Assembly of the Cardiac Pacemaking Complex: Electrogenic Principles of Sinoatrial Node Morphogenesis
Abstract
1. Introduction
2. Physiological Barriers to Sinoatrial Node Function/Source-Sink Relationships in the Sinoatrial Node
3. Electro-Anatomical Features That Support Sinoatrial Node Function
3.1. Synchronization
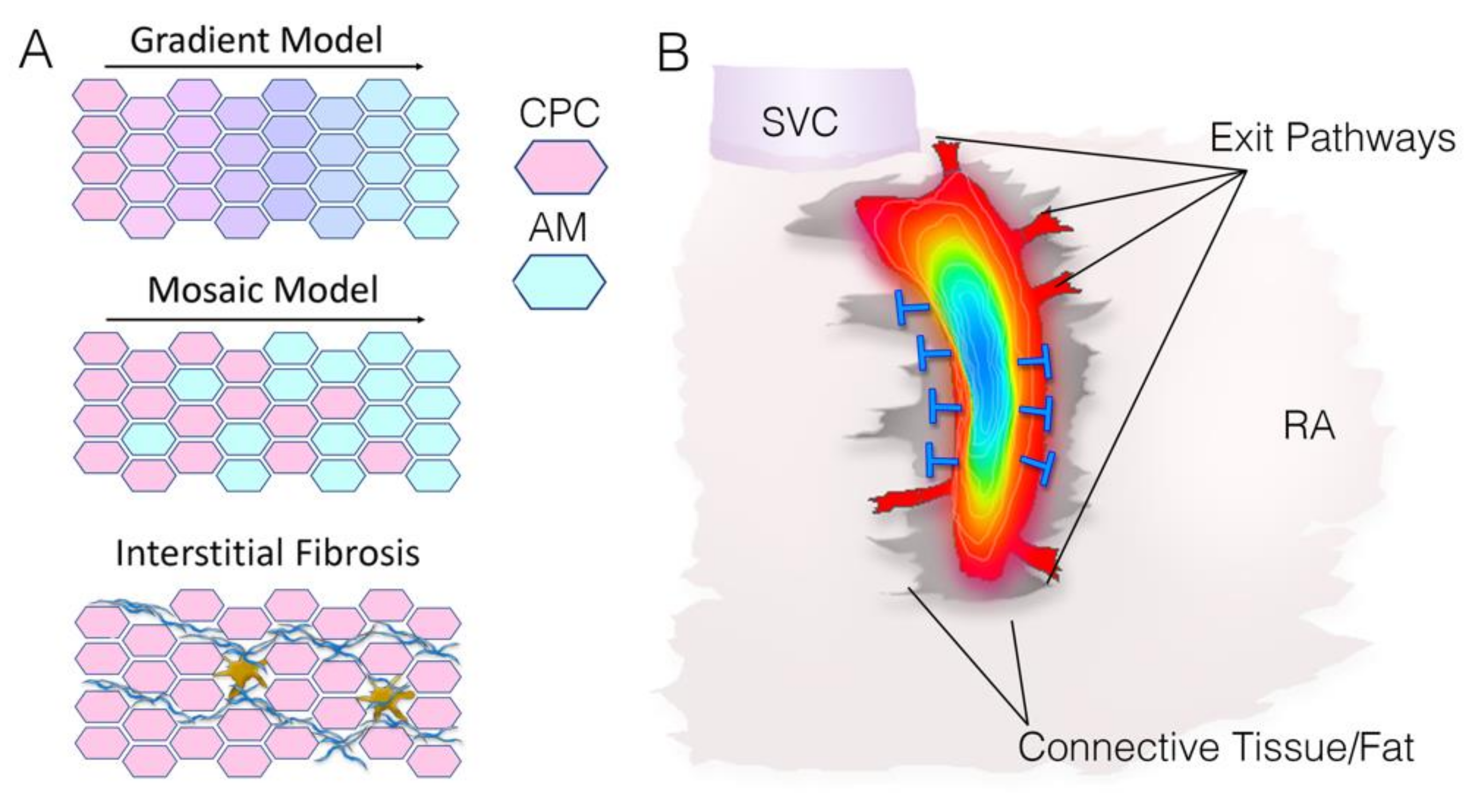
3.2. Sinoatrial Node Structure—Interstitial Fibrosis
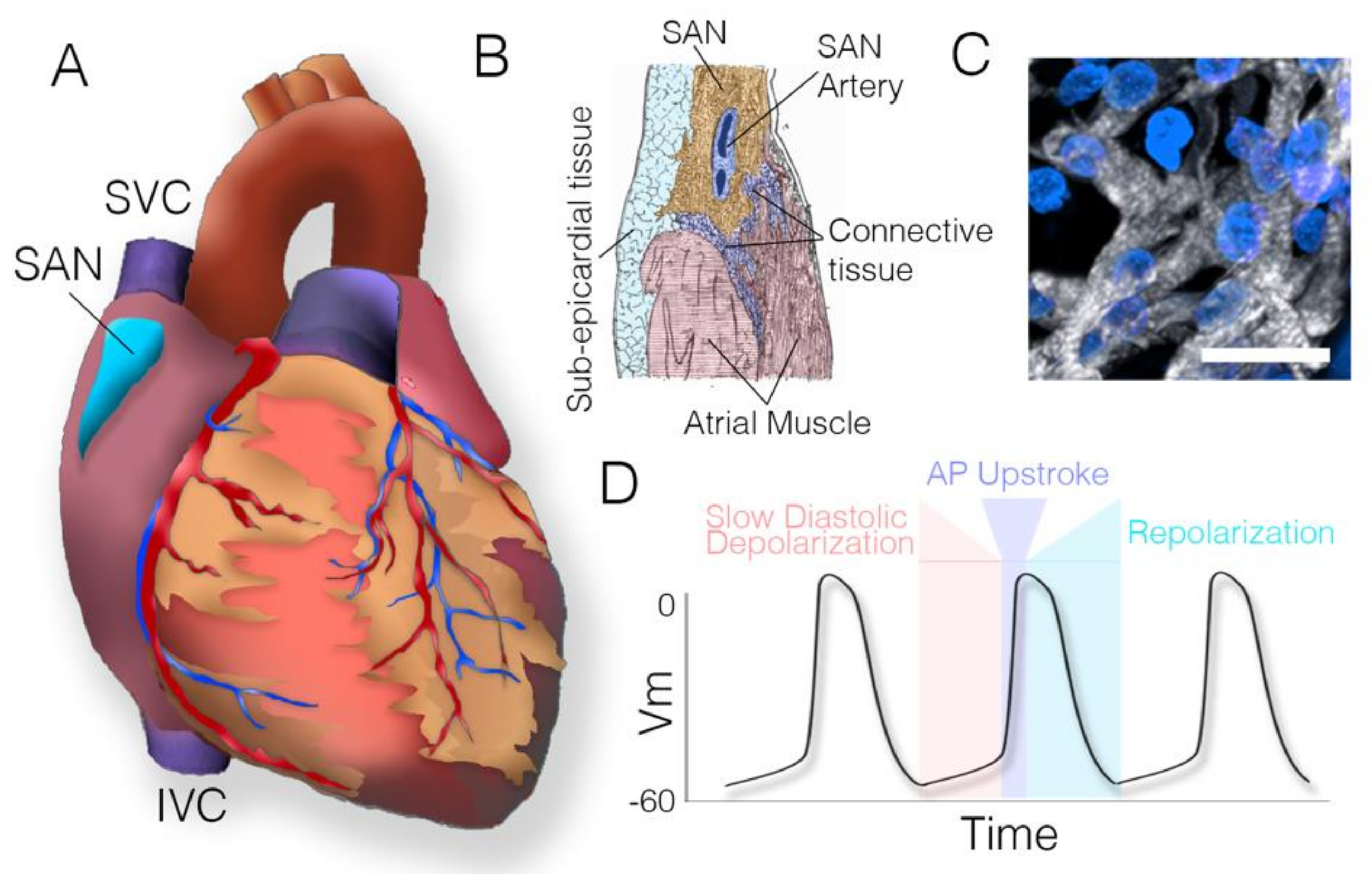
3.3. Sinoatrial Node Structure—Atrial Interface
4. Sinoatrial Node Morphogenesis
4.1. Pacing in the Early Heart
4.2. Formation of the Pacemaker Cell Microenvironment
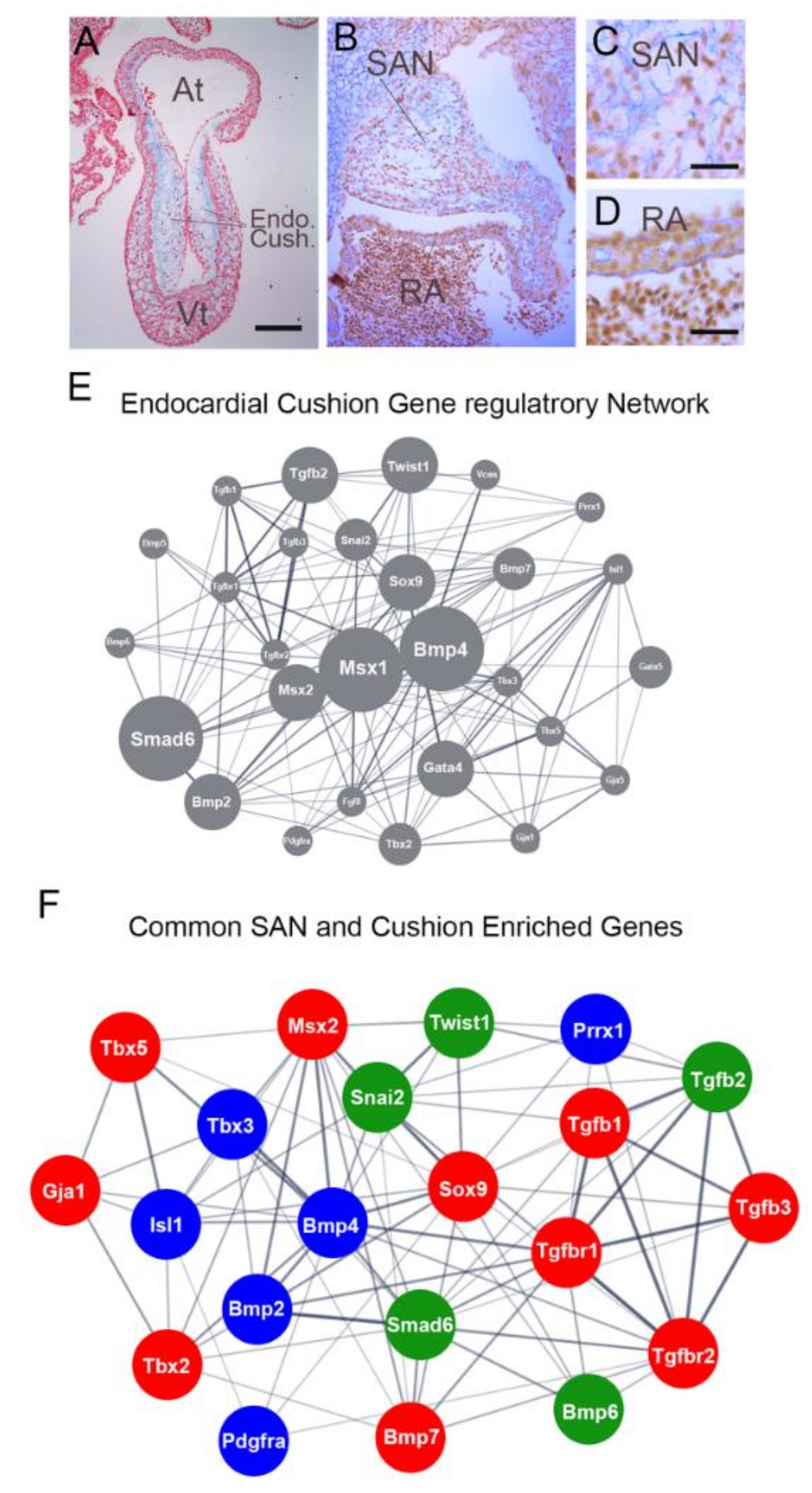
4.3. Signaling Environment of the Embryonic Sinoatrial Node
4.4. Potential Roles for TGFb/BMP Signaling in Sinoatrial Node Morphogenesis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouman, L.N.; Jongsma, H.J. Structure and function of the sino-atrial node: A review. Eur. Heart J. 1986, 7, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Keith, A.; Flack, M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J. Anat. Physiol. 1907, 41, 172–189. [Google Scholar] [PubMed]
- Hirschhorn, R. Adenosine deaminase deficiency. Immunodefic. Rev. 1990, 2, 175–198. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Kumar, R.; Malik, S.; Raj, T.; Kumar, P. Analysis of Heart rate Variability and Implication of Different Factors on Heart Rate Variability. Curr. Cardiol. Rev. 2020. [Google Scholar] [CrossRef]
- Jensen, P.N.; Gronroos, N.N.; Chen, L.Y.; Folsom, A.R.; de Filippi, C.; Heckbert, S.R.; Alonso, A. Incidence of and risk factors for sick sinus syndrome in the general population. J. Am. Coll Cardiol 2014, 64, 531–538. [Google Scholar] [CrossRef]
- Mann, D.L.; Zipes, D.P.; Libby, P.; Bonow, R.O.; Braunwald, E. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine, 10th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Kusumoto, F.M.; Schoenfeld, M.H.; Barrett, C.; Edgerton, J.R.; Ellenbogen, K.A.; Gold, M.R.; Goldschlager, N.F.; Hamilton, R.M.; Joglar, J.A.; Kim, R.J.; et al. 2018 ACC/AHA/HRS Guideline on the Evaluation and Management of Patients with Bradycardia and Cardiac Conduction Delay: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2019, 140, e382–e482. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Maltsev, V.A.; Vinogradova, T.M. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ. Res. 2010, 106, 659–673. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Lakatta, E.G. The funny current in the context of the coupled-clock pacemaker cell system. Heart Rhythm 2012, 9, 302–307. [Google Scholar] [CrossRef]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 2015, 6, 28. [Google Scholar] [CrossRef]
- Joyner, R.W.; Wilders, R.; Wagner, M.B. Propagation of pacemaker activity. Med. Biol Eng. Comput 2007, 45, 177–187. [Google Scholar] [CrossRef]
- Li, P.; Lines, G.; Maleckar, M.; Tveito, A. Mathematical models of cardiac pacemaking function. Front. Phys. 2013, 1. [Google Scholar] [CrossRef][Green Version]
- Ye, W.; Song, Y.; Huang, Z.; Zhang, Y.; Chen, Y. Genetic Regulation of Sinoatrial Node Development and Pacemaker Program in the Venous Pole. J. Cardiovasc Dev. Dis 2015, 2, 282–298. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.; Hoogaars, W.M.; Prall, O.W.; de Gier-de Vries, C.; Wiese, C.; Clout, D.E.; Papaioannou, V.E.; Brown, N.A.; Harvey, R.P.; Moorman, A.F.; et al. Molecular pathway for the localized formation of the sinoatrial node. Circ. Res. 2007, 100, 354–362. [Google Scholar] [CrossRef]
- Van Eif, V.W.W.; Devalla, H.D.; Boink, G.J.J.; Christoffels, V.M. Transcriptional regulation of the cardiac conduction system. Nat. Rev. Cardiol 2018, 15, 617–630. [Google Scholar] [CrossRef]
- Liang, X.; Evans, S.M.; Sun, Y. Development of the cardiac pacemaker. Cell Mol. Life Sci. 2017, 74, 1247–1259. [Google Scholar] [CrossRef]
- Vedantham, V. New Approaches to Biological Pacemakers: Links to Sinoatrial Node Development. Trends Mol. Med. 2015, 21, 749–761. [Google Scholar] [CrossRef]
- Adler, C.P.; Costabel, U. Cell number in human heart in atrophy, hypertrophy, and under the influence of cytostatics. Recent Adv. Stud. Card. Struct. Metab. 1975, 6, 343–355. [Google Scholar]
- Herget, G.W.; Neuburger, M.; Plagwitz, R.; Adler, C.P. DNA content, ploidy level and number of nuclei in the human heart after myocardial infarction. Cardiovasc. Res. 1997, 36, 45–51. [Google Scholar] [CrossRef]
- Kleber, A.G.; Rudy, Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol. Rev. 2004, 84, 431–488. [Google Scholar] [CrossRef]
- Issa, Z.F.; Miller, J.M.; Zipes, D.P. Molecular Mechanisms of Cardiac Electrical Activity. In Clinical Arrhythmology and Electrophysiology, 3rd ed.; Issa, Z.F., Miller, J.M., Zipes, D.P., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 1–14. [Google Scholar] [CrossRef]
- Kleber, A.G. Gap junctions and conduction of cardiac excitation. Heart Rhythm 2011, 8, 1981–1984. [Google Scholar] [CrossRef]
- Unudurthi, S.D.; Wolf, R.M.; Hund, T.J. Role of sinoatrial node architecture in maintaining a balanced source-sink relationship and synchronous cardiac pacemaking. Front. Physiol. 2014, 5, 446. [Google Scholar] [CrossRef]
- Joyner, R.W.; van Capelle, F.J. Propagation through electrically coupled cells. How a small SA node drives a large atrium. Biophys J. 1986, 50, 1157–1164. [Google Scholar] [CrossRef]
- Bottani, S. Pulse-coupled relaxation oscillators: From biological synchronization to self-organized criticality. Phys. Rev. Lett 1995, 74, 4189–4192. [Google Scholar] [CrossRef]
- Winfree, A.T. Biological rhythms and the behavior of populations of coupled oscillators. J. Biol. 1967, 16, 15–42. [Google Scholar] [CrossRef]
- Mirollo, R.E.; Strogatz, S.H. Synchronization of Pulse-Coupled Biological Oscillators. Siam J. Appl. Math. 1990, 50, 1645–1662. [Google Scholar] [CrossRef]
- Glass, L. Synchronization and rhythmic processes in physiology. Nature 2001, 410, 277–284. [Google Scholar] [CrossRef]
- Monfredi, O.; Tsutsui, K.; Ziman, B.; Stern, M.D.; Lakatta, E.G.; Maltsev, V.A. Electrophysiological heterogeneity of pacemaker cells in the rabbit intercaval region, including the SA node: Insights from recording multiple ion currents in each cell. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H403–H414. [Google Scholar] [CrossRef]
- Kim, M.S.; Maltsev, A.V.; Monfredi, O.; Maltseva, L.A.; Wirth, A.; Florio, M.C.; Tsutsui, K.; Riordon, D.R.; Parsons, S.P.; Tagirova, S.; et al. Heterogeneity of calcium clock functions in dormant, dysrhythmically and rhythmically firing single pacemaker cells isolated from SA node. Cell Calcium. 2018, 74, 168–179. [Google Scholar] [CrossRef]
- Wilders, R.; Jongsma, H.J. Beating irregularity of single pacemaker cells isolated from the rabbit sinoatrial node. Biophys. J. 1993, 65, 2601–2613. [Google Scholar] [CrossRef][Green Version]
- Lyashkov, A.E.; Juhaszova, M.; Dobrzynski, H.; Vinogradova, T.M.; Maltsev, V.A.; Juhasz, O.; Spurgeon, H.A.; Sollott, S.J.; Lakatta, E.G. Calcium Cycling Protein Density and Functional Importance to Automaticity of Isolated Sinoatrial Nodal Cells Are Independent of Cell Size. Circ. Res. 2007, 100, 1723–1731. [Google Scholar] [CrossRef]
- Bleeker, W.K.; Mackaay, A.J.; Masson-Pevet, M.; Bouman, L.N.; Becker, A.E. Functional and morphological organization of the rabbit sinus node. Circ. Res. 1980, 46, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Clay, J.R.; DeHaan, R.L. Fluctuations in interbeat interval in rhythmic heart-cell clusters. Role of membrane voltage noise. Biophys. J. 1979, 28, 377–389. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Peron, T.K.D.; Ji, P.; Kurths, J. The Kuramoto model in complex networks. Phys. Rep. 2016, 610, 1–98. [Google Scholar] [CrossRef]
- Kuramoto, Y. Mutual Entrainment. In Chemical Oscillations, Waves, and Turbulence; Springer: Berlin/Heidelberg, Germany, 1984; pp. 60–88. [Google Scholar] [CrossRef]
- Jalife, J. Mutual entrainment and electrical coupling as mechanisms for synchronous firing of rabbit sino-atrial pace-maker cells. J. Physiol. 1984, 356, 221–243. [Google Scholar] [CrossRef]
- Anumonwo, J.M.; Delmar, M.; Vinet, A.; Michaels, D.C.; Jalife, J. Phase resetting and entrainment of pacemaker activity in single sinus nodal cells. Circ. Res. 1991, 68, 1138–1153. [Google Scholar] [CrossRef]
- Verheijck, E.E.; Wilders, R.; Joyner, R.W.; Golod, D.A.; Kumar, R.; Jongsma, H.J.; Bouman, L.N.; van Ginneken, A.C. Pacemaker synchronization of electrically coupled rabbit sinoatrial node cells. J. Gen. Physiol. 1998, 111, 95–112. [Google Scholar] [CrossRef]
- Cai, D.; Winslow, R.L.; Noble, D. Effects of gap junction conductance on dynamics of sinoatrial node cells: Two-cell and large-scale network models. IEEE Trans. Biomed. Eng. 1994, 41, 217–231. [Google Scholar] [CrossRef]
- Van Meerwijk, W.P.; de Bruin, G.; Van Ginneken, C.G.; Van Hartevelt, J.; Jongsma, H.J.; Kruyt, E.W.; Scott, S.S.; Ypey, D.L. Phase resetting properties of cardiac pacemaker cells. J. Gen. Physiol. 1984, 83, 613–629. [Google Scholar] [CrossRef]
- Michaels, D.C.; Matyas, E.P.; Jalife, J. Dynamic interactions and mutual synchronization of sinoatrial node pacemaker cells. A mathematical model. Circ. Res. 1986, 58, 706–720. [Google Scholar] [CrossRef]
- Nicolás Mata, A.; Román Alonso, G.; López Garza, G.; Godinez Fernández, J.R.; Castro García, M.A.; Castellanos Ábrego, N.P. Parallel simulation of the synchronization of heterogeneous cells in the sinoatrial node. Concurr. Comput. Pract. Exp. 2020, 32, e5317. [Google Scholar] [CrossRef]
- Michaels, D.C.; Matyas, E.P.; Jalife, J. Mechanisms of sinoatrial pacemaker synchronization: A new hypothesis. Circ. Res. 1987, 61, 704–714. [Google Scholar] [CrossRef]
- Gratz, D.; Onal, B.; Dalic, A.; Hund, T.J. Synchronization of Pacemaking in the Sinoatrial Node: A Mathematical Modeling Study. Front. Phys. 2018, 6. [Google Scholar] [CrossRef]
- Ly, C.; Weinberg, S.H. Analysis of heterogeneous cardiac pacemaker tissue models and traveling wave dynamics. J. Biol. 2018, 459, 18–35. [Google Scholar] [CrossRef]
- Karpaev, A.A.; Syunyaev, R.A.; Aliev, R.R. Effects of fibroblast-myocyte coupling on the sinoatrial node activity: A computational study. Int. J. Numer. Method Biomed. Eng. 2018, 34, e2966. [Google Scholar] [CrossRef]
- Sehgal, S.; Patel, N.D.; Malik, A.; Roop, P.S.; Trew, M.L. Resonant model-A new paradigm for modeling an action potential of biological cells. PLoS ONE 2019, 14, e0216999. [Google Scholar] [CrossRef]
- Opthof, T. The mammalian sinoatrial node. Cardiovasc. Drugs Ther. 1988, 1, 573–597. [Google Scholar] [CrossRef]
- Lev, M. Aging changes in the human sinoatrial node. J. Gerontol. 1954, 9, 1–9. [Google Scholar] [CrossRef]
- James, T.N. Anatomy of the human sinus node. Anat. Rec. 1961, 141, 109–139. [Google Scholar] [CrossRef]
- Truex, R.C.; Smythe, M.Q.; Taylor, M.J. Reconstruction of the human sinoatrial node. Anat. Rec. 1967, 159, 371–378. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, Y.; Zhang, X.; Nirmalan, M.; Davies, L.; Konstantinou, D.; Yin, F.; Dobrzynski, H.; Wang, X.; Grace, A.; et al. TGF-β1-mediated fibrosis and ion channel remodeling are key mechanisms in producing the sinus node dysfunction associated with SCN5A deficiency and aging. Circ. Arrhythm. Electrophysiol. 2011, 4, 397–406. [Google Scholar] [CrossRef]
- Glukhov, A.V.; Kalyanasundaram, A.; Lou, Q.; Hage, L.T.; Hansen, B.J.; Belevych, A.E.; Mohler, P.J.; Knollmann, B.C.; Periasamy, M.; Györke, S.; et al. Calsequestrin 2 deletion causes sinoatrial node dysfunction and atrial arrhythmias associated with altered sarcoplasmic reticulum calcium cycling and degenerative fibrosis within the mouse atrial pacemaker complex1. Eur. Heart J. 2015, 36, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Opthof, T.; de Jonge, B.; Masson-Pevet, M.; Jongsma, H.J.; Bouman, L.N. Functional and morphological organization of the cat sinoatrial node. J. Mol. Cell. Cardiol. 1986, 18, 1015–1031. [Google Scholar] [CrossRef]
- Shiraishi, I.; Takamatsu, T.; Minamikawa, T.; Onouchi, Z.; Fujita, S. Quantitative histological analysis of the human sinoatrial node during growth and aging. Circulation 1992, 85, 2176–2184. [Google Scholar] [CrossRef] [PubMed]
- Kohl, P.; Kamkin, A.G.; Kiseleva, I.S.; Noble, D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: Interaction with cardiomyocytes and possible role. Exp. Physiol. 1994, 79, 943–956. [Google Scholar] [CrossRef]
- Oren, R.V.; Clancy, C.E. Determinants of heterogeneity, excitation and conduction in the sinoatrial node: A model study. PLoS Comput. Biol. 2010, 6, e1001041. [Google Scholar] [CrossRef]
- Miragoli, M.; Gaudesius, G.; Rohr, S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ. Res. 2006, 98, 801–810. [Google Scholar] [CrossRef]
- Fahrenbach, J.P.; Mejia-Alvarez, R.; Banach, K. The relevance of non-excitable cells for cardiac pacemaker function. J. Physiol. 2007, 585, 565–578. [Google Scholar] [CrossRef]
- Miragoli, M.; Salvarani, N.; Rohr, S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ. Res. 2007, 101, 755–758. [Google Scholar] [CrossRef]
- Camelliti, P. Fibroblast Network in Rabbit Sinoatrial Node: Structural and Functional Identification of Homogeneous and Heterogeneous Cell Coupling. Circ. Res. 2004, 94, 828–835. [Google Scholar] [CrossRef]
- Gaudesius, G.; Miragoli, M.; Thomas, S.P.; Rohr, S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ. Res. 2003, 93, 421–428. [Google Scholar] [CrossRef]
- De Mazière, A.M.G.L.; van Ginneken, A.C.G.; Wilders, R.; Jongsma, H.J.; Bouman, L.N. Spatial and functional relationship between myocytes and fibroblasts in the rabbit sinoatrial node. J. Mol. Cell. Cardiol. 1992, 24, 567–578. [Google Scholar] [CrossRef]
- Joyner, R.W.; Veenstra, R.; Rawling, D.; Chorro, A. Propagation through electrically coupled cells. Effects of a resistive barrier. Biophys. J. 1984, 45, 1017–1025. [Google Scholar] [CrossRef]
- Joyner, R.W. Effects of the discrete pattern of electrical coupling on propagation through an electrical syncytium. Circ. Res. 1982, 50, 192–200. [Google Scholar] [CrossRef]
- Rohr, S.; Kucera, J.P.; Fast, V.G.; Kleber, A.G. Paradoxical improvement of impulse conduction in cardiac tissue by partial cellular uncoupling. Science 1997, 275, 841–844. [Google Scholar] [CrossRef]
- Huang, X.; Cui, X. The Functions of Atrial Strands Interdigitating with and Penetrating into Sinoatrial Node: A Theoretical Study of the Problem. PLoS ONE 2015, 10, e0118623. [Google Scholar] [CrossRef][Green Version]
- Anumonwo, J.M.; Wang, H.Z.; Trabka-Janik, E.; Dunham, B.; Veenstra, R.D.; Delmar, M.; Jalife, J. Gap junctional channels in adult mammalian sinus nodal cells. Immunolocalization and electrophysiology. Circ. Res. 1992, 71, 229–239. [Google Scholar] [CrossRef]
- Verheule, S.; van Batenburg, C.A.; Coenjaerts, F.E.; Kirchhoff, S.; Willecke, K.; Jongsma, H.J. Cardiac conduction abnormalities in mice lacking the gap junction protein connexin40. J. Cardiovasc. Electrophysiol. 1999, 10, 1380–1389. [Google Scholar] [CrossRef]
- Boyett, M.R.; Honjo, H.; Kodama, I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc. Res. 2000, 47, 658–687. [Google Scholar] [CrossRef]
- Verheule, S.; van Kempen, M.J.; te Welscher, P.H.; Kwak, B.R.; Jongsma, H.J. Characterization of gap junction channels in adult rabbit atrial and ventricular myocardium. Circ. Res. 1997, 80, 673–681. [Google Scholar] [CrossRef]
- Jongsma, H.J.; Wilders, R. Gap Junctions in Cardiovascular Disease. Circ. Res. 2000, 86, 1193–1197. [Google Scholar] [CrossRef]
- Ten Velde, I.; de Jonge, B.; Verheijck, E.E.; van Kempen, M.J.; Analbers, L.; Gros, D.; Jongsma, H.J. Spatial distribution of connexin43, the major cardiac gap junction protein, visualizes the cellular network for impulse propagation from sinoatrial node to atrium. Circ. Res. 1995, 76, 802–811. [Google Scholar] [CrossRef]
- Lovell, N.H.; Cloherty, S.L.; Celler, B.G.; Dokos, S. A gradient model of cardiac pacemaker myocytes. Prog. Biophys. Mol. Biol. 2004, 85, 301–323. [Google Scholar] [CrossRef]
- Inada, S.; Zhang, H.; Tellez, J.O.; Shibata, N.; Nakazawa, K.; Kamiya, K.; Kodama, I.; Mitsui, K.; Dobrzynski, H.; Boyett, M.R.; et al. Importance of gradients in membrane properties and electrical coupling in sinoatrial node pacing. PLoS ONE 2014, 9, e94565. [Google Scholar] [CrossRef]
- Zhang, H.; Holden, A.V.; Boyett, M.R. Gradient model versus mosaic model of the sinoatrial node. Circulation 2001, 103, 584–588. [Google Scholar] [CrossRef]
- López Garza, G.; Castellanos, N.P.; Godínez, R. Cell-to-cell modeling of the interface between atrial and sinoatrial anisotropic heterogeneous nets. Comput. Biol. Chem. 2017, 68, 245–259. [Google Scholar] [CrossRef]
- Bromberg, B.I.; Hand, D.E.; Schuessler, R.B.; Boineau, J.P. Primary negativity does not predict dominant pacemaker location: Implications for sinoatrial conduction. Am. J. Physiol. 1995, 269, H877–H887. [Google Scholar] [CrossRef]
- Schuessler, R.B. Abnormal sinus node function in clinical arrhythmias. J. Cardiovasc. Electrophysiol. 2003, 14, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Glukhov, A.V.; Chang, R.; Kostecki, G.; Aferol, H.; Hucker, W.J.; Wuskell, J.P.; Loew, L.M.; Schuessler, R.B.; Moazami, N.; et al. Optical mapping of the isolated coronary-perfused human sinus node. J. Am. Coll. Cardiol. 2010, 56, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, V.V.; Schuessler, R.B.; Hemphill, M.; Ambrosi, C.M.; Chang, R.; Voloshina, A.S.; Brown, K.; Hucker, W.J.; Efimov, I.R. Structural and functional evidence for discrete exit pathways that connect the canine sinoatrial node and atria. Circ. Res. 2009, 104, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, A.; Li, N.; Hansen, B.J.; Zhao, J.; Fedorov, V.V. Canine and human sinoatrial node: Differences and similarities in the structure, function, molecular profiles, and arrhythmia. J. Vet. Cardiol. 2019, 22, 2–19. [Google Scholar] [CrossRef] [PubMed]
- Csepe, T.A.; Zhao, J.; Hansen, B.J.; Li, N.; Sul, L.V.; Lim, P.; Wang, Y.; Simonetti, O.P.; Kilic, A.; Mohler, P.J.; et al. Human sinoatrial node structure: 3D microanatomy of sinoatrial conduction pathways. Prog. Biophys. Mol. Biol. 2016, 120, 164–178. [Google Scholar] [CrossRef]
- Li, N.; Hansen, B.J.; Csepe, T.A.; Zhao, J.; Ignozzi, A.J.; Sul, L.V.; Zakharkin, S.O.; Kalyanasundaram, A.; Davis, J.P.; Biesiadecki, B.J.; et al. Redundant and diverse intranodal pacemakers and conduction pathways protect the human sinoatrial node from failure. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef]
- Lou, Q.; Hansen, B.J.; Fedorenko, O.; Csepe, T.A.; Kalyanasundaram, A.; Li, N.; Hage, L.T.; Glukhov, A.V.; Billman, G.E.; Weiss, R.; et al. Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping. Circulation 2014, 130, 315–324. [Google Scholar] [CrossRef]
- Ho, S.Y.; Sánchez-Quintana, D. Anatomy and pathology of the sinus node. J. Interv. Card. Electrophysiol. 2016, 46, 3–8. [Google Scholar] [CrossRef]
- Chandler, N.J.; Greener, I.D.; Tellez, J.O.; Inada, S.; Musa, H.; Molenaar, P.; Difrancesco, D.; Baruscotti, M.; Longhi, R.; Anderson, R.H.; et al. Molecular architecture of the human sinus node: Insights into the function of the cardiac pacemaker. Circulation 2009, 119, 1562–1575. [Google Scholar] [CrossRef]
- Sánchez-Quintana, D.; Cabrera, J.A.; Farré, J.; Climent, V.; Anderson, R.H.; Ho, S.Y. Sinus node revisited in the era of electroanatomical mapping and catheter ablation. Heart 2005, 91, 189–194. [Google Scholar] [CrossRef]
- Matsuyama, T.-A.; Inoue, S.; Kobayashi, Y.; Sakai, T.; Saito, T.; Katagiri, T.; Ota, H. Anatomical diversity and age-related histological changes in the human right atrial posterolateral wall. EP Eur. 2004, 6, 307–315. [Google Scholar] [CrossRef]
- Monfredi, O.; Dobrzynski, H.; Mondal, T.; Boyett, M.R.; Morris, G.M. The anatomy and physiology of the sinoatrial node—A contemporary review. Pacing Clin. Electrophysiol. 2010, 33, 1392–1406. [Google Scholar] [CrossRef]
- Kharche, S.R.; Vigmond, E.; Efimov, I.R.; Dobrzynski, H. Computational assessment of the functional role of sinoatrial node exit pathways in the human heart. PLoS ONE 2017, 12, e0183727. [Google Scholar] [CrossRef]
- Kamino, K.; Hirota, A.; Fujii, S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature 1981, 290, 595–597. [Google Scholar] [CrossRef]
- Van Mierop, L.H. Location of pacemaker in chick embryo heart at the time of initiation of heartbeat. Am. J. Physiol. 1967, 212, 407–415. [Google Scholar] [CrossRef]
- Bressan, M.; Liu, G.; Mikawa, T. Early mesodermal cues assign avian cardiac pacemaker fate potential in a tertiary heart field. Science 2013, 340, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Patten, B.M. Initiation and early changes in the character of the heart beat in vertebrate embryos. Physiol. Rev. 1949, 29, 31–47. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.; Dominguez, J.N.; Wiese, C.; Norden, J.; de Gier-de Vries, C.; Burch, J.B.; Kispert, A.; Brown, N.A.; Moorman, A.F.; Christoffels, V.M. The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc. Res. 2010, 87, 92–101. [Google Scholar] [CrossRef]
- Dominguez, J.N.; Meilhac, S.M.; Bland, Y.S.; Buckingham, M.E.; Brown, N.A. Asymmetric fate of the posterior part of the second heart field results in unexpected left/right contributions to both poles of the heart. Circ. Res. 2012, 111, 1323–1335. [Google Scholar] [CrossRef]
- Wiese, C.; Grieskamp, T.; Airik, R.; Mommersteeg, M.T.; Gardiwal, A.; de Gier-de Vries, C.; Schuster-Gossler, K.; Moorman, A.F.; Kispert, A.; Christoffels, V.M. Formation of the sinus node head and differentiation of sinus node myocardium are independently regulated by Tbx18 and Tbx3. Circ. Res. 2009, 104, 388–397. [Google Scholar] [CrossRef]
- Hoogaars, W.M.; Engel, A.; Brons, J.F.; Verkerk, A.O.; de Lange, F.J.; Wong, L.Y.; Bakker, M.L.; Clout, D.E.; Wakker, V.; Barnett, P.; et al. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007, 21, 1098–1112. [Google Scholar] [CrossRef]
- Blaschke, R.J.; Hahurij, N.D.; Kuijper, S.; Just, S.; Wisse, L.J.; Deissler, K.; Maxelon, T.; Anastassiadis, K.; Spitzer, J.; Hardt, S.E.; et al. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation 2007, 115, 1830–1838. [Google Scholar] [CrossRef]
- Ye, W.; Wang, J.; Song, Y.; Yu, D.; Sun, C.; Liu, C.; Chen, F.; Zhang, Y.; Wang, F.; Harvey, R.P.; et al. A common Shox2-Nkx2-5 antagonistic mechanism primes the pacemaker cell fate in the pulmonary vein myocardium and sinoatrial node. Development 2015, 142, 2521–2532. [Google Scholar] [CrossRef]
- Espinoza-Lewis, R.A.; Yu, L.; He, F.; Liu, H.; Tang, R.; Shi, J.; Sun, X.; Martin, J.F.; Wang, D.; Yang, J.; et al. Shox2 is essential for the differentiation of cardiac pacemaker cells by repressing Nkx2-5. Dev. Biol. 2009, 327, 376–385. [Google Scholar] [CrossRef]
- Bressan, M.; Henley, T.; Louie, J.D.; Liu, G.; Christodoulou, D.; Bai, X.; Taylor, J.; Seidman, C.E.; Seidman, J.G.; Mikawa, T. Dynamic Cellular Integration Drives Functional Assembly of the Heart’s Pacemaker Complex. CELREP 2018, 23, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Steijn, R.; Kolditz, D.P.; Mahtab, E.A.F.; Askar, S.F.A.; Bax, N.A.M.; van der Graaf, L.M.; Wisse, L.J.; Passier, R.; Pijnappels, D.A.; Schalij, M.J.; et al. Electrical Activation of Sinus Venosus Myocardium and Expression Patterns of RhoA and Isl-1 in the Chick Embryo. J. Cardiovasc. Electrophysiol. 2010, 21, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Steijn, R.; Passier, R.; Wisse, L.J.; Schalij, M.J.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; Jongbloed, M.R. Funny current channel HCN4 delineates the developing cardiac conduction system in chicken heart. Heart Rhythm 2011, 8, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.; Paes de Carvalho, A. The Electrophysiological Organization of the Embryonic Chick Heart. J. Gen. Physiol. 1965, 49, 351–363. [Google Scholar] [CrossRef]
- Sperelakis, N.; Shigenobu, K. Changes in membrane properties of chick embryonic hearts during development. J. Gen. Physiol. 1972, 60, 430–453. [Google Scholar] [CrossRef]
- Bressan, M.; Yang, P.B.; Louie, J.D.; Navetta, A.M.; Garriock, R.J.; Mikawa, T. Reciprocal myocardial-endocardial interactions pattern the delay in atrioventricular junction conduction. Development 2014, 141, 4149–4157. [Google Scholar] [CrossRef]
- Williams, E.H.; DeHaan, R.L. Electrical coupling among heart cells in the absence of ultrastructurally defined gap junctions. J. Membr. Biol. 1981, 60, 237–248. [Google Scholar] [CrossRef]
- Gros, D.; Mocquard, J.P.; Challice, C.E.; Schrevel, J. Formation and growth of gap junctions in mouse myocardium during ontogenesis: Quantitative data and their implications on the development of intercellular communication. J. Mol. Cell. Cardiol. 1979, 11, 543–554. [Google Scholar] [CrossRef]
- Christoffels, V.M.; Mommersteeg, M.T.; Trowe, M.O.; Prall, O.W.; de Gier-de Vries, C.; Soufan, A.T.; Bussen, M.; Schuster-Gossler, K.; Harvey, R.P.; Moorman, A.F.; et al. Formation of the venous pole of the heart from an Nkx2-5-negative precursor population requires Tbx18. Circ. Res. 2006, 98, 1555–1563. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.L.; Chen, J.; Evans, S.M. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007, 304, 286–296. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Q.; Cattaneo, P.; Zhuang, S.; Gong, X.; Spann, N.J.; Jiang, C.; Cao, X.; Zhao, X.; Zhang, X.; et al. Transcription factor ISL1 is essential for pacemaker development and function. J. Clin. Investig. 2015, 125, 3256–3268. [Google Scholar] [CrossRef]
- Manner, J.; Merkel, N. Early morphogenesis of the sinuatrial region of the chick heart: A contribution to the understanding of the pathogenesis of direct pulmonary venous connections to the right atrium and atrial septal defects in hearts with right isomerism of the atrial appendages. Anat. Rec. 2007, 290, 168–180. [Google Scholar] [CrossRef]
- Quiring, D.P. The development of the sino-atrial region of the chick heart. J. Morphol. 1933, 55, 81–118. [Google Scholar] [CrossRef]
- Niderla-BieliŃska, J.; Jankowska-Steifer, E.; Flaht-Zabost, A.; Gula, G.; Czarnowska, E.; Ratajska, A. Proepicardium: Current Understanding of its Structure, Induction, and Fate. Anat. Rec. 2019, 302, 893–903. [Google Scholar] [CrossRef]
- Maya-Ramos, L.; Cleland, J.; Bressan, M.; Mikawa, T. Induction of the Proepicardium. J. Dev. Biol. 2013, 1, 82–91. [Google Scholar] [CrossRef]
- Schlueter, J.; Brand, T. Subpopulation of Proepicardial Cells Is Derived From the Somatic Mesoderm in the Chick Embryo. Circ. Res. 2013, 113, 1128–1137. [Google Scholar] [CrossRef]
- Dueñas, A.; Aranega, A.E.; Franco, D. More than Just a Simple Cardiac Envelope; Cellular Contributions of the Epicardium. Front. Cell Dev. Biol. 2017, 5. [Google Scholar] [CrossRef]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. 1951. Dev. Dyn. 1992, 195, 231–272. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Qu, Z.; Weiss, J.N. Cardiac fibrosis and arrhythmogenesis: The road to repair is paved with perils. J. Mol. Cell Cardiol. 2014, 70, 83–91. [Google Scholar] [CrossRef]
- Tveito, A.; Lines, G.T. A condition for setting off ectopic waves in computational models of excitable cells. Math. Biosci. 2008, 213, 141–150. [Google Scholar] [CrossRef]
- Xie, Y.; Sato, D.; Garfinkel, A.; Qu, Z.; Weiss, J.N. So little source, so much sink: Requirements for afterdepolarizations to propagate in tissue. Biophys. J. 2010, 99, 1408–1415. [Google Scholar] [CrossRef]
- Fast, V.G.; Kléber, A.G. Block of impulse propagation at an abrupt tissue expansion: Evaluation of the critical strand diameter in 2- and 3-dimensional computer models. Cardiovasc. Res. 1995, 30, 449–459. [Google Scholar] [CrossRef]
- Rohr, S.; Salzberg, B.M. Characterization of impulse propagation at the microscopic level across geometrically defined expansions of excitable tissue: Multiple site optical recording of transmembrane voltage (MSORTV) in patterned growth heart cell cultures. J. Gen. Physiol. 1994, 104, 287–309. [Google Scholar] [CrossRef]
- Li, H.; Li, D.; Wang, Y.; Huang, Z.; Xu, J.; Yang, T.; Wang, L.; Tang, Q.; Cai, C.L.; Huang, H.; et al. Nkx2-5 defines a subpopulation of pacemaker cells and is essential for the physiological function of the sinoatrial node in mice. Development 2019, 146. [Google Scholar] [CrossRef]
- Vedantham, V.; Galang, G.; Evangelista, M.; Deo, R.C.; Srivastava, D. RNA sequencing of mouse sinoatrial node reveals an upstream regulatory role for Islet-1 in cardiac pacemaker cells. Circ. Res. 2015, 116, 797–803. [Google Scholar] [CrossRef]
- Van Eif, V.W.W.; Stefanovic, S.; van Duijvenboden, K.; Bakker, M.; Wakker, V.; de Gier-de Vries, C.; Zaffran, S.; Verkerk, A.O.; Boukens, B.J.; Christoffels, V.M. Transcriptome analysis of mouse and human sinoatrial node cells reveals a conserved genetic program. Development 2019, 146, dev173161-173129. [Google Scholar] [CrossRef]
- Goodyer, W.R.; Beyersdorf, B.M.; Paik, D.T.; Tian, L.; Li, G.; Buikema, J.W.; Chirikian, O.; Choi, S.; Venkatraman, S.; Adams, E.L.; et al. Transcriptomic Profiling of the Developing Cardiac Conduction System at Single-Cell Resolution. Circ. Res. 2019, 125, 379–397. [Google Scholar] [CrossRef]
- Linscheid, N.; Logantha, S.J.R.J.; Poulsen, P.C.; Zhang, S.; Schrölkamp, M.; Egerod, K.L.; Thompson, J.J.; Kitmitto, A.; Galli, G.; Humphries, M.J.; et al. Quantitative proteomics and single-nucleus transcriptomics of the sinus node elucidates the foundation of cardiac pacemaking. Nat. Commun. 2019, 10, 1–19. [Google Scholar] [CrossRef]
- Brennan, J.A.; Chen, Q.; Gams, A.; Dyavanapalli, J.; Mendelowitz, D.; Peng, W.; Efimov, I.R. Evidence of Superior and Inferior Sinoatrial Nodes in the Mammalian Heart. JACC Clin. Electrophysiol. 2020, 6, 1827–1840. [Google Scholar] [CrossRef]
- Kruithof, B.P.; Duim, S.N.; Moerkamp, A.T.; Goumans, M.J. TGFβ and BMP signaling in cardiac cushion formation: Lessons from mice and chicken. Differentiation 2012, 84, 89–102. [Google Scholar] [CrossRef]
- Dronkers, E.; Wauters, M.M.M.; Goumans, M.J.; Smits, A.M. Epicardial TGFβ and BMP Signaling in Cardiac Regeneration: What Lesson Can We Learn from the Developing Heart? Biomolecules 2020, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Garside, V.C.; Chang, A.C.; Karsan, A.; Hoodless, P.A. Co-ordinating Notch, BMP, and TGF-β signaling during heart valve development. Cell. Mol. Life Sci. 2013, 70, 2899–2917. [Google Scholar] [CrossRef] [PubMed]
- Euler-Taimor, G.; Heger, J. The complex pattern of SMAD signaling in the cardiovascular system. Cardiovasc. Res. 2006, 69, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.J.; Doetschman, T.; Azhar, M. The Inter-Relationship of Periostin, TGFβ, and BMP in Heart Valve Development and Valvular Heart Diseases. Sci. World J. 2011, 11, 574370. [Google Scholar] [CrossRef]
- Van Wijk, B.; Moorman, A.F.M.; van den Hoff, M.J.B. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovasc. Res. 2007, 74, 244–255. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. The Role of the TGF-β Superfamily in Myocardial Infarction. Front. Cardiovasc. Med. 2019, 6. [Google Scholar] [CrossRef]
- Jiao, K.; Langworthy, M.; Batts, L.; Brown, C.B.; Moses, H.L.; Baldwin, H.S. Tgfbeta signaling is required for atrioventricular cushion mesenchyme remodeling during in vivo cardiac development. Development 2006, 133, 4585–4593. [Google Scholar] [CrossRef][Green Version]
- Morabito, C.J.; Dettman, R.W.; Kattan, J.; Collier, J.M.; Bristow, J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev. Biol. 2001, 234, 204–215. [Google Scholar] [CrossRef]
- Person, A.D.; Klewer, S.E.; Runyan, R.B. Cell biology of cardiac cushion development. Int. Rev. Cytol 2005, 243, 287–335. [Google Scholar] [CrossRef]
- Ishii, Y.; Garriock, R.J.; Navetta, A.M.; Coughlin, L.E.; Mikawa, T. BMP Signals Promote Proepicardial Protrusion Necessary for Recruitment of Coronary Vessel and Epicardial Progenitors to the Heart. Dev. Cell 2010, 19, 307–316. [Google Scholar] [CrossRef]
- Hill, C.R.; Sanchez, N.S.; Love, J.D.; Arrieta, J.A.; Hong, C.C.; Brown, C.B.; Austin, A.F.; Barnett, J.V. BMP2 signals loss of epithelial character in epicardial cells but requires the Type III TGFβ receptor to promote invasion. Cell. Signal. 2012, 24, 1012–1022. [Google Scholar] [CrossRef][Green Version]
- Craig, E.A.; Austin, A.F.; Vaillancourt, R.R.; Barnett, J.V.; Camenisch, T.D. TGFβ2-mediated production of hyaluronan is important for the induction of epicardial cell differentiation and invasion. Exp. Cell Res. 2010, 316, 3397–3405. [Google Scholar] [CrossRef]
- De Laughter, D.M.; Clark, C.R.; Christodoulou, D.C.; Seidman, C.E.; Baldwin, H.S.; Seidman, J.G.; Barnett, J.V. Transcriptional Profiling of Cultured, Embryonic Epicardial Cells Identifies Novel Genes and Signaling Pathways Regulated by TGFβR3 In Vitro. PLoS ONE 2016, 11, e0159710. [Google Scholar] [CrossRef]
- Gluck, J.M.; Herren, A.W.; Yechikov, S.; Kao, H.K.J.; Khan, A.; Phinney, B.S.; Chiamvimonvat, N.; Chan, J.W.; Lieu, D.K. Biochemical and biomechanical properties of the pacemaking sinoatrial node extracellular matrix are distinct from contractile left ventricular matrix. PLoS ONE 2017, 12, e0185125. [Google Scholar] [CrossRef]
- Yanni, J.; Tellez, J.O.; Sutyagin, P.V.; Boyett, M.R.; Dobrzynski, H. Structural remodelling of the sinoatrial node in obese old rats. J. Mol. Cell. Cardiol. 2010, 48, 653–662. [Google Scholar] [CrossRef]
- De Laughter, D.M.; Christodoulou, D.C.; Robinson, J.Y.; Seidman, C.E.; Baldwin, H.S.; Seidman, J.G.; Barnett, J.V. Spatial transcriptional profile of the chick and mouse endocardial cushions identify novel regulators of endocardial EMT in vitro. J. Mol. Cell. Cardiol. 2013, 59, 196–204. [Google Scholar] [CrossRef]
- Schroeder, J.A.; Jackson, L.F.; Lee, D.C.; Camenisch, T.D. Form and function of developing heart valves: Coordination by extracellular matrix and growth factor signaling. J. Mol. Med. 2003, 81, 392–403. [Google Scholar] [CrossRef]
- Singh, R.; Hoogaars, W.M.; Barnett, P.; Grieskamp, T.; Rana, M.S.; Buermans, H.; Farin, H.F.; Petry, M.; Heallen, T.; Martin, J.F.; et al. Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation. Cell Mol. Life Sci. 2012, 69, 1377–1389. [Google Scholar] [CrossRef]
- Bujak, M.; Frangogiannis, N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007, 74, 184–195. [Google Scholar] [CrossRef]
- Mezzano, V.; Liang, Y.; Wright, A.T.; Lyon, R.C.; Pfeiffer, E.; Song, M.Y.; Gu, Y.; Dalton, N.D.; Scheinman, M.; Peterson, K.L.; et al. Desmosomal junctions are necessary for adult sinus node function. Cardiovasc. Res. 2016, 111, 274–286. [Google Scholar] [CrossRef]
- Desmoulière, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Wang, S.; Sun, A.; Li, L.; Zhao, G.; Jia, J.; Wang, K.; Ge, J.; Zou, Y. Up-regulation of BMP-2 antagonizes TGF-β1/ROCK-enhanced cardiac fibrotic signalling through activation of Smurf1/Smad6 complex. J. Cell Mol. Med. 2012, 16, 2301–2310. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Jiang, B.; Liu, D. Bone Morphogenetic Protein-7 Antagonizes Myocardial Fibrosis Induced by Atrial Fibrillation by Restraining Transforming Growth Factor-β (TGF-β)/Smads Signaling. Med. Sci. Monit. 2016, 22, 3457–3468. [Google Scholar] [CrossRef]
- Morine, K.J.; Qiao, X.; York, S.; Natov, P.S.; Paruchuri, V.; Zhang, Y.; Aronovitz, M.J.; Karas, R.H.; Kapur, N.K. Bone Morphogenetic Protein 9 Reduces Cardiac Fibrosis and Improves Cardiac Function in Heart Failure. Circulation 2018, 138, 513–526. [Google Scholar] [CrossRef]
- Greenspon, A.J.; Patel, J.D.; Lau, E.; Ochoa, J.A.; Frisch, D.R.; Ho, R.T.; Pavri, B.B.; Kurtz, S.M. Trends in Permanent Pacemaker Implantation in the United States From 1993 to 2009. J. Am. Coll. Cardiol. 2012, 60, 1540–1545. [Google Scholar] [CrossRef]
- Mond, H.G.; Proclemer, A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: Calendar year 2009—A World Society of Arrhythmia’s project. Pacing Clin. Electrophysiol. 2011, 34, 1013–1027. [Google Scholar] [CrossRef]
- Miake, J.; Marbán, E.; Nuss, H.B. Biological pacemaker created by gene transfer. Nature 2002, 419, 132–133. [Google Scholar] [CrossRef]
- Cingolani, E. Biological pacemakers: Ready for the clinic? Trends Cardiovasc. Med. 2015, 25, 674–675. [Google Scholar] [CrossRef]
- Cingolani, E.; Goldhaber, J.I.; Marbán, E. Next-generation pacemakers: From small devices to biological pacemakers. Nat. Rev. Cardiol. 2017, 15, 139–150. [Google Scholar] [CrossRef]
- Grijalva, S.I.; Gu, J.M.; Li, J.; Fernandez, N.; Fan, J.; Sung, J.H.; Lee, S.Y.; Herndon, C.; Buckley, E.M.; Park, S.J.; et al. Engineered Cardiac Pacemaker Nodes Created by TBX18 Gene Transfer Overcome Source-Sink Mismatch. Adv. Sci. 2019, 6, 1901099. [Google Scholar] [CrossRef]
- Boink, G.J.J.; Verkerk, A.O.; van Amersfoorth, S.C.M.; Tasseron, S.J.; van der Rijt, R.; Bakker, D.; Linnenbank, A.C.; van der Meulen, J.; de Bakker, J.M.T.; Seppen, J.; et al. Engineering physiologically controlled pacemaker cells with lentiviral HCN4 gene transfer. J. Gene Med. 2008, 10, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Kashiwakura, Y.; Cho, H.C.; Barth, A.S.; Azene, E.; Marbán, E. Gene transfer of a synthetic pacemaker channel into the heart: A novel strategy for biological pacing. Circulation 2006, 114, 1682–1686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hashem, S.I.; Claycomb, W.C. Genetic isolation of stem cell-derived pacemaker-nodal cardiac myocytes. Mol. Cell. Biochem. 2013, 383, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Chauveau, S.; Brink, P.R.; Cohen, I.S. Stem cell-based biological pacemakers from proof of principle to therapy: A review. J. Cytotherapy 2014, 16, 873–880. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Easterling, M.; Rossi, S.; Mazzella, A.J.; Bressan, M. Assembly of the Cardiac Pacemaking Complex: Electrogenic Principles of Sinoatrial Node Morphogenesis. J. Cardiovasc. Dev. Dis. 2021, 8, 40. https://doi.org/10.3390/jcdd8040040
Easterling M, Rossi S, Mazzella AJ, Bressan M. Assembly of the Cardiac Pacemaking Complex: Electrogenic Principles of Sinoatrial Node Morphogenesis. Journal of Cardiovascular Development and Disease. 2021; 8(4):40. https://doi.org/10.3390/jcdd8040040
Chicago/Turabian StyleEasterling, Marietta, Simone Rossi, Anthony J Mazzella, and Michael Bressan. 2021. "Assembly of the Cardiac Pacemaking Complex: Electrogenic Principles of Sinoatrial Node Morphogenesis" Journal of Cardiovascular Development and Disease 8, no. 4: 40. https://doi.org/10.3390/jcdd8040040
APA StyleEasterling, M., Rossi, S., Mazzella, A. J., & Bressan, M. (2021). Assembly of the Cardiac Pacemaking Complex: Electrogenic Principles of Sinoatrial Node Morphogenesis. Journal of Cardiovascular Development and Disease, 8(4), 40. https://doi.org/10.3390/jcdd8040040





