin I at presentation in patients with suspected acute coronary syndrome: A cohort study. Lancet 2015, 386, 2481–2488. [Google Scholar] [CrossRef] [Green Version]
- Than, M.P.; Pickering, J.W.; Sandoval, Y.; Shah, A.S.V.; Tsanas, A.; Apple, F.S.; Blankenberg, S.; Cullen, L.; Mueller, C.; Neumann, J.T.; et al. Machine Learning to Predict the Likelihood of Acute Myocardial Infarction. Circulation 2019, 140, 899–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavsak, P.A.; Cerasuolo, J.O.; Ko, D.T.; Ma, J.; Sherbino, J.; Mondoux, S.E.; Perez, R.; Seow, H.; Worster, A. High-Sensitivity Cardiac Troponin I vs a Clinical Chemistry Score for Predicting All-Cause Mortality in an Emergency Department Population. CJC Open 2020, 2, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavsak, P.A.; Cerasuolo, J.O.; Ko, D.T.; Ma, J.; Sherbino, J.; Mondoux, S.E.; Clayton, N.; Hill, S.A.; McQueen, M.; Griffith, L.E.; et al. Using the clinical chemistry score in the emergency department to detect adverse cardiac events: A diagnostic accuracy study. CMAJ Open 2020, 8, E676–E684. [Google Scholar] [CrossRef]
- Anand, A.; Lee, K.K.; Chapman, A.R.; Ferry, A.V.; Adamson, P.D.; Strachan, F.E.; Berry, C.; Findlay, I.; Cruickshank, A.; Reid, A.; et al. High-Sensitivity Cardiac Troponin on Presentation to Rule Out Myocardial Infarction: A Stepped-Wedge Cluster Randomized Controlled Trial. Circulation 2021, 143, 2214–2224. [Google Scholar] [CrossRef]
- Pickering, J.W. The Need to Improve Derivation and Description of Algorithms to Rule-Out Patients with Possible Myocardial Infarction. Circulation 2019, 139, 1351–1353. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavsak, P.A.; Neumann, J.T.; Cullen, L.; Than, M.; Shortt, C.; Greenslade, J.H.; Pickering, J.W.; Ojeda, F.; Ma, J.; Clayton, N.; et al. Clinical chemistry score versus high-sensitivity cardiac troponin I and T tests alone to identify patients at low or high risk for myocardial infarction or death at presentation to the emergency department. CMAJ Can. Med. Assoc. J. 2018, 190, E974–E984. [Google Scholar] [CrossRef] [Green Version]
- Kavsak, P.A.; Cerasuolo, J.O.; Mondoux, S.E.; Sherbino, J.; Ma, J.; Hoard, B.K.; Perez, R.; Seow, H.; Ko, D.T.; Worster, A. Risk Stratification for Patients with Chest Pain Discharged Home from the Emergency Department. J. Clin. Med. 2020, 9, 2948. [Google Scholar] [CrossRef]
- Shortt, C.; Ma, J.; Clayton, N.; Sherbino, J.; Whitlock, R.; Pare, G.; Hill, S.A.; McQueen, M.; Mehta, S.R.; Devereaux, P.J.; et al. Rule-In and Rule-Out of Myocardial Infarction Using Cardiac Troponin and Glycemic Biomarkers in Patients with Symptoms Suggestive of Acute Coronary Syndrome. Clin. Chem. 2017, 63, 403–414. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Worster, A.; Ma, J.; Shortt, C.; Clayton, N.; Sherbino, J.; Hill, S.A.; McQueen, M.; Mehta, S.R.; Devereaux, P.J. High-Sensitivity Cardiac Troponin Risk Cutoffs for Acute Cardiac Outcomes at Emergency Department Presentation. Can. J. Cardiol. 2017, 33, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.; Koh, M.; Kavsak, P.A.; Schull, M.J.; Armstrong, D.W.J.; Udell, J.A.; Austin, P.C.; Wang, X.; Ko, D.T. Clinical outcomes for chest pain patients discharged home from emergency departments using high-sensitivity versus conventional cardiac troponin assays. Am. Heart J. 2020, 221, 84–94. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Pardhan, A.; Krizmanich, W.; Worster, A. Hospital Admission and Myocardial Injury Prevalence after the Clinical Introduction of a High-Sensitivity Cardiac Troponin I Assay. Clin. Chem. 2015, 61, 1209–1210. [Google Scholar] [CrossRef] [Green Version]
- Kufaishi, H.; Pardhan, A.; Krizmanich, W.; Worster, A.; Hill, S.; Kavsak, P.A. Adopting ‘ng/L’ as the units for high-sensitivity cardiac troponin assays and commitment by the entire health-care team could be the key for adopting recommendations. Ann. Clin. Biochem. 2016, 53 Pt 4, 516–517. [Google Scholar] [CrossRef] [Green Version]
- Kavsak, P.A.; Mondoux, S.E.; Ma, J.; Sherbino, J.; Hill, S.A.; Clayton, N.; Mehta, S.R.; Griffith, L.E.; McQueen, M.; Devereaux, P.J.; et al. Comparison of two biomarker only algorithms for early risk stratification in patients with suspected acute coronary syndrome. Int. J. Cardiol. 2020, 319, 140–143. [Google Scholar] [CrossRef]
- Hickman, P.E.; Koerbin, G.; Badrick, T.; Oakman, C.; Potter, J.M. The importance of low level QC for high sensitivity troponin assays. Clin. Biochem. 2018, 58, 60–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildi, K.; Boeddinghaus, J.; Nestelberger, T.; Haaf, P.; Koechlin, L.; Ayala Lopez, P.; Walter, J.; Badertscher, P.; Ratmann, P.D.; Miró, Ò.; et al. External validation of the clinical chemistry score. Clin. Biochem. 2021, 91, 16–25. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Jaffe, A.S.; Greene, D.N.; Christenson, R.H.; Apple, F.S.; Wu, A.H.B. Total Analytic Error for Low Cardiac Troponin Concentrations (≤10 ng/L) by Use of a High-Sensitivity Cardiac Troponin Assay. Clin. Chem. 2017, 63, 1043–1045. [Google Scholar] [CrossRef]
- Duceppe, E.; Borges, F.; Tiboni, M.; Pearse, R.; Chan, M.; Srinathan, S.; Kavsak, P.; Szalay, D.; Garg, A.; Sessler, D.; et al. Association between high-sensitivity troponin I and major cardiovascular events after non-cardiac surgery. J. Am. Coll. Cardiol. 2020, 75 (Suppl. 1), 110. [Google Scholar] [CrossRef]
- Pickering, J.W.; Than, M.P.; Cullen, L.; Aldous, S.; Ter Avest, E.; Body, R.; Carlton, E.W.; Collinson, P.; Dupuy, A.M.; Ekelund, U.; et al. Rapid rule-out of acute myocardial Infarction with a single high-sensitivity cardiac troponin T measurement below the limit of detection: A Collaborative Meta-analysis. Ann. Intern. Med. 2017, 166, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.R.; Lee, K.K.; McAllister, D.A.; Cullen, L.; Greenslade, J.H.; Parsonage, W.; Worster, A.; Kavsak, P.A.; Blankenberg, S.; Neumann, J.; et al. Association of high-sensitivity cardiac troponin I concentration with cardiac outcomes in patients with suspected acute coronary syndrome. JAMA J. Am. Med. Assoc. 2017, 318, 1913–1924. [Google Scholar] [CrossRef] [PubMed]
- Hammarsten, O.; Jacobsson, C.E.; Widegren, M.; Danylchenko, T.; Jaffe, A.S. Long-time quality assessment of the Elecsys Troponin T hs assay. Clin. Biochem. 2013, 46, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Clifford-Mobley, O. Using the European Society of Cardiology 1-h algorithm for ruling out non-ST-segment elevated myocardial infarction to define acceptable analytical performance limits for a cardiac troponin T assay. Ann. Clin. Biochem. 2021, 58, 157. [Google Scholar] [CrossRef] [PubMed]
- Kavsak, P.A.; Clark, L.; Martin, J.; Mark, C.T.; Paré, G.; Mondoux, S.; Chetty, V.T.; Ainsworth, C.; Worster, A. Acute Phase Response and Non-Reproducible Elevated Concentrations with a High-Sensitivity Cardiac Troponin I Assay. J. Clin. Med. 2021, 10, 1014. [Google Scholar] [CrossRef]
- Kavsak, P.A.; Mondoux, S.E.; Martin, J.; Hewitt, M.K.; Clark, L.; Caruso, N.; Mark, C.T.; Chetty, V.T.; Ainsworth, C.; Worster, A. Disagreement between Cardiac Troponin Tests Yielding a Higher Incidence of Myocardial Injury in the Emergency Setting. J. Cardiovasc. Dev. Dis. 2021, 8, 31. [Google Scholar] [CrossRef]
- Sandoval, Y.; Chapman, A.R.; Mills, N.L.; Than, M.; Pickering, J.W.; Worster, A.; Kavsak, P.; Apple, F.S. Sex-Specific Kinetics of High-Sensitivity Cardiac Troponin I and T following Symptom Onset and Early Presentation in Non-ST-Segment Elevation Myocardial Infarction. Clin. Chem. 2021, 67, 321–324. [Google Scholar] [CrossRef]
- Sandoval, Y.; Nowak, R.; deFilippi, C.R.; Christenson, R.H.; Peacock, W.F.; McCord, J.; Limkakeng, A.T.; Sexter, A.; Apple, F.S. Myocardial Infarction Risk Stratification with a Single Measurement of High-Sensitivity Troponin I. J. Am. Coll. Cardiol. 2019, 74, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Meah, M.N.; Denvir, M.A.; Mills, N.L.; Norrie, J.; Newby, D.E. Clinical endpoint adjudication. Lancet 2020, 395, 1878–1882. [Google Scholar] [CrossRef]
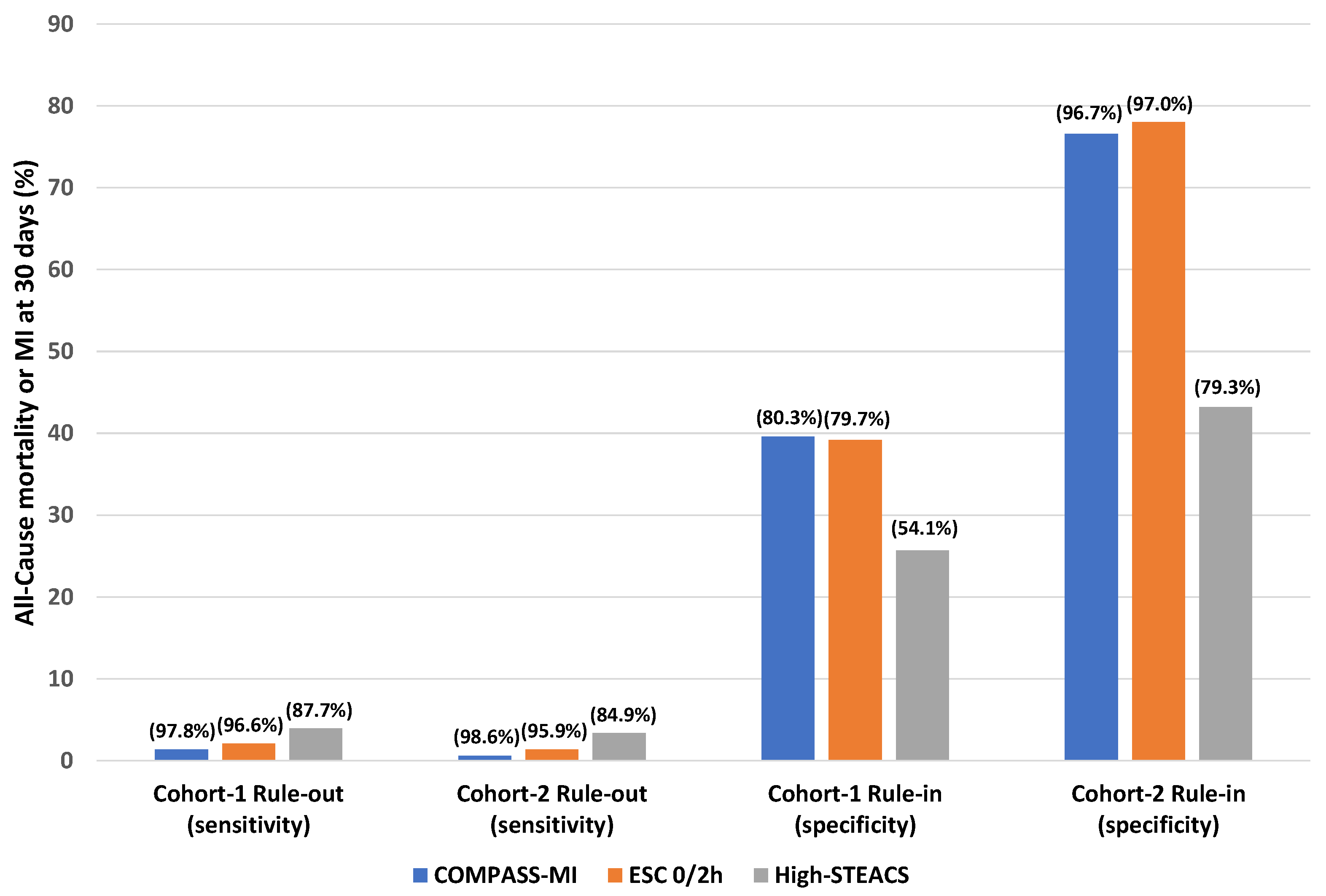
| ESC 0/2 h algorithm | Rule-out: if 1st hsTnI < 6 ng/L and absolute change between 2nd and 1st sample < 2 ng/L | Rule-in: if 1st ≥ 64 ng/L or absolute change ≥ 15 ng/L between 2nd and 1st sample |
| COMPASS-MI algorithm | Rule-out: if 1st hsTnI < 4 ng/L and absolute change between 2nd and 1st sample < 4 ng/L | Rule-in: if 1st ≥ 60 ng/L or absolute change ≥ 18 ng/L between 2nd and 1st sample |
| High-STEACS | Rule-out: if 1st hsTnI ≤ sex-specific URLs and absolute change between 2nd and 1st sample < 3 ng/L | Rule-in: if 1st hsTnI > sex-specific URLs or absolute change between 2nd and 1st sample ≥ 3 ng/L |
| Variable | Value | Cohort-1 (n = 2966) | Cohort-2 (n = 935) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Uncertain | Rule-In Group | Rule-Out Group | p-Value | Uncertain | Rule-In Group | Rule-Out Group | p-Value | ||
| ESC absolute criteria | |||||||||
| Number | Count | 1264 | 837 | 865 | - | 401 | 109 | 425 | - |
| Age (years) | Median (IQR) | 77 (65–84) | 78 (66–86) | 60 (50–71) | <0.001 | 77 (64–85) | 77 (63–85) | 59 (48–70) | <0.001 |
| Sex | Female (%) | 612 (48.4%) | 397 (47.4%) | 453 (52.4%) | 0.09 | 210 (52.4%) | 49 (45.0%) | 237 (55.8%) | 0.12 |
| History of arrhythmia | Count (%) | 311 (24.6%) | 203 (24.3%) | 81 (9.4%) | <0.001 | 131 (32.7%) | 39 (35.8%) | 56 (13.2%) | <0.001 |
| History of heart failure | Count (%) | 417 (33.0%) | 307 (36.7%) | 93 (10.8%) | <0.001 | 114 (28.4%) | 40(36.7%) | 27 (6.4%) | <0.001 |
| History of diabetes | Count (%) | 509 (40.3%) | 356 (42.5%) | 220 (25.4%) | <0.001 | 137 (34.2%) | 45 (41.3%) | 92 (21.7%) | <0.001 |
| History of myocardial infarction | Count (%) | 217 (17.2%) | 167 (20.0%) | 61 (7.1%) | <0.001 | 175 (43.6%) | 54 (49.5%) | 109 (25.7%) | <0.001 |
| COMPASS-MI absolute criteria | |||||||||
| Number | Count | 1448 | 821 | 697 | - | 488 | 111 | 336 | - |
| Age (years) | Median (IQR) | 76 (65–84) | 78 (66–86) | 57 (48–67) | <0.001 | 76 (63–84) | 77 (64–85) | 56 (46–67) | <0.001 |
| Sex | Female (%) | 711 (49.1%) | 386 (47.0%) | 365 (52.4%) | 0.113 | 266 (54.5%) | 50 (45.1%) | 180 (53.6%) | 0.191 |
| History of arrhythmia | Count (%) | 340 (23.5%) | 201 (24.5%) | 54 (7.7%) | <0.001 | 142 (29.1%) | 42 (37.8%) | 42 (12.5%) | <0.001 |
| History of heart failure | Count (%) | 464 (32.0%) | 300 (36.5%) | 53 (7.6%) | <0.001 | 122 (25.0%) | 43 (38.7%) | 16 (4.8%) | <0.001 |
| History of diabetes | Count (%) | 566 (39.1%) | 352 (42.9%) | 167 (24.0%) | <0.001 | 156 (32.0%) | 45 (40.5%) | 73 (21.7%) | 0.001 |
| History of myocardial infarction | Count (%) | 238 (16.4%) | 157 (19.1%) | 50 (7.2%) | <0.001 | 210 (43.0%) | 53 (47.8%) | 75 (22.3%) | <0.001 |
| High-STEACS absolute criteria | |||||||||
| Number | Count | 1551 | 1415 | - | 287 | 648 | - | ||
| Age (years) | Median (IQR) | 78 (66–85) | 66 (54–78) | <0.001 | 78 (64–85) | 64 (53–77) | <0.001 | ||
| Sex | Female (%) | 771 (49.7%) | 691 (48.8%) | 0.634 | 160 (55.8%) | 336 (51.9%) | 0.271 | ||
| History of arrhythmia | Count (%) | 374 (24.1%) | 221 (15.6%) | <0.001 | 99 (34.5%) | 127 (19.6%) | <0.001 | ||
| History of heart failure | Count (%) | 550 (35.5%) | 267 (18.9%) | <0.001 | 97 (33.8%) | 84 (13.0%) | <0.001 | ||
| History of diabetes | Count (%) | 646 (41.7%) | 439 (31.0%) | <0.001 | 102 (35.5%) | 172 (26.5%) | 0.015 | ||
| History of myocardial infarction | Count (%) | 287 (18.5%) | 158 (11.2%) | <0.001 | 136 (47.4%) | 202 (31.2%) | <0.001 | ||
| Variable | Value | Cohort-1 (n = 2966) | Cohort-2 (n = 935) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Uncertain | Rule-In Group | Rule-Out Group | p-Value | Uncertain | Rule-In Group | Rule-Out Group | p-Value | ||
| ESC absolute criteria | |||||||||
| Number | Count | 1264 | 837 | 865 | - | 401 | 109 | 425 | - |
| Time elapsed (hours) between 1st and 2nd hsTnI | Median (IQR) | 8 (6–13) | 8 (7–11) | 6 (4–9) | <0.001 | 3 (3–3) | 3 (3–3) | 3 (3–3) | <0.001 |
| 1st hsTnI (ng/L) | Median (IQR) | 13 (8–26) | 89 (35–235) | 3 (2–4) | <0.001 | 12 (7–23) | 99 (41–229) | 2 (1–4) | <0.001 |
| 2nd hsTnI (ng/L) | Median (IQR) | 14 (9–26) | 137 (67–492) | 3 (2–4) | <0.001 | 13 (8–5) | 188 (80–695) | 2 (1–4) | <0.001 |
| Absolute difference hsTnI (ng/L) between samples | Median (IQR) | 3 (1–6) | 56 (21–250) | 1 (0–1) | <0.001 | 2 (1–3) | 52 (15–288) | 0 (0–1) | <0.001 |
| All-cause mortality or MI at 30 days | Count (%) | 108 (8.5%) | 328 (39.2%) | 18 (2.1%) | <0.001 | 55 (13.7%) | 85 (78.0%) | 6 (1.4%) | <0.001 |
| COMPASS-MI absolute criteria | |||||||||
| Number | Count | 1448 | 821 | 697 | - | 488 | 111 | 336 | - |
| Time elapsed (hours) between 1st and 2nd hsTnI | Median (IQR) | 8 (6–12) | 8 (7–10) | 6 (4–9) | <0.001 | 3 (3–3) | 3 (3–3) | 3 (3–3) | <0.001 |
| 1st hsTnI (ng/L) | Median (IQR) | 11 (6–23) | 93 (39–241) | 2 (1–3) | <0.001 | 10 (5–19) | 90 (50–229) | 2 (1–3) | <0.001 |
| 2nd hsTnI (ng/L) | Median (IQR) | 12 (7–23) | 142 (72–506) | 2 (2–3) | <0.001 | 11 (6–21) | 182 (77–695) | 2 (1–3) | <0.001 |
| Absolute difference hsTnI (ng/L) between samples | Median (IQR) | 2 (1–5) | 56 (22–255) | 1 (0–1) | <0.001 | 1 (0–3) | 51 (11–288) | 0 (0–1) | <0.001 |
| All-cause mortality or MI at 30 days | Count (%) | 119 (8.2%) | 325 (39.6%) | 10 (1.4%) | <0.001 | 59 (12.1%) | 85 (76.6%) | 2 (0.6%) | <0.001 |
| High-STEACS absolute criteria | |||||||||
| Number | Count | 1551 | 1415 | - | 287 | 648 | - | ||
| Time elapsed (hours) between 1st and 2nd hsTnI | Median (IQR) | 8 (7–12) | 7 (4–10) | <0.001 | 3 (3–3) | 3 (3–3) | <0.001 | ||
| 1st hsTnI (ng/L) | Median (IQR) | 38 (16–100) | 4 (2–8) | <0.001 | 30 (15–66) | 3 (2–7) | <0.001 | ||
| 2nd hsTnI (ng/L) | Median (IQR) | 48 (21–157) | 5 (2–8) | <0.001 | 38 (21–100) | 4 (2–7) | <0.001 | ||
| Absolute difference hsTnI (ng/L) between samples | Median (IQR) | 14 (5–64) | 1 (0–1) | <0.001 | 6 (3–22) | 1 (0–1) | <0.001 | ||
| All-cause mortality or MI at 30 days | Count (%) | 398 (25.7%) | 56 (4.0%) | <0.001 | 124 (43.2%) | 22 (3.4%) | <0.001 | ||
| Group | Sensitivity | Specificity | PPV | NPV | Positive LR | Negative LR | Sensitivity | Specificity | PPV | NPV | Positive LR | Negative LR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort-1 ESC absolute criteria | Cohort-2 ESC absolute criteria | |||||||||||
| Rule-out | 96.0% (93.8–97.6%) | 33.7% (31.9–35.6%) | 20.8% (19.0–22.6%) | 97.9% (96.7–98.8%) | 1.45 (1.40–1.50) | 0.12 (0.06–0.17) | 95.9% (91.3–98.5%) | 53.1% (49.6–56.6%) | 27.5% (25.9–29.1%) | 98.6% (97.0–99.4%) | 2.04 (1.88–2.22) | 0.08 (0.04–0.17) |
| Rule-in | 72.2% (67.9–76.3%) | 79.7% (78.1–81.3%) | 39.2% (35.9–42.6%) | 94.1% (93.0–95.0%) | 3.57 (3.22–3.91) | 0.35 (0.30–0.40) | 58.2% (49.8–66.3%) | 97.0% (95.5–98.0%) | 78.0% (70.0–84.3%) | 92.6% (91.2–93.3%) | 19.14 (12.61–29.05) | 0.43 (0.36–0.52) |
| Cohort-1 COMPASS-MI absolute criteria | Cohort-2 COMPASS-MI absolute criteria | |||||||||||
| Rule-out | 97.8% (96.0–98.9%) | 27.3% (25.6–29.1%) | 19.6% (18.0–21.3%) | 98.6% (97.4–99.3%) | 1.35 (1.31–1.38) | 0.08 (0.03–0.13) | 98.6% (95.1–99.8%) | 42.3% (38.9–45.9%) | 24.0% (22.9–25.2%) | 99.4% (97.7–99.9%) | 1.71 (1.61–1.82) | 0.03 (0.01–0.13) |
| Rule-in | 71.6% (67.2–75.7%) | 80.3% (78.6–81.8%) | 39.6% (36.2–43.0%) | 94.0% (92.9–95.0%) | 3.63 (3.27–3.98) | 0.35 (0.30–0.41) | 58.2% (49.8–66.3%) | 96.7% (95.2–97.8%) | 76.6% (68.6–83.0%) | 92.6% (91.2–93.8%) | 17.67 (11.82–26.41) | 0.43 (0.36–0.52) |
| Cohort-1 High-STEACS absolute criteria | Cohort-2 High-STEACS absolute criteria | |||||||||||
| Rule-out and Rule-in | 87.7% (84.2–90.5%) | 54.1% (52.1–56.1%) | 25.7% (23.5–27.9%) | 96.0% (94.9–97.0%) | 1.91 (1.81–2.01) | 0.23 (0.17–0.29) | 84.9% (78.1–90.3%) | 79.3% (76.4–82.1%) | 43.2% (39.5–47.0%) | 96.6% (95.1–97.7%) | 4.11 (3.53–4.79) | 0.19 (0.13–0.28) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavsak, P.A.; Hewitt, M.K.; Mondoux, S.E.; Cerasuolo, J.O.; Ma, J.; Clayton, N.; McQueen, M.; Griffith, L.E.; Perez, R.; Seow, H.; et al. Diagnostic Performance of Serial High-Sensitivity Cardiac Troponin Measurements in the Emergency Setting. J. Cardiovasc. Dev. Dis. 2021, 8, 97. https://doi.org/10.3390/jcdd8080097
Kavsak PA, Hewitt MK, Mondoux SE, Cerasuolo JO, Ma J, Clayton N, McQueen M, Griffith LE, Perez R, Seow H, et al. Diagnostic Performance of Serial High-Sensitivity Cardiac Troponin Measurements in the Emergency Setting. Journal of Cardiovascular Development and Disease. 2021; 8(8):97. https://doi.org/10.3390/jcdd8080097
Chicago/Turabian StyleKavsak, Peter A., Mark K. Hewitt, Shawn E. Mondoux, Joshua O. Cerasuolo, Jinhui Ma, Natasha Clayton, Matthew McQueen, Lauren E. Griffith, Richard Perez, Hsien Seow, and et al. 2021. "Diagnostic Performance of Serial High-Sensitivity Cardiac Troponin Measurements in the Emergency Setting" Journal of Cardiovascular Development and Disease 8, no. 8: 97. https://doi.org/10.3390/jcdd8080097
APA StyleKavsak, P. A., Hewitt, M. K., Mondoux, S. E., Cerasuolo, J. O., Ma, J., Clayton, N., McQueen, M., Griffith, L. E., Perez, R., Seow, H., Ainsworth, C., Ko, D. T., & Worster, A. (2021). Diagnostic Performance of Serial High-Sensitivity Cardiac Troponin Measurements in the Emergency Setting. Journal of Cardiovascular Development and Disease, 8(8), 97. https://doi.org/10.3390/jcdd8080097





