Muscularization of the Mesenchymal Outlet Septum during Cardiac Development
Abstract
1. Introduction
2. Septation of the Arterial Pole
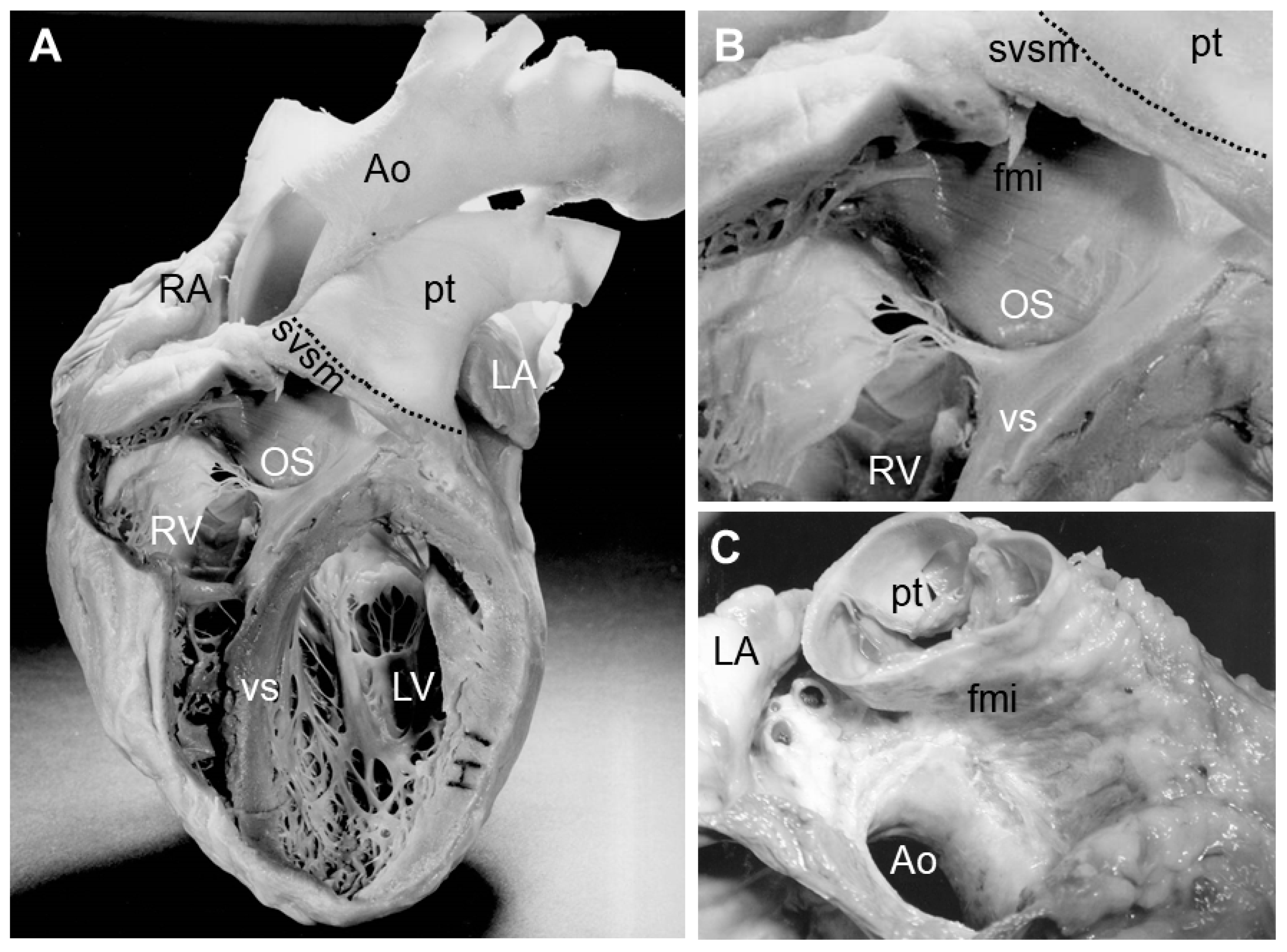
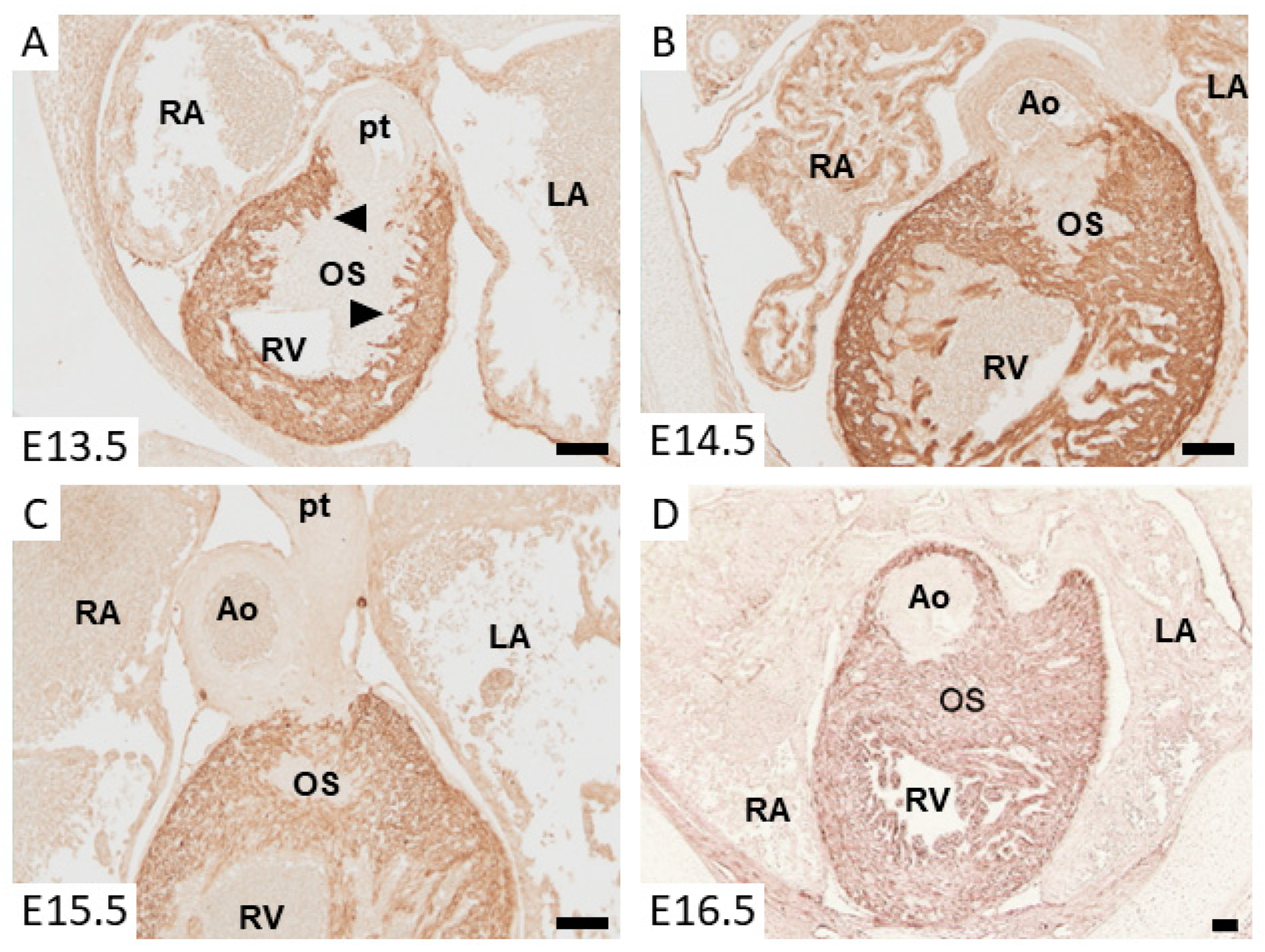
3. Myocardializaton of the Outlet Septum
4. Congenital Cardiac Abnormalities and Absence of Myocardializaton
5. Mechanism of Cardiac Muscle Formation in the Outlet Septum: Muscularization vs. Differentiation
6. Molecular Regulation of Myocardium Formation in the Outlet Septum
7. Muscularization of the Dorsal Mesenchymal Protrusion
8. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Sylva, M.; van den Hoff, M.J.B.; Moorman, A.F.M. Development of the human heart. Am. J. Med. Genet. Part A 2014, 164A, 1347–1371. [Google Scholar] [CrossRef] [PubMed]
- Buijtendijk, M.F.J.; Barnett, P.; van den Hoff, M.J.B. Development of the human heart. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Freedom, R.M. Normal and abnormal structure of the ventriculo-arterial junctions. Cardiol. Young 2005, 15 (Suppl. 1), 3–16. [Google Scholar] [CrossRef]
- Anderson, R.H.; Brown, N.A.; Webb, S.; Chaudhry, B.; Henderson, D.; Moorman, A.F.M. Morphology of the Developing Cardiac Outflow Tract. In Shaping the Heart in Development and Disease; Mikhailov, A.T., Torrado, M., Eds.; Transworld Research Network: Kerala, India, 2010. [Google Scholar]
- Anderson, R.H.; Chaudhry, B.; Mohun, T.J.; Bamforth, S.D.; Hoyland, D.; Phillips, H.M.; Webb, S.; Moorman, A.F.; Brown, N.A.; Henderson, D.J. Normal and Abnormal Development of the Intrapericardial Arterial Trunks in Man and Mouse. Cardiovasc. Res. 2012, 95, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Mori, S.; Spicer, D.E.; Brown, N.A.; Mohun, T.J. Development and Morphology of the Ventricular Outflow Tracts. World J. Pediatr. Congenit. Hear. Surg. 2016, 7, 561–577. [Google Scholar] [CrossRef] [PubMed]
- Moorman, A.F.M.; Anderson, R.H.; Christoffels, V.M. The ballooning model for formation of the cardiac chambers. In Heart Development and Regeneration; Harvey, R.P., Rosenthal, N., Eds.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Moorman, A.F.M.; Christoffels, V.M. Cardiac chamber formation: Development, genes and evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.G.; Brown, N.A.; Buckingham, M.E. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev. Cell 2001, 1, 435–440. [Google Scholar] [CrossRef]
- Mjaatvedt, C.H.; Nakaoka, T.; Moreno-Rodriguez, R.A.; Norris, R.A.; Kern, M.J.; Eisenberg, C.A.; Turner, D.; Markwald, R.R. The outflow of the heart is recruited from a novel heart forming field. Dev. Biol. 2001, 238, 97–109. [Google Scholar] [CrossRef]
- Waldo, K.L.; Kumiski, D.H.; Wallis, K.T.; Stadt, H.A.; Hutson, M.R.; Platt, D.H.; Kirby, M.L. Conotruncal myocardium arises from a secondary heart field. Development 2001, 128, 3179–3188. [Google Scholar] [PubMed]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the mammalian heart from two sources of myocardial cells. Nat. Rev. Genet. 2005, 6, 826–837. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; Christoffels, V.M.; Anderson, R.H.; van den Hoff, M.J.B. The heart-forming fields: One or multiple? Philos. Trans. R. Soc. B 2007, 362, 1257–1265. [Google Scholar] [CrossRef]
- Rana, M.S.; Horsten, N.C.A.; Tesink-Taekema, S.; Lamers, W.H.; Moorman, A.F.M.; van den Hoff, M.J.B. Trabeculated right ventricular free wall in the chicken heart forms by ventricularization of the myocardium initially forming the outflow tract. Circ. Res. 2007, 100, 1000–1007. [Google Scholar] [CrossRef]
- Verzi, M.P.; McCulley, D.J.; De, V.S.; Dodou, E.; Black, B.L. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev. Biol. 2005, 287, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; van den Hoff, M.J.; de Boer, P.A.; Tesink-Taekema, S.; Franco, D.; Moorman, A.F.; Lamers, W.H. Normal development of the outflow tract in the rat. Circ. Res. 1998, 82, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Lamers, W.H.; Mohun, T.J.; Brown, N.A.; Anderson, R.H.; Moorman, A.F. Three-dimensional and molecular analysis of the arterial pole of the developing human heart. J. Anat. 2012, 220, 336–349. [Google Scholar] [CrossRef]
- Rana, M.S.; Sizarov, A.; Christoffels, V.M.; Moorman, A.F. Development of the Human Aortic Arch System Captured in an Interactive Three-Dimensional Reference Model. Am. J. Med. Genet. A 2014, 164, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Choudry, A.; Berlan, M.; Singal, A.; Siwik, E.; Mohr, S.; Fisher, S.A. Developmental remodeling and shortening of the cardiac outflow tract involves programmed cell death. Development 1998, 125, 3809–3820. [Google Scholar]
- van den Hoff, M.J.B.; van den Eijnde, S.M.; Virágh, S.; Moorman, A.F.M. Programmed cell death in the developing heart. Cardiovasc. Res. 2000, 45, 603–620. [Google Scholar] [CrossRef]
- Wirrig, E.E.; Snarr, B.S.; Chintalapudi, M.R.; O’Neal, J.L.; Phelps, A.L.; Barth, J.L.; Fresco, V.M.; Kern, C.B.; Mjaatvedt, C.H.; Toole, B.P.; et al. Cartilage link protein 1 (Crtl1), an extracellular matrix component playing an important role in heart development. Dev. Biol. 2007, 310, 291–303. [Google Scholar] [CrossRef]
- Waldo, K.; Miyagawa-Tomita, S.; Kumiski, D.; Kirby, M.L. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: Aortic sac to ventricular septal closure. Dev. Biol. 1998, 196, 129–144. [Google Scholar] [CrossRef]
- de Lange, F.J.; Moorman, A.F.M.; Anderson, R.H.; Manner, J.; Soufan, A.T.; de Gier-de Vries, C.; Schneider, M.D.; Webb, S.; van den Hoff, M.J.B.; Christoffels, V.M. Lineage and morphogenetic analysis of the cardiac valves. Circ. Res 2004, 95, 645–654. [Google Scholar] [CrossRef]
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607–1616. [Google Scholar]
- Cheng, G.; Wessels, A.; Gourdie, R.G.; Thompson, R.P. Spatiotemporal and tissue specific distribution of apoptosis in the developing chick heart. Dev. Dyn. 2002, 223, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Poelmann, R.E.; Gittenberger-de Groot, A.C. Apoptosis as an instrument in cardiovascular development. Birth Defects Res. C Embryo Today. 2005, 75, 305–313. [Google Scholar] [CrossRef]
- Lamers, W.H.; Wessels, A.; Verbeek, F.J.; Moorman, A.F.M.; Virágh, S.; Wenink, A.C.G.; Gittenberger-de Groot, A.C.; Anderson, R.H. New findings concerning ventricular septation in the human heart. Implications for maldevelopment. Circulation 1992, 86, 1194–1205. [Google Scholar] [CrossRef]
- van den Hoff, M.J.B.; Moorman, A.F.M.; Ruijter, J.M.; Lamers, W.H.; Bennington, R.W.; Markwald, R.R.; Wessels, A. Myocardialization of the cardiac outflow tract. Dev. Biol. 1999, 212, 477–490. [Google Scholar] [CrossRef]
- Kruithof, B.P.T.; van den Hoff, M.J.B.; Wessels, A.; Moorman, A.F.M. Cardiac muscle cell formation after development of the linear heart tube. Dev. Dyn. 2003, 227, 1–13. [Google Scholar] [CrossRef] [PubMed]
- van den Hoff, M.J.B.; Kruithof, B.P.T.; Moorman, A.F.M.; Markwald, R.R.; Wessels, A. Formation of myocardium after the initial development of the linear heart tube. Dev. Biol. 2001, 240, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Ho, S.Y.; Wilcox, B.R. The surgical anatomy of ventricular septal defect part IV: Double outlet ventricle. J. Card. Surg. 1996, 11, 2–11. [Google Scholar] [CrossRef]
- McCarthy, K.P.; Ching Leung, P.K.; Ho, S.Y. Perimembranous and muscular ventricular septal defects—Morphology revisited in the era of device closure. J. Interv. Cardiol. 2005, 18, 507–513. [Google Scholar] [CrossRef]
- Colas, J.F.; Lawson, A.; Schoenwolf, G.C. Evidence that translation of smooth muscle alpha-actin mRNA is delayed in the chick promyocardium until fusion of the bilateral heart-forming regions. Dev. Dyn. 2000, 218, 316–330. [Google Scholar] [CrossRef]
- Kruithof, B.P.T.; van den Hoff, M.J.B.; Tesink-Taekema, S.; Moorman, A.F.M. Recruitment of intra- and extracardiac cells into the myocardial lineage during mouse development. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2003, 271, 303–314. [Google Scholar] [CrossRef]
- Moralez, I.; Phelps, A.; Riley, B.; Raines, M.; Wirrig, E.; Snarr, B.; Jin, J.-P.; van den Hoff, M.J.B.; Hoffman, S.; Wessels, A. Muscularizing tissues in the endocardial cushions of the avian heart are characterized by the expression of h1-calponin. Dev. Dyn. 2006, 235, 1648–1658. [Google Scholar] [CrossRef]
- Okagawa, H.; Markwald, R.R.; Sugi, Y. Functional BMP receptor in endocardial cells is required in atrioventricular cushion mesenchymal cell formation in chick. Dev. Biol. 2007, 306, 179–192. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Mikawa, T.; Gittenberger-de Groot, A.C. Neural crest cells in outflow tract septation of the embryonic chicken herat: Differentiation and apoptosis. Dev. Dyn. 1998, 212, 373–384. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.L.; Chen, J.; Evans, S.M. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007, 304, 286–296. [Google Scholar] [CrossRef]
- Cai, C.L.; Liang, X.; Shi, Y.; Chu, P.H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef]
- Stanley, E.G.; Biben, C.; Elefanty, A.; Barnett, L.; Koentgen, F.; Robb, L.; Harvey, R.P. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3’UTR-ires-Cre allele of the homeobox gene Nkx2-5. Int. J. Dev. Biol. 2002, 46, 431–439. [Google Scholar]
- van den Hoff, M.J.B.; Kruithof, B.P.T.; Wessels, A.; Markwald, R.R.; Moorman, A.F.M. Regulation of myocardium formation after the initial development of the linear heart tube. In Cardiovascular Development and Congenital Malformations; Molecular and Genetic Mechanisms; Artman, M., Benson, D.W., Srivastava, D., Nakazawa, M., Eds.; Blackwell Publishing, Inc.: Malden, MA, USA, 2005; pp. 37–40. [Google Scholar]
- Mercado-Pimentel, M.E.; Runyan, R.B. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 2007, 185, 146–156. [Google Scholar] [CrossRef]
- Doetschman, T.; Barnett, J.V.; Runyan, R.B.; Camenisch, T.D.; Heimark, R.L.; Granzier, H.L.; Conway, S.J.; Azhar, M. Transforming growth factor beta signaling in adult cardiovascular diseases and repair. Cell Tissue Res. 2012, 347, 203–223. [Google Scholar] [CrossRef]
- Sanford, L.P.; Ormsby, I.; Gittenberger-de Groot, A.C.; Sariola, H.; Friedman, R.; Boivin, G.P.; Cardell, E.L.; Doetschman, T. TGFb2 knockout mice have multiple developmental defects that are non-overlapping with other TGFb knockout phenotypes. Development 1997, 124, 2659–2670. [Google Scholar] [PubMed]
- Bartram, U.; Molin, D.G.; Wisse, L.J.; Mohamad, A.; Sanford, L.P.; Doetschman, T.; Speer, C.P.; Poelmann, R.E.; Gittenberger-de Groot, A.C. Double-outlet right ventricle and overriding tricuspid valve reflect disturbances of looping, myocardialization, endocardial cushion differentiation, and apoptosis in TGF-ß2-knockout mice. Circulation 2001, 103, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, B.P.T. Myocardium Formation after the Initial Development of the Linear Heart Tube. Ph.D. Thesis, University of Amsterdam, Amsterdam, The Netherlands, 2003. [Google Scholar]
- Proetzel, G.; Pawlowski, S.A.; Wiles, M.V.; Yin, M.; Boivin, G.P.; Howles, P.N.; Ding, J.; Ferguson, M.W.; Doetschman, T. Transforming growth factor-beta 3 is required for secondary palate fusion. Nat. Genet. 1995, 11, 409–414. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Al-Sammarraie, N.; Gebere, M.G.; Bhattacharya, A.; Chopra, S.; Johnson, J.; Pena, E.A.; Eberth, J.F.; Poelmann, R.E.; Gittenberger-de Groot, A.C.; et al. Transforming Growth Factor Beta3 is Required for Cardiovascular Development. J. Cardiovasc. Dev. Dis. 2020, 7, 19. [Google Scholar] [CrossRef]
- Lakkis, M.M.; Epstein, J.A. Neurofibromin modulation of ras activity is required for normal endocardial-mesenchymal transformation in the developing heart. Development 1998, 125, 4359–4367. [Google Scholar]
- Flagg, A.E.; Earley, J.U.; Svensson, E.C. FOG-2 attenuates endothelial-to-mesenchymal transformation in the endocardial cushions of the developing heart. Dev. Biol. 2007, 304, 308–316. [Google Scholar] [CrossRef]
- Plein, A.; Calmont, A.; Fantin, A.; Denti, L.; Anderson, N.A.; Scambler, P.J.; Ruhrberg, C. Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation. J. Clin. Inv. 2015, 125, 2661–2676. [Google Scholar] [CrossRef]
- Waller, B.R.; McQuinn, T.; Phelps, A.; Markwald, R.R.; Lo, C.W.; Thompson, R.P.; Wessels, A. Conotruncal anomalies in the trisomy 16 mouse: An immunohistochemical analysis with emphasis on the involvement of the neural crest. Anat. Rec. 2000, 260, 279–293. [Google Scholar] [CrossRef]
- Huang, G.Y.; Wessels, A.; Smith, B.R.; Linask, K.K.; Ewart, J.L.; Lo, C.W. Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development. Dev. Biol. 1998, 198, 32–44. [Google Scholar] [CrossRef]
- Rhee, D.Y.; Zhao, X.Q.; Francis, R.J.; Huang, G.Y.; Mably, J.D.; Lo, C.W. Connexin 43 regulates epicardial cell polarity and migration in coronary vascular development. Development 2009, 136, 3185–3193. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Villalba, A.; Hoppler, S.; van den Hoff, M.J. Wnt signaling in the heart fields: Variations on a common theme. Dev. Dyn. 2015. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.M.; Hildreth, V.; Peat, J.D.; Murdoch, J.N.; Kobayashi, K.; Chaudhry, B.; Henderson, D.J. Non-cell-autonomous roles for the planar cell polarity gene Vangl2 in development of the coronary circulation. Circ. Res. 2008, 102, 615–623. [Google Scholar] [CrossRef]
- Phillips, H.M.; Murdoch, J.N.; Chaudhry, B.; Copp, A.J.; Henderson, D.J. Vangl2 Acts via RhoA Signaling to Regulate Polarized Cell Movements During Development of the Proximal Outflow Tract. Circ. Res. 2005, 96, 292–299. [Google Scholar] [CrossRef]
- Henderson, D.J.; Phillips, H.M.; Chaudhry, B. Vang-like 2 and noncanonical Wnt signaling in outflow tract development. Trends Cardiovasc. Med. 2006, 16, 38–45. [Google Scholar] [CrossRef]
- Gibbs, B.C.; Damerla, R.R.; Vladar, E.K.; Chatterjee, B.; Wan, Y.; Liu, X.; Cui, C.; Gabriel, G.C.; Zahid, M.; Yagi, H.; et al. Prickle1 mutation causes planar cell polarity and directional cell migration defects associated with cardiac outflow tract anomalies and other structural birth defects. Biol. Open 2016, 5, 323–335. [Google Scholar] [CrossRef]
- Qi, C.H.; Zhao, X.Q.; Ma, D.; Ma, X.J.; Zhou, G.M.; Huang, G.Y. Downregulation of Rho associated coiled-coil forming protein kinase 1 in the process of delayed myocardialization of cardiac proximal outflow tract septum in connexin 43 knockout mice embryo. Chin. Med. J. 2011, 124, 2021–2027. [Google Scholar]
- Nagy, I.I.; Railo, A.; Rapila, R.; Hast, T.; Sormunen, R.; Tavi, P.; Rasanen, J.; Vainio, S.J. Wnt-11 signalling controls ventricular myocardium development by patterning N-cadherin and beta-catenin expression. Cardiovasc. Res. 2010, 85, 100–109. [Google Scholar] [CrossRef]
- Zhou, W.; Lin, L.; Majumdar, A.; Li, X.; Zhang, X.; Liu, W.; Etheridge, L.; Shi, Y.; Martin, J.; Van de Ven, W.; et al. Modulation of morphogenesis by noncanonical Wnt signaling requires ATF/CREB family-mediated transcriptional activation of TGFbeta2. Nat. Genet. 2007, 39, 1225–1234. [Google Scholar] [CrossRef]
- Schleiffarth, J.R.; Person, A.D.; Martinsen, B.J.; Sukovich, D.J.; Neumann, A.; Baker, C.V.; Lohr, J.L.; Cornfield, D.N.; Ekker, S.C.; Petryk, A. Wnt5a is required for cardiac outflow tract septation in mice. Pediatr. Res. 2007, 61, 386–391. [Google Scholar] [CrossRef]
- Matsuda, T.; Nomi, M.; Ikeya, M.; Kani, S.; Oishi, I.; Terashima, T.; Takada, S.; Minami, Y. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech. Dev. 2001, 105, 153–156. [Google Scholar] [CrossRef]
- Yuan, Y.; Niu, C.C.; Deng, G.; Li, Z.Q.; Pan, J.; Zhao, C.; Yang, Z.L.; Si, W.K. The Wnt5a/Ror2 noncanonical signaling pathway inhibits canonical Wnt signaling in K562 cells. Int. J. Mol. Med. 2011, 27, 63–69. [Google Scholar] [CrossRef]
- Takeuchi, S.; Takeda, K.; Oishi, I.; Nomi, M.; Ikeya, M.; Itoh, K.; Tamura, S.; Ueda, T.; Hatta, T.; Otani, H.; et al. Mouse Ror2 receptor tyrosine kinase is required for the heart development and limb formation. Genes Cells 2000, 5, 71–78. [Google Scholar] [CrossRef]
- Nomi, M.; Oishi, I.; Kani, S.; Suzuki, H.; Matsuda, T.; Yoda, A.; Kitamura, M.; Itoh, K.; Takeuchi, S.; Takeda, K.; et al. Loss of mRor1 enhances the heart and skeletal abnormalities in mRor2-deficient mice: Redundant and pleiotropic functions of mRor1 and mRor2 receptor tyrosine kinases. Mol. Cell Biol. 2001, 21, 8329–8335. [Google Scholar] [CrossRef]
- Verkaar, F.; Zaman, G.J. A model for signaling specificity of Wnt/Frizzled combinations through co-receptor recruitment. FEBS Lett. 2010, 584, 3850–3854. [Google Scholar] [CrossRef] [PubMed]
- DeRossi, C.; Laiosa, M.D.; Silverstone, A.E.; Holdener, B.C. Mouse fzd4 maps within a region of chromosome 7 important for thymus and cardiac development. Genesis 2000, 27, 64–75. [Google Scholar] [CrossRef]
- Hamblet, N.S.; Lijam, N.; Ruiz-Lozano, P.; Wang, J.; Yang, Y.; Luo, Z.; Mei, L.; Chien, K.R.; Sussman, D.J.; Wynshaw-Boris, A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development 2002, 129, 5827–5838. [Google Scholar] [CrossRef] [PubMed]
- Hakim, Z.S.; DiMichele, L.A.; Doherty, J.T.; Homeister, J.W.; Beggs, H.E.; Reichardt, L.F.; Schwartz, R.J.; Brackhan, J.; Smithies, O.; Mack, C.P.; et al. Conditional deletion of focal adhesion kinase leads to defects in ventricular septation and outflow tract alignment. Mol. Cell. Biol. 2007, 27, 5352–5364. [Google Scholar] [CrossRef]
- Armstrong, P.B.; Armstrong, M.T. Intercellular invasion and the organizational stability of tissues: A role for fibronectin. Biochim. Biophys. Acta 2000, 1470, O9–O20. [Google Scholar] [CrossRef]
- Burns, T.A.; Dours-Zimmermann, M.T.; Zimmermann, D.R.; Krug, E.L.; Comte-Walters, S.; Reyes, L.; Davis, M.A.; Schey, K.L.; Schwacke, J.H.; Kern, C.B.; et al. Imbalanced expression of Vcan mRNA splice form proteins alters heart morphology and cellular protein profiles. PLoS ONE 2014, 9, e89133. [Google Scholar] [CrossRef]
- Burns, T.; Yang, Y.; Hiriart, E.; Wessels, A. The Dorsal Mesenchymal Protrusion and the Pathogenesis of Atrioventricular Septal Defects. J. Cardiovasc. Dev. Dis. 2016, 3, 29. [Google Scholar] [CrossRef]
- Snarr, B.S.; O’Neal, J.L.; Chintalapudi, M.R.; Wirrig, E.E.; Phelps, A.L.; Kubalak, S.W.; Wessels, A. Isl1 Expression at the Venous Pole Identifies a Novel Role for the Second Heart Field in Cardiac Development. Circ. Res. 2007, 101, 971–974. [Google Scholar] [CrossRef]
- Snarr, B.S.; Wirrig, E.E.; Phelps, A.L.; Trusk, T.C.; Wessels, A. A spatiotemporal evaluation of the contribution of the dorsal mesenchymal protrusion to cardiac development. Dev. Dyn. 2007, 236, 1287–1294. [Google Scholar] [CrossRef]
- Kim, J.S.; Virágh, S.; Moorman, A.F.M.; Anderson, R.H.; Lamers, W.H. Development of the myocardium of the atrioventricular canal and the vestibular spine in the human heart. Circ. Res. 2001, 88, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yuan, L.; Goss, A.M.; Wang, T.; Yang, J.; Lepore, J.J.; Zhou, D.; Schwartz, R.J.; Patel, V.; Cohen, E.D.; et al. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev. Cell 2010, 18, 275–287. [Google Scholar] [CrossRef]
- Briggs, L.E.; Phelps, A.L.; Brown, E.; Kakarla, J.; Anderson, R.H.; van den Hoff, M.J.; Wessels, A. Expression of the BMP Receptor Alk3 in the Second Heart Field is Essential for Development of the Dorsal Mesenchymal Protrusion and Atrioventricular Septation. Circ. Res. 2013, 112, 1420–4132. [Google Scholar] [CrossRef]
- Briggs, L.E.; Burns, T.A.; Lockhart, M.M.; Phelps, A.L.; van den Hoff, M.J.; Wessels, A. The Wnt/beta-catenin and Sonic Hedgehog pathways interact in the regulation of the development of the Dorsal Mesenchymal Protrusion. Dev. Dyn. 2016, 245, 103–113. [Google Scholar] [CrossRef]
- van Vliet, P.P.; Lin, L.; Boogerd, C.J.; Martin, J.F.; Andelfinger, G.; Grossfeld, P.D.; Evans, S.M. Tissue specific requirements for WNT11 in developing outflow tract and dorsal mesenchymal protrusion. Dev. Biol. 2017, 429, 249–259. [Google Scholar] [CrossRef]
- Li, D.; Angermeier, A.; Wang, J. Planar cell polarity signaling regulates polarized second heart field morphogenesis to promote both arterial and venous pole septation. Development 2019, 146, dev181719. [Google Scholar] [CrossRef]
- Gunthel, M.; Barnett, P.; Christoffels, V.M. Development, Proliferation, and Growth of the Mammalian Heart. Mol. Ther. 2018, 26, 1599–1609. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Hoff, M.J.B.; Wessels, A. Muscularization of the Mesenchymal Outlet Septum during Cardiac Development. J. Cardiovasc. Dev. Dis. 2020, 7, 51. https://doi.org/10.3390/jcdd7040051
van den Hoff MJB, Wessels A. Muscularization of the Mesenchymal Outlet Septum during Cardiac Development. Journal of Cardiovascular Development and Disease. 2020; 7(4):51. https://doi.org/10.3390/jcdd7040051
Chicago/Turabian Stylevan den Hoff, Maurice J. B., and Andy Wessels. 2020. "Muscularization of the Mesenchymal Outlet Septum during Cardiac Development" Journal of Cardiovascular Development and Disease 7, no. 4: 51. https://doi.org/10.3390/jcdd7040051
APA Stylevan den Hoff, M. J. B., & Wessels, A. (2020). Muscularization of the Mesenchymal Outlet Septum during Cardiac Development. Journal of Cardiovascular Development and Disease, 7(4), 51. https://doi.org/10.3390/jcdd7040051





