An Appreciation of Anatomy in the Molecular World
Abstract
1. Introduction
2. Early and Highly Perceptive Insights
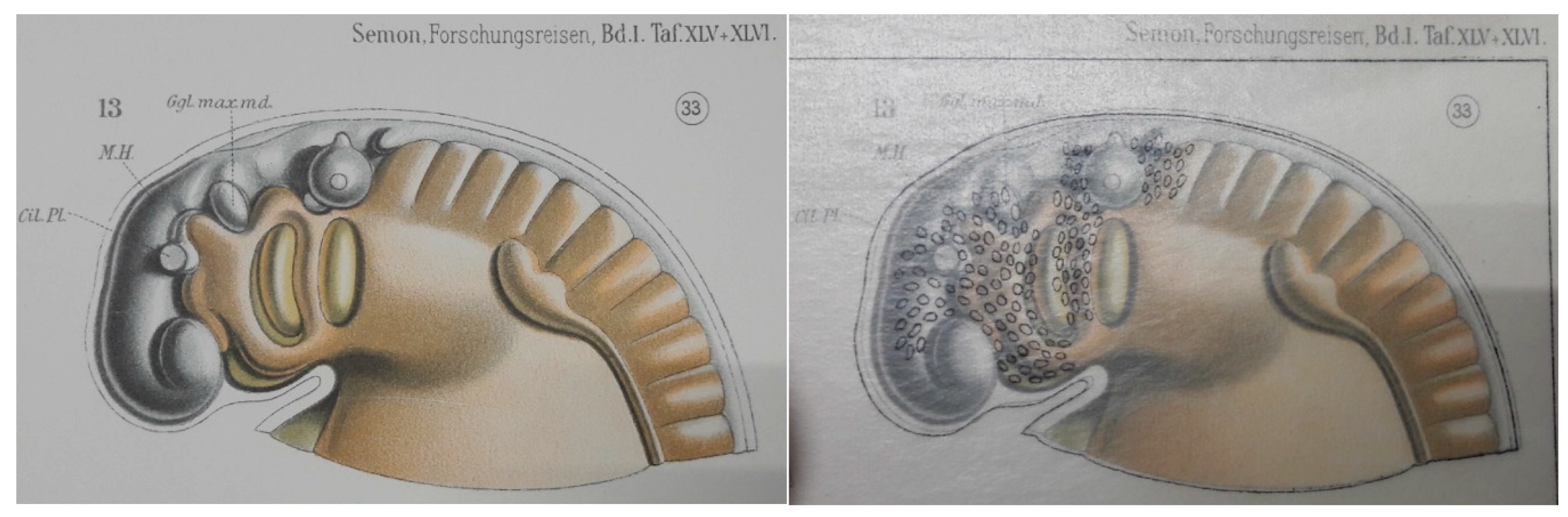
2.1. Neural Crest Cells
2.2. Cardio-Pharyngeal Mesoderm or Second Heart Field
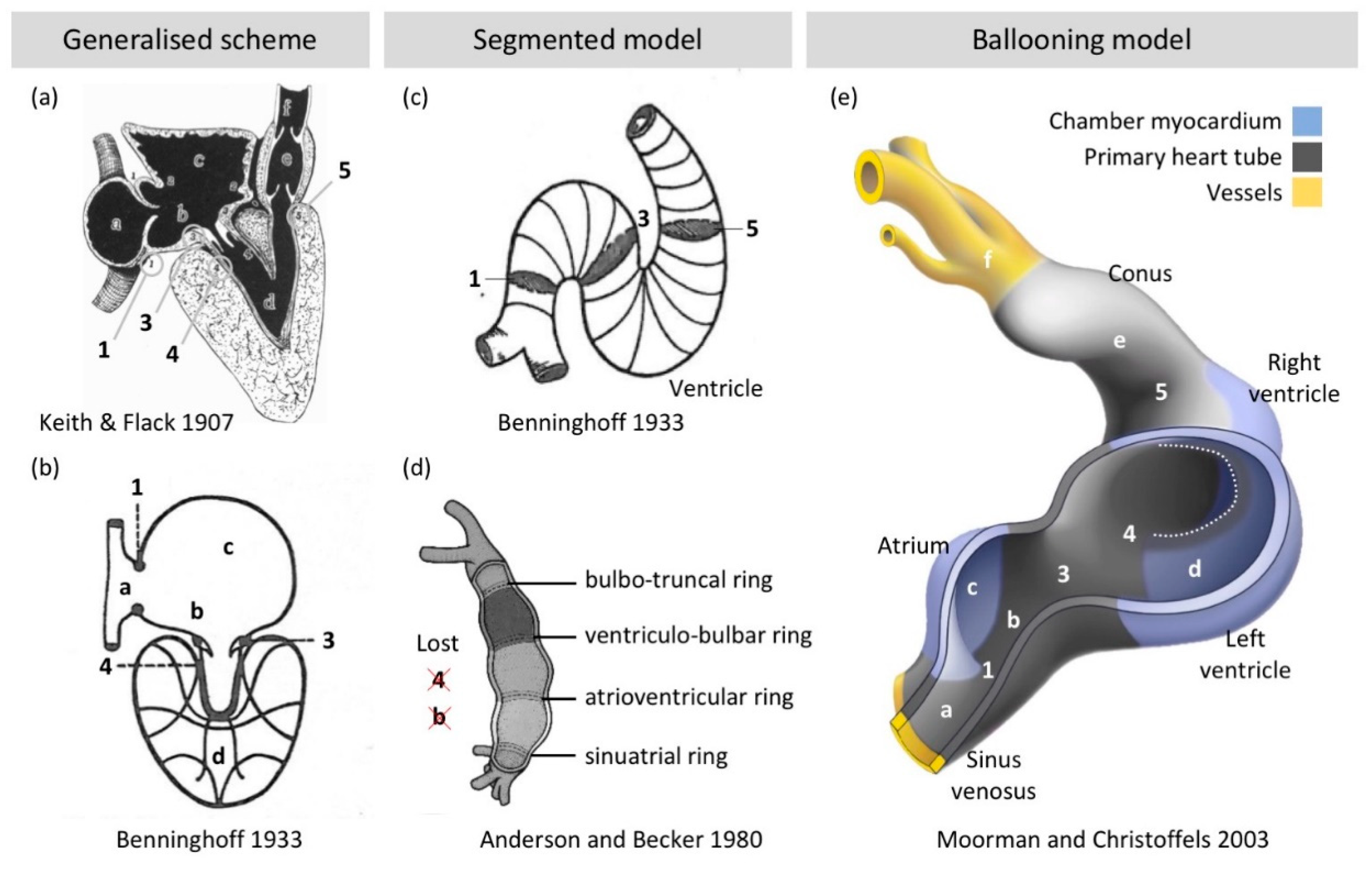
2.3. Chamber Formation: Bulges, Segments, and Finally Balloons
3. Detailed Analyses of Ventricular Structure, But How Does It Relate to Function?
3.1. Myocyte Orientation of the Compact Wall
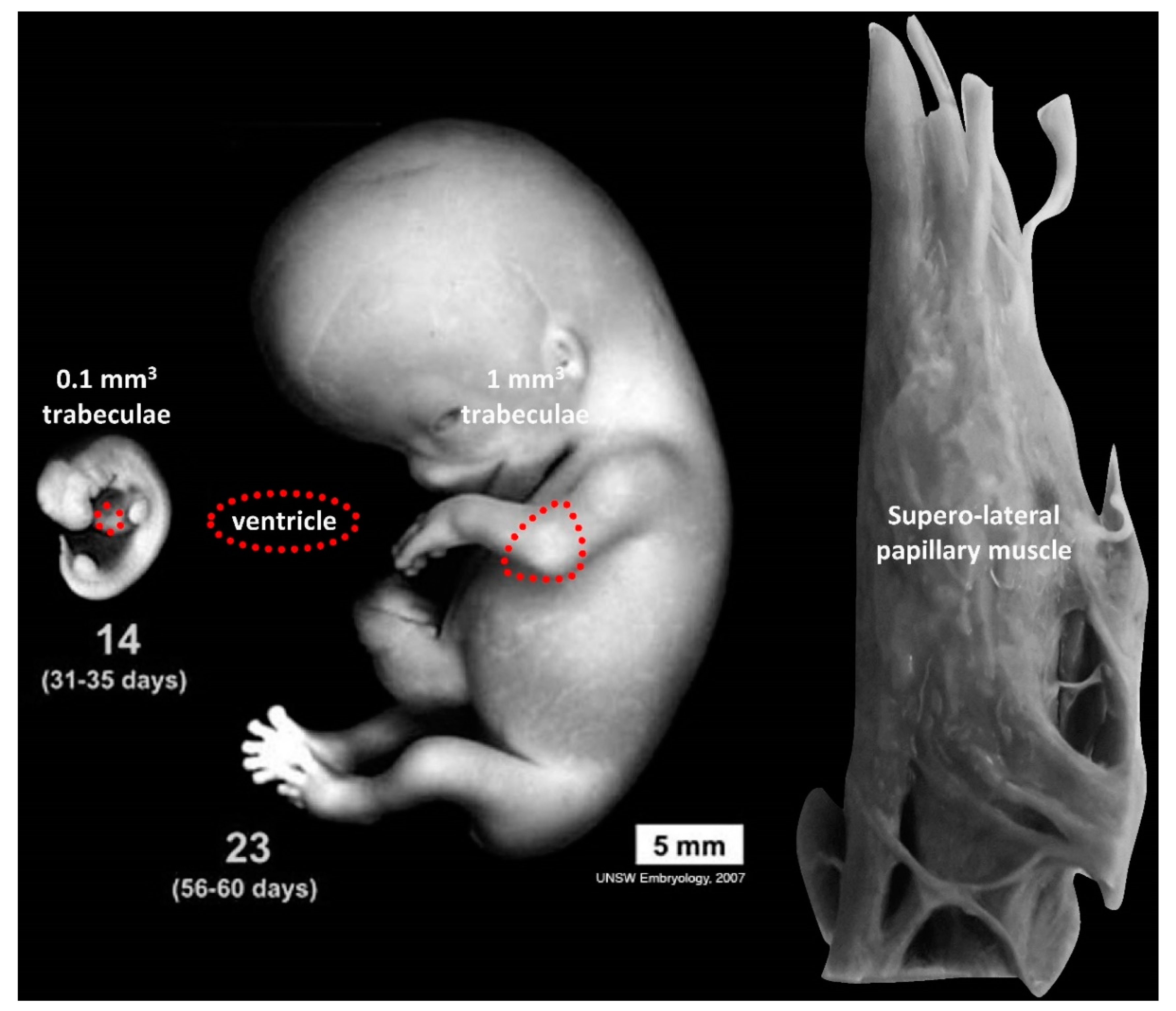
3.2. Trabeculae, Compaction and Differential Growth as a Mechanism for Shape Change
4. What Makes an Atrial Septum?
5. The Special Case of the Cardiac Conduction System
Funding
Conflicts of Interest
References
- Gladka, M.M.; Molenaar, B.; De Ruiter, H.; Van Der Elst, S.; Tsui, H.; Versteeg, D.; Lacraz, G.P.A.; Huibers, M.M.H.; Van Oudenaarden, A.; Van Rooij, E. Single-cell sequencing of the healthy and diseased heart reveals cytoskeleton-associated protein 4 as a new modulator of fibroblasts activation. Circulation 2018, 138, 166–180. [Google Scholar] [CrossRef] [PubMed]
- DeLaughter, D.M.; Bick, A.G.; Wakimoto, H.; Mckean, D.; Gorham, J.M.; Kathiriya, I.S.; Hinson, J.T.; Homsy, J.; Gray, J.; Pu, W.; et al. Single-Cell Resolution of Temporal Gene Expression during Heart Development. Dev. Cell 2016, 39, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Nyhart, L. The Disciplinary Breakdown of German Morphology, 1870–1900. Isis 1987, 78, 365–389. [Google Scholar] [CrossRef] [PubMed]
- Gifford, C.A.; Ranade, S.S.; Samarakoon, R.; Salunga, H.T.; De Soysa, T.Y.; Huang, Y.; Zhou, P.; Elfenbein, A.; Wyman, S.K.; Bui, Y.K.; et al. Oligogenic inheritance of a human heart disease involving a genetic modifier. Science 2019, 364, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.V.; Dawes, T.J.W.; Serrani, M.; Bai, W.; Tokarczuk, P.; Cai, J.; De Marvao, A.; Henry, A.; Lumbers, T.; Gierten, J.; et al. Genetic and functional insights into the fractal structure of the heart. Nature 2020, 584, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Hulsmans, M.; Clauss, S.; Xiao, L.; Aguirre, A.D.; King, K.R.; Hanley, A.; Hucker, W.J.; Wülfers, E.M.; Seemann, G.; Courties, G.; et al. Macrophages Facilitate Electrical Conduction in the Heart. Cell 2017, 169, 510–522.e20. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.; KarimianPour, A.; Collier, P.; Krasuski, R.A. Isolated Noncompaction of the Left Ventricle in Adults. J. Am. Coll. Cardiol. 2015, 66, 578–585. [Google Scholar] [CrossRef]
- Greil, A. Entwickelungsgeschichte des Kopfes und des Blutgefässsystemes von Ceratodus forsteri. I. Gesammtenentwickelung bis zum Beginn der Blutzirkulation. Denkschriften der Medizinisch-Naturwissenschaftlichen Gesellschaft zu Jena 4; Fischer: Jena, Germany, 1908; pp. 661–934. (In German) [Google Scholar]
- Ericsson, R.; Joss, J.; Olsson, L. The fate of cranial neu1ral crest cells in the Australian lungfish, Neoceratodus forsteri. J. Exp. Zool. 2008, 310, 345–354. [Google Scholar] [CrossRef]
- Lopez, D.; Durán, A.C.; De Andrés, A.V.; Guerrero, A.; Blasco, M.; Sans-Coma, V. Formation of cartilage in the heart of the Spanish terrapin, Mauremys leprosa (Reptilia, Chelonia). J. Morphol. 2003, 258, 97–105. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Groot, A.C.G.-D.; Biermans, M.W.M.; Dolfing, A.I.; Jagessar, A.; Van Hattum, S.; Hoogenboom, A.; Wisse, L.J.; Vicente-Steijn, R.; De Bakker, M.; et al. Outflow tract septation and the aortic arch system in reptiles: Lessons for understanding the mammalian heart. EvoDevo 2017, 8, 9. [Google Scholar] [CrossRef]
- Le Douarin, N. A biological cell labeling technique and its use in expermental embryology. Dev. Biol. 1973, 30, 217–222. [Google Scholar] [CrossRef]
- Kirby, M.L.; Gale, T.F.; E Stewart, D. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983, 220, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Greil, A. Beitrage zur vergelichenden anatomie und entwicklungsgeschichte des herzens und des trauncus arteriosus der wirbelthiere. Morph. Jahrb. 1903, 31, 123–310. [Google Scholar]
- Keith, A. Schorstein lecture on the fate of the bulbus cordis in the human heart. Lancet 1924, 204, 1267–1273. [Google Scholar] [CrossRef]
- De La Cruz, M.V.; Gómez, C.S.; Arteaga, M.M.; Argüello, C. Experimental study of the development of the truncus and the conus in the chick embryo. J. Anat. 1977, 123, 661–686. [Google Scholar]
- Virágh, S.; Challice, C. Origin and differentiation of cardiac muscle cells in the mouse. J. Ultrastruct. Res. 1973, 42, 1–24. [Google Scholar] [CrossRef]
- Kelly, R.G.; Brown, N.A.; Buckingham, M.E. The Arterial Pole of the Mouse Heart Forms from Fgf10-Expressing Cells in Pharyngeal Mesoderm. Dev. Cell 2001, 1, 435–440. [Google Scholar] [CrossRef]
- Diogo, R.; Kelly, R.G.; Christiaen, L.; Levine, M.; Ziermann, J.M.; Molnar, J.L.; Noden, D.M.; Tzahor, E. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nat. Cell Biol. 2015, 520, 466–473. [Google Scholar] [CrossRef]
- Mommersteeg, M.T.; Domínguez, J.N.; Wiese, C.; Norden, J.; Vries, C.D.G.-D.; Burch, J.B.; Kispert, A.; Brown, N.A.; Moorman, A.F.M.; Christoffels, V.M. The sinus venosus progenitors separate and diversify from the first and second heart fields early in development. Cardiovasc. Res. 2010, 87, 92–101. [Google Scholar] [CrossRef]
- Meilhac, S.M.; Lescroart, F.; Blanpain, C.; Buckingham, M. Cardiac Cell Lineages that Form the Heart. Cold Spring Harb. Perspect. Med. 2014, 4, a013888. [Google Scholar] [CrossRef]
- Bressan, M.; Liu, G.; Mikawa, T. Early Mesodermal Cues Assign Avian Cardiac Pacemaker Fate Potential in a Tertiary Heart Field. Science 2013, 340, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Abu-Issa, R.; Waldo, K.; Kirby, M.L. Heart fields: One, two or more? Dev. Biol. 2004, 272, 281–285. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; Christoffels, V.M.; Anderson, R.H.; Hoff, M.J.V.D. The heart-forming fields: One or multiple? Philos. Trans. R. Soc. B 2007, 362, 1257–1265. [Google Scholar] [CrossRef]
- Gaskell, W.H. On the Innervation of the Heart, with especial reference to the Heart of the Tortoise. J. Physiol. 1883, 4, 43–230. [Google Scholar] [CrossRef]
- Bakker, M.L.; Christoffels, V.M.; Moorman, A.F.M. The Cardiac Pacemaker and Conduction System Develops From Embryonic Myocardium that Retains Its Primitive Phenotype. J. Cardiovasc. Pharmacol. 2010, 56, 6–15. [Google Scholar] [CrossRef]
- Keith, A.; Flack, M. The auriculo-ventricular bundle of the human heart. Lancet 1906, 168, 359–364. [Google Scholar] [CrossRef]
- Robertson, J.I. Memoirs: The development of the heart and vascular system of Lepidosiren paradoxa, in Quart. J. Cell. Sci. 1913, 59, 53–132. [Google Scholar]
- Moorman, A.F.M.; Christoffels, V.M. Cardiac Chamber Formation: Development, Genes, and Evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef]
- Keith, A.; Flack, M. The Form and Nature of the Muscular Connections between the Primary Divisions of the Vertebrate Heart. J. Anat. Physiol. 1907, 41, 172–189. [Google Scholar]
- Benninghoff, A. Das Herz. In Handbuch der vergleichende Anatomie der Wirbeltiere; Bolk, L., Ed.; Urban & Schwarzenberg: Berlin, Germany, 1933; pp. 467–555. [Google Scholar]
- Anderson, R.H.; Becker, A.E. Cardiac Anatomy: Integrated Text and Colour Atlas; Gower Medical Pub.: London, UK, 1980. [Google Scholar]
- Anderson, R.H.; Becker, A.E.; Wenink, A.C.G.; Janse, M.J. The Development of the Cardiac Specialized Tissue. In The Conduction System of the Heart: Structure, Function and Clinical Implications; Wellens, H.J.J., Lie, K.I., Janse, M.J., Eds.; Springer: Dordrecht, The Netherlands, 1978; pp. 3–28. [Google Scholar]
- Wenink, A.C.G. Development of the human cardiac conducting system. J. Anat. 1976, 121, 617–631. [Google Scholar]
- Bettex, D.A.; Prêtre, R.; Chassot, P.-G. Is our heart a well-designed pump? The heart along animal evolution. Eur. Hear. J. 2014, 35, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, A. Über die Beziehungen des Reizleitungssystems und der papillarmuskeln zu den Konturfasern des Herzschlauches. Anat. Anz 1923, 57, 185–208. [Google Scholar]
- Srivastava, D.; Olson, E.N. A genetic blueprint for cardiac development. Nat. Cell Biol. 2000, 407, 221–226. [Google Scholar] [CrossRef]
- Wessels, A.; Vermeulen, J.L.M.; Verbeek, F.J.; Virágh, S.Z.; Kálmán, F.; Lamers, W.H.; Moorman, A.F.M. Spatial distribution of “tissue-specific” antigens in the developing human heart and skeletal muscle III. An immunohistochemical analysis of the distribution of the neural tissue antigen G1N2 in the embryonic heart; implications for the development of the atrioventricular conduction system. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1992, 232, 97–111. [Google Scholar] [CrossRef]
- Anderson, R.H. Simplifying the understanding of congenital malformations of the heart. Int. J. Cardiol. 1991, 32, 131–142. [Google Scholar] [CrossRef]
- Ivanovitch, K.; Esteban, I.; Torres, M. Growth and Morphogenesis during Early Heart Development in Amniotes. J. Cardiovasc. Dev. Dis. 2017, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Sizarov, A.; Ya, J.; De Boer, B.A.; Lamers, W.H.; Christoffels, V.M.; Moorman, A.F.M. Formation of the Building Plan of the Human Heart. Circulation 2011, 123, 1125–1135. [Google Scholar] [CrossRef]
- Sizarov, A.; Devalla, H.D.; Anderson, R.H.; Passier, R.; Christoffels, V.M.; Moorman, A.F.M. Molecular Analysis of Patterning of Conduction Tissues in the Developing Human Heart. Circ. Arrhythmia Electrophysiol. 2011, 4, 532–542. [Google Scholar] [CrossRef]
- Pettigrew, J.B. XIV. On the arrangement of the muscular fibres in the ventricles of the vertebrate heart, with physiological remarks. Philos. Trans. R. Soc. Lond. 1864, 154, 445–500. [Google Scholar] [CrossRef]
- Streeter, D.D., Jr. Gross Morphology and Fiber Geometry of the Heart. Handb. Physiol. 1979, 61–112. [Google Scholar]
- Goodrich, E.S. Note on the Reptilian Heart. J. Anat. 1919, 53, 298–304. [Google Scholar] [PubMed]
- Jensen, B.; Christoffels, V.M. Reptiles as a Model System to Study Heart Development. Cold Spring Harb. Perspect. Biol. 2019, 12, a037226. [Google Scholar] [CrossRef]
- Shaner, R.F. On the Muscular Architecture of the Vertebrate Ventricle. J. Anat. 1923, 58, 59–70. [Google Scholar]
- Lunkenheimer, P.P.; Niederer, P.; Sanchez-Quintana, D.; Murillo, M.; Smerup, M. Models of Ventricular Structure and Function Reviewed for Clinical Cardiologists. J. Cardiovasc. Transl. Res. 2012, 6, 176–186. [Google Scholar] [CrossRef]
- Kelly, A.; Salerno, S.; Connolly, A.; Bishop, M.; Charpentier, F.; Stølen, T.; Smith, G.L. Normal interventricular differences in tissue architecture underlie right ventricular susceptibility to conduction abnormalities in a mouse model of Brugada syndrome. Cardiovasc. Res. 2017, 114, 724–736. [Google Scholar] [CrossRef]
- Meilhac, S.M.; Esner, M.; Kelly, R.G.; Nicolas, J.-F.; Buckingham, M.E. The Clonal Origin of Myocardial Cells in Different Regions of the Embryonic Mouse Heart. Dev. Cell 2004, 6, 685–698. [Google Scholar] [CrossRef]
- Meilhac, S.M.; Esner, M.; Kerszberg, M.; Moss, J.E.; Buckingham, M.E. Oriented clonal cell growth in the developing mouse myocardium underlies cardiac morphogenesis. J. Cell Biol. 2004, 164, 97–109. [Google Scholar] [CrossRef]
- Shaner, R.F. The Development of the Muscular Arrangement in the Ventricles of the Heart. Can. Med Assoc. J. 1929, 20, 386–390. [Google Scholar] [PubMed]
- Garcia-Cañadilla, P.; Dejea, H.; Bonnin, A.; Balicevic, V.; Loncaric, S.; Zhang, C.; Butakoff, C.; Aguado-Sierra, J.; Vázquez, M.; Jackson, L.H.; et al. Complex Congenital Heart Disease Associated with Disordered Myocardial Architecture in a Midtrimester Human Fetus. Circ. Cardiovasc. Imaging 2018, 11, 007753. [Google Scholar] [CrossRef]
- Torrent-Guasp, F.; Buckberg, G.D.; Clemente, C.; Cox, J.L.; Coghlan, H.C.; Gharib, M. The Structure and Function of the Helical Heart and Its Buttress Wrapping. I. The Normal Macroscopic Structure of the Heart. Semin. Thorac. Cardiovasc. Surg. 2001, 13, 301–319. [Google Scholar] [CrossRef]
- Buckberg, G.D.; Nanda, N.C.; Nguyen, C.; Kocica, M.J. What Is the Heart? Anatomy, Function, Pathophysiology, and Misconceptions. J. Cardiovasc. Dev. Dis. 2018, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H. Spatial orientation of the ventricular muscle band. J. Thorac. Cardiovasc. Surg. 2002, 124, 389–392. [Google Scholar] [CrossRef]
- MacIver, D.H.; Partridge, J.B.; Agger, P.; Stephenson, R.S.; Boukens, B.J.D.; Omann, C.; Jarvis, J.C.; Zhang, H. The end of the unique myocardial band: Part. II. Clinical and functional considerations. Eur. J. Cardiothorac. Surg. 2018, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- MacIver, D.H.; Stephenson, R.S.; Jensen, B.; Damián Sánchez-Quintana, P.A.; Jarvis, J.C.; Partridge, J.B.; Anderson, R.H. The end of the unique myocardial band: Part. I. Anatomical considerations. Eur. J. Cardiothorac. Surg. 2018, 53, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, A.; Adams, J.W.; Vaccarezza, M. The vertebrate heart: An evolutionary perspective. J. Anat. 2017, 231, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H. Evolution of the vertebrate heart. J. Anat. 2018, 232, 886–887. [Google Scholar] [CrossRef]
- Agger, P.; Omann, C.; Laustsen, C.; Stephenson, R.S.; Anderson, R.H. Anatomically correct assessment of the orientation of the cardiomyocytes using diffusion tensor imaging. NMR Biomed. 2019, 33, 4205. [Google Scholar] [CrossRef]
- Agger, P.; Ilkjær, C.; Laustsen, C.; Smerup, M.; Frandsen, J.R.; Ringgaard, S.; Pedersen, M.; Partridge, J.B.; Anderson, R.H.; Hjortdal, V. Changes in overall ventricular myocardial architecture in the setting of a porcine animal model of right ventricular dilation. J. Cardiovasc. Magn. Reson. 2017, 19, 93. [Google Scholar] [CrossRef]
- Ariga, R.; Tunnicliffe, E.M.; Manohar, S.G.; Mahmod, M.; Raman, B.; Piechnik, S.K.; Francis, J.M.; Robson, M.D.; Neubauer, S.; Watkins, H. Identification of Myocardial Disarray in Patients with Hypertrophic Cardiomyopathy and Ventricular Arrhythmias. J. Am. Coll. Cardiol. 2019, 73, 2493–2502. [Google Scholar] [CrossRef]
- Chin, T.K.; Perloff, J.K.; Williams, R.G.; Jue, K.; Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 1990, 82, 507–513. [Google Scholar] [CrossRef]
- Jenni, R.; Oechslin, E.; Schneider, J.; Jost, C.A.; A Kaufmann, P. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Jenni, R.; Rojas, J.; Oechslin, E. Isolated Noncompaction of the Myocardium. N. Engl. J. Med. 1999, 340, 966–967. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left Ventricular Non-Compaction. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Captur, G.; Flett, A.S.; Jacoby, D.L.; Moon, J.R. Left ventricular non-noncompaction: The mitral valve prolapse of the 21st century? Int. J. Cardiol. 2013, 164, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Grothoff, M.; Pachowsky, M.; Hoffmann, J.; Posch, M.; Klaassen, S.; Lehmkuhl, L.; Gutberlet, M. Value of cardiovascular MR in diagnosing left ventricular non-compaction cardiomyopathy and in discriminating between other cardiomyopathies. Eur. Radiol. 2012, 22, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Halaney, D.L.; Sanyal, A.; Nafissi, N.A.; Escobedo, D.; Goros, M.; Michalek, J.E.; Acevedo, P.; Pérez, W.; Escobar, G.P.; Feldman, M.D.; et al. The Effect of Trabeculae Carneae on Left Ventricular Diastolic Compliance: Improvement in Compliance with Trabecular Cutting. J. Biomech. Eng. 2017, 139, 0310121–0310128. [Google Scholar] [CrossRef]
- Shave, R.E.; Lieberman, D.E.; Drane, A.L.; Brown, M.G.; Batterham, A.M.; Worthington, S.; Atencia, R.; Feltrer, Y.; Neary, J.; Weiner, R.B.; et al. Selection of endurance capabilities and the trade-off between pressure and volume in the evolution of the human heart. Proc. Natl. Acad. Sci. USA 2019, 116, 19905–19910. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Sanchez-Quintana, D.; A Cabrera, J.; Anderson, R.H. Anatomy of the left atrium: Implications for radiofrequency ablation of atrial fibrillation. J. Cardiovasc. Electrophysiol. 1999, 10, 1525–1533. [Google Scholar]
- Rillig, A.; Tilz, R.; Lin, T.; Fink, T.; Heeger, C.-H.; Arya, A.; Metzner, A.; Mathew, S.; Wissner, E.; Makimoto, H.; et al. Unexpectedly High Incidence of Stroke and Left Atrial Appendage Thrombus Formation After Electrical Isolation of the Left Atrial Appendage for the Treatment of Atrial Tachyarrhythmias. Circ. Arrhythmia Electrophysiol. 2016, 9, e003461. [Google Scholar] [CrossRef]
- Sedmera, D.; Pexieder, T.; Vuillemin, M.; Thompson, R.P.; Anderson, R.H. Developmental patterning of the myocardium. Anat. Rec. 2000, 258, 319–337. [Google Scholar] [CrossRef]
- Feldt, R.H.; Rahimtoola, S.H.; Davis, G.D.; Swan, H.; Titus, J.L. Anomalous ventricular myocardial patterns in a child with complex congenital heart disease. Am. J. Cardiol. 1969, 23, 732–734. [Google Scholar] [CrossRef]
- Angelini, A.; Melacini, P.; Barbero, F.; Thiene, G. Evolutionary persistence of spongy myocardium in humans. Circulation 1999, 99, 2475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freedom, R.M.; Yoo, S.-J.; Perrin, D.; Taylor, G.; Petersen, S.; Anderson, R.H. The morphological spectrum of ventricular noncompaction. Cardiol. Young 2005, 15, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Towbin, J.A.; Jefferies, J.L. Cardiomyopathies Due to Left Ventricular Noncompaction, Mitochondrial and Storage Diseases, and Inborn Errors of Metabolism. Circ. Res. 2017, 121, 838–854. [Google Scholar] [CrossRef] [PubMed]
- Zemrak, F.; Ahlman, M.A.; Captur, G.; Mohiddin, S.A.; Kawel-Boehm, N.; Prince, M.R.; Moon, J.C.; Hundley, W.G.; Lima, J.A.; Bluemke, D.A.; et al. The Relationship of Left Ventricular Trabeculation to Ventricular Function and Structure Over a 9.5-Year Follow-Up. J. Am. Coll. Cardiol. 2014, 64, 1971–1980. [Google Scholar] [CrossRef]
- Weir-McCall, J.R.; Yeap, P.M.; Papagiorcopulo, C.; Fitzgerald, K.; Gandy, S.J.; Lambert, M.; Belch, J.J.F.; Cavin, I.; Littleford, R.; Macfarlane, J.A.; et al. Left Ventricular Noncompaction. J. Am. Coll. Cardiol. 2016, 68, 2157–2165. [Google Scholar] [CrossRef]
- Ivanov, A.; Dabiesingh, D.S.; Bhumireddy, G.P.; Mohamed, A.; Asfour, A.; Briggs, W.M.; Ho, J.; Khan, S.A.; Grossman, A.; Klem, I.; et al. Prevalence and Prognostic Significance of Left Ventricular Noncompaction in Patients Referred for Cardiac Magnetic Resonance Imaging. Circ. Cardiovasc. Imaging 2017, 10, e006174. [Google Scholar] [CrossRef]
- Oechslin, E.N.; Jenni, R.; Klaassen, S. Left ventricular noncompaction is a myocardial phenotype: Cardiomyopathy—Yes or no? Can. J. Cardiol. 2020, in press. [Google Scholar] [CrossRef]
- Van Waning, J.I.; Caliskan, K.; Chelu, R.G.; Van Der Velde, N.; Pezzato, A.; Michels, M.; Van Slegtenhorst, M.A.; Boersma, E.; Nieman, K.; Majoor-Krakauer, D.; et al. Diagnostic CMR Imaging Criteria in Noncompaction Cardiomyopathy and the Yield of Genetic Testing. Can. J. Cardiol. 2020, in press. [Google Scholar] [CrossRef]
- De Bakker, B.S.; De Jong, K.H.; Hagoort, J.; De Bree, K.; Besselink, C.T.; De Kanter, F.E.C.; Veldhuis, T.; Bais, B.; Schildmeijer, R.; Ruijter, J.M.; et al. An interactive three-dimensional digital atlas and quantitative database of human development. Science 2016, 354, aag0053. [Google Scholar] [CrossRef]
- E Blausen, B.; Johannes, R.S.; Hutchins, G.M. Computer-based reconstructions of the cardiac ventricles of human embryos. Am. J. Cardiovasc. Pathol. 1990, 3, 37–43. [Google Scholar]
- Miquerol, L.; Moreno-Rascon, N.; Beyer, S.; Dupays, L.; Meilhac, S.M.; Buckingham, M.E.; Franco, D.; Kelly, R.G. Biphasic Development of the Mammalian Ventricular Conduction System. Circ. Res. 2010, 107, 153–161. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; He, L.; Zhang, H.; Huang, X.; Liu, Q.; Pu, W.; Zhang, L.; Li, Y.; Zhao, H.; et al. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat. Commun. 2017, 8, 87. [Google Scholar] [CrossRef]
- Sissman, N.J. Developmental landmarks in cardiac morphogenesis: Comparative chronology. Am. J. Cardiol. 1970, 25, 141–148. [Google Scholar] [CrossRef]
- Anderson, R.H.; Jensen, B.; Mohun, T.J.; Petersen, S.E.; Aung, N.; Zemrak, F.; Planken, R.N.; Maciver, D.H. Key Questions Relating to Left Ventricular Noncompaction Cardiomyopathy: Is the Emperor Still Wearing Any Clothes? Can. J. Cardiol. 2017, 33, 747–757. [Google Scholar] [CrossRef]
- Oechslin, E.; Jenni, R. Left Ventricular Noncompaction. J. Am. Coll. Cardiol. 2018, 71, 723–726. [Google Scholar] [CrossRef]
- Luxán, G.; Casanova, J.C.; Martínez-Poveda, B.; Prados, B.; D’Amato, G.; MacGrogan, D.; Gonzalez-Rajal, A.; Dobarro, D.; Torroja, C.; Martinez, F.; et al. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 2013, 19, 193–201. [Google Scholar] [CrossRef]
- D’Amato, G.; Luxán, G.; Del Monte-Nieto, G.; Martínez-Poveda, B.; Torroja, C.; Walter, W.; Bochter, M.S.; Benedito, R.; Cole, S.; Martinez, F.; et al. Sequential Notch activation regulates ventricular chamber development. Nat. Cell Biol. 2015, 18, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Del Monte-Nieto, G.; Ramialison, M.; Adam, A.A.S.; Wu, B.; Aharonov, A.; D’Uva, G.; Bourke, L.M.; Pitulescu, M.E.; Chen, H.; De La Pompa, J.L.; et al. Control of cardiac jelly dynamics by NOTCH1 and NRG1 defines the building plan for trabeculation. Nat. Cell Biol. 2018, 557, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Sandireddy, R.; Cibi, D.M.; Gupta, P.; Singh, A.; Tee, N.; Uemura, A.; Epstein, J.A.; Singh, M.K. Semaphorin 3E/PlexinD1 signaling is required for cardiac ventricular compaction. JCI Insight 2019, 4, e125908. [Google Scholar] [CrossRef] [PubMed]
- E Sigvardsen, P.; Fuchs, A.; Kühl, J.T.; Afzal, S.; Køber, L.; Nordestgaard, B.G.; Kofoed, K.F. Left ventricular trabeculation and major adverse cardiovascular events: The Copenhagen General Population Study. Eur. Hear. J. Cardiovasc. Imaging 2020, jeaa110. [Google Scholar] [CrossRef] [PubMed]
- Röse, C. Beitrage zur vergleichenden Anatomie des Herzens der Wirbelthiere. Morphol. Jahrb. 1890, 16, 27–96. [Google Scholar]
- Rowlatt, U. Comparative anatomy of the heart of mammals. Zool. J. Linn. Soc. 1990, 98, 73–110. [Google Scholar] [CrossRef]
- Odgers, P.N.B. The Formation of the Venous Valves, the Foramen Secundum and the Septum Secundum in the Human Heart. J. Anat. 1935, 69, 412–422.5. [Google Scholar]
- Waterston, D. XII.—The Development of the Heart in Man. Trans. R. Soc. Edinb. 1919, 52, 257–302. [Google Scholar] [CrossRef]
- Patten, B.M. Developmental defects at the foramen ovale. Am. J. Pathol. 1938, 14, 135–162.9. [Google Scholar]
- Patten, B.M. The changes in circulation following birth. Am. Hear. J. 1930, 6, 192–205. [Google Scholar] [CrossRef]
- Crick, S.J.; Sheppard, M.N.; Ho, S.Y.; Gebstein, L.; Anderson, R.H. Anatomy of the pig heart: Comparisons with normal human cardiac structure. J. Anat. 1998, 193, 105–119. [Google Scholar] [CrossRef]
- Hara, H.; Virmani, R.; Ladich, E.; Mackey-Bojack, S.; Titus, J.L.; Karnicki, K.; Stewart, M.; Pelzel, J.M.; Schwartz, R.S. Patent foramen ovale: Standards for a preclinical model of prevalence, structure, and histopathologic comparability to human hearts. Catheter. Cardiovasc. Interv. 2007, 69, 266–273. [Google Scholar] [CrossRef]
- Hara, H.; Virmani, R.; Ladich, E.; Mackey-Bojack, S.; Titus, J.; Reisman, M.; Gray, W.; Nakamura, M.; Mooney, M.; Poulose, A.; et al. Patent Foramen Ovale: Current Pathology, Pathophysiology, and Clinical Status. J. Am. Coll. Cardiol. 2005, 46, 1768–1776. [Google Scholar] [CrossRef]
- Calvert, P.A.; Rana, B.S.; Kydd, A.C.; Shapiro, L.M. Patent foramen ovale: Anatomy, outcomes, and closure. Nat. Rev. Cardiol. 2011, 8, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Homma, S.; Messé, S.R.; Rundek, T.; Sun, Y.-P.; Franke, J.; Davidson, K.; Sievert, H.; Sacco, R.L.; Di Tullio, M.R. Patent foramen ovale. Nat. Rev. Dis. Prim. 2016, 2, 15087. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Joyce, W.; Gregorovicova, M.; Sedmera, D.; Wang, T.; Christoffels, V.M. Low incidence of atrial septal defects in nonmammalian vertebrates. Evol. Dev. 2019, 22, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Brown, N.A. The anatomy of the heart revisited. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 1996, 246, 1–7. [Google Scholar] [CrossRef]
- Anderson, R.H.; Spicer, D.E.; Brown, N.A.; Mohun, T.J. The Development of Septation in the Four-Chambered Heart. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2014, 297, 1414–1429. [Google Scholar] [CrossRef]
- Naqvi, N.; McCarthy, K.P.; Ho, S.Y. Anatomy of the atrial septum and interatrial communications. J. Thorac. Dis. 2018, 10, S2837–S2847. [Google Scholar] [CrossRef]
- Wessels, A. Inflow Tract Development, in Congenital Heart Diseases: The Broken Heart: Clinical Features, Human Genetics and Molecular Pathways; Springer: Vienna, Austria, 2016; pp. 55–62. [Google Scholar]
- Jensen, B.; Wang, T.; Moorman, A.F. Evolution and Development of the Atrial Septum. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2018, 302, 32–48. [Google Scholar] [CrossRef]
- Sharratt, G.P.; Webb, S.; Anderson, R.H. The vestibular defect: An interatrial communication due to a deficiency in the atrial septal component derived from the vestibular spine. Cardiol. Young 2003, 13, 184–190. [Google Scholar] [CrossRef]
- Snarr, B.S.; O’Neal, J.L.; Chintalapudi, M.R.; Wirrig, E.E.; Phelps, A.L.; Kubalak, S.W.; Wessels, A. Isl1 Expression at the Venous Pole Identifies a Novel Role for the Second Heart Field in Cardiac Development. Circ. Res. 2007, 101, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Mommersteeg, M.T. Two Distinct Pools of Mesenchyme Contribute to the Development of the Atrial Septum. Circ. Res. 2006, 99, 351–353. [Google Scholar] [CrossRef]
- Loomba, R.S.; Tretter, J.T.; Mohun, T.J.; Anderson, R.H.; Kramer, S.; Spicer, D.E. Identification and Morphogenesis of Vestibular Atrial Septal Defects. J. Cardiovasc. Dev. Dis. 2020, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.E.; Anderson, R.H.; Durrer, D.; Wellens, H.J. The anatomical substrates of wolff-parkinson-white syndrome. A clinicopathologic correlation in seven patients. Circulation 1978, 57, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.H.; Becker, A.E.; Arnold, R.; Wilkinson, J.L. The Conducting Tissues in Congenitally Corrected Transposition. Circulation 1974, 50, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Piazza, N.; De Jaegere, P.; Schultz, C.; Becker, A.E.; Serruys, P.W.; Anderson, R.H. Anatomy of the Aortic Valvar Complex and Its Implications for Transcatheter Implantation of the Aortic Valve. Circ. Cardiovasc. Interv. 2008, 1, 74–81. [Google Scholar] [CrossRef]
- Li, Y.; Chen, K.; Dai, Y.; Li, C.; Sun, Q.; Chen, R.; Gold, M.R.; Zhang, S. Left bundle branch pacing for symptomatic bradycardia: Implant success rate, safety, and pacing characteristics. Hear. Rhythm. 2019, 16, 1758–1765. [Google Scholar] [CrossRef]
- Ali, N.; Shin, M.S.; Whinnett, Z. The Emerging Role of Cardiac Conduction System Pacing as a Treatment for Heart Failure. Curr. Hear. Fail. Rep. 2020, 17, 288–298. [Google Scholar] [CrossRef]
- Sedmera, D.; Gourdie, R.G. Why do we have Purkinje fibers deep in our heart? Physiol. Res. 2014, 63, 9–18. [Google Scholar] [CrossRef]
- Anderson, R.H.; Mori, S. Wilhelm His Junior and his bundle. J. Electrocardiol. 2016, 49, 637–643. [Google Scholar] [CrossRef]
- De Almeida, M.C.; Sanchez-Quintana, D.; Anderson, R.H. Sunao Tawara: Further musings on his tribulations in providing the basis for the modern-day understanding of cardiac electrophysiology. Anat. Sci. Int. 2020, 95, 381–386. [Google Scholar] [CrossRef]
- Sanders, E.; Groot, I.J.M.; Geerts, W.J.C.; De Jong, F.; A Van Horssen, A.; Los, J.A.; Moorman, A.F.M. The local expression of adult chicken heart myosins during development. Brain Struct. Funct. 1986, 174, 187–193. [Google Scholar] [CrossRef]
- Gorza, L.; Schiaffino, S.; Vitadello, M. Heart conduction system: A neural crest derivative? Brain Res. 1988, 457, 360–366. [Google Scholar] [CrossRef]
- Anderson, R.H.; Mori, S.; Spicer, D.E.; Sanchez-Quintana, D.; Jensen, B. The Anatomy, Development, and Evolution of the Atrioventricular Conduction Axis. J. Cardiovasc. Dev. Dis. 2018, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Dobrzynski, H.; Anderson, R.H.; Atkinson, A.; Borbas, Z.; D’Souza, A.; Fraser, J.F.; Inada, S.; Logantha, S.J.; Monfredi, O.J.; Morris, G.M.; et al. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol. Ther. 2013, 139, 260–288. [Google Scholar] [CrossRef]
- Delorme, B.; Dahl, E.; Jarry-Guichard, T.; Marics, I.; Briand, J.-P.; Willecke, K.; Gros, D.; Théveniau-Ruissy, M. Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system. Dev. Dyn. 1995, 204, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Oosthoek, P.W.; Virágh, S.; Lamers, W.H.; Moorman, A.F. Immunohistochemical delineation of the conduction system. II: The atrioventricular node and Purkinje fibers. Circ. Res. 1993, 73, 482–491. [Google Scholar] [CrossRef]
- Krogh, A. The progress of physiology. Science 1929, 70, 200–204. [Google Scholar] [CrossRef]
- Mummery, C. Perspectives on the Use of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in Biomedical Research. Stem Cell Rep. 2018, 11, 1306–1311. [Google Scholar] [CrossRef]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef]
- Kvon, E.Z.; Kamneva, O.K.; Melo, U.S.; Barozzi, I.; Osterwalder, M.; Mannion, B.J.; Tissières, V.; Pickle, C.S.; Plajzer-Frick, I.; Lee, E.A.; et al. Progressive Loss of Function in a Limb Enhancer during Snake Evolution. Cell 2016, 167, 633–642. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, B.; Christoffels, V.M.; Moorman, A.F.M. An Appreciation of Anatomy in the Molecular World. J. Cardiovasc. Dev. Dis. 2020, 7, 44. https://doi.org/10.3390/jcdd7040044
Jensen B, Christoffels VM, Moorman AFM. An Appreciation of Anatomy in the Molecular World. Journal of Cardiovascular Development and Disease. 2020; 7(4):44. https://doi.org/10.3390/jcdd7040044
Chicago/Turabian StyleJensen, Bjarke, Vincent M. Christoffels, and Antoon F. M. Moorman. 2020. "An Appreciation of Anatomy in the Molecular World" Journal of Cardiovascular Development and Disease 7, no. 4: 44. https://doi.org/10.3390/jcdd7040044
APA StyleJensen, B., Christoffels, V. M., & Moorman, A. F. M. (2020). An Appreciation of Anatomy in the Molecular World. Journal of Cardiovascular Development and Disease, 7(4), 44. https://doi.org/10.3390/jcdd7040044




