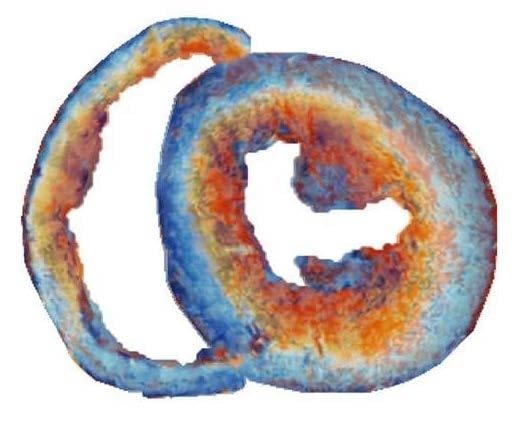
Resolving the True Ventricular Mural Architecture
Abstract
1. Introduction
2. Previous Studies of Ventricular Mural Architecture
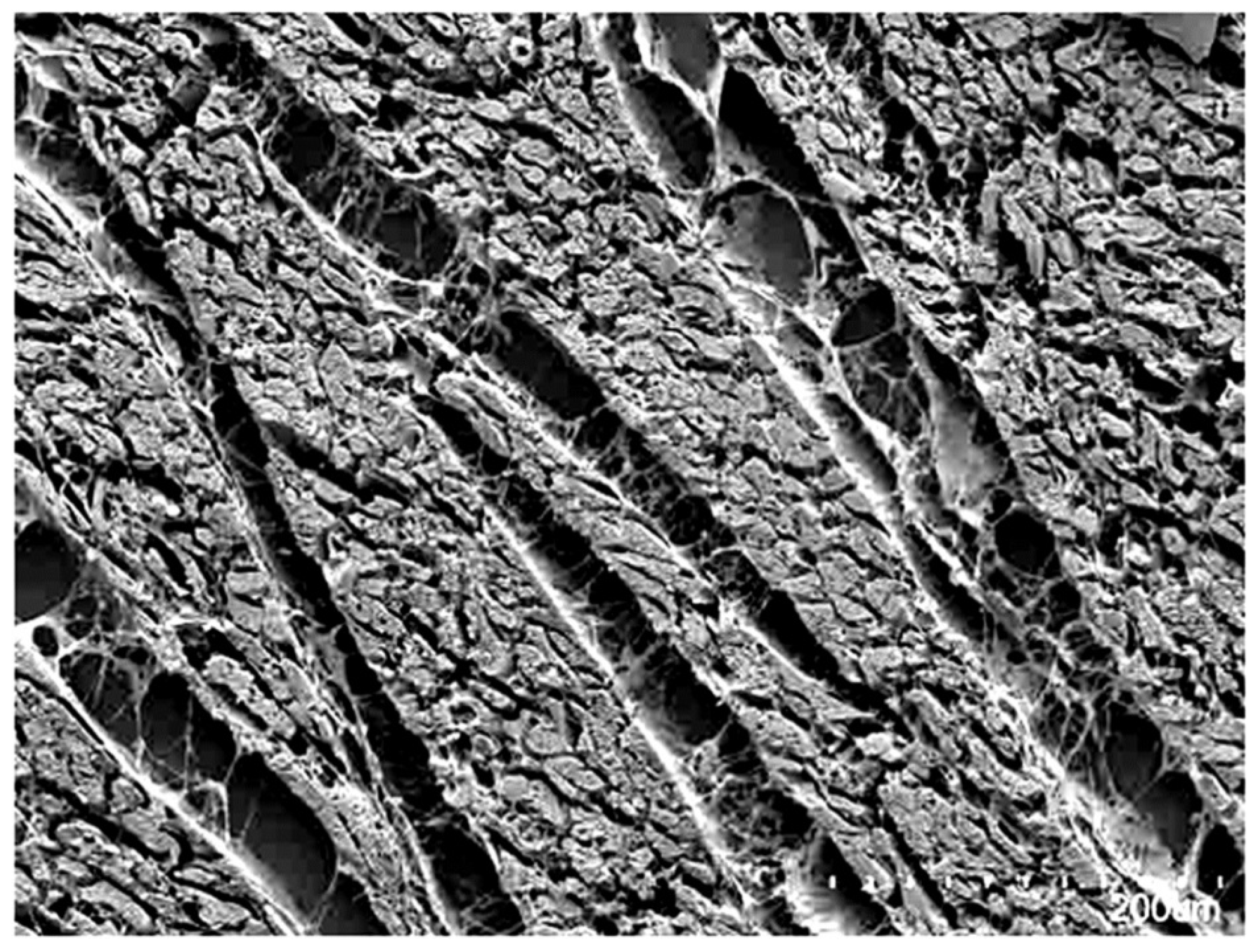
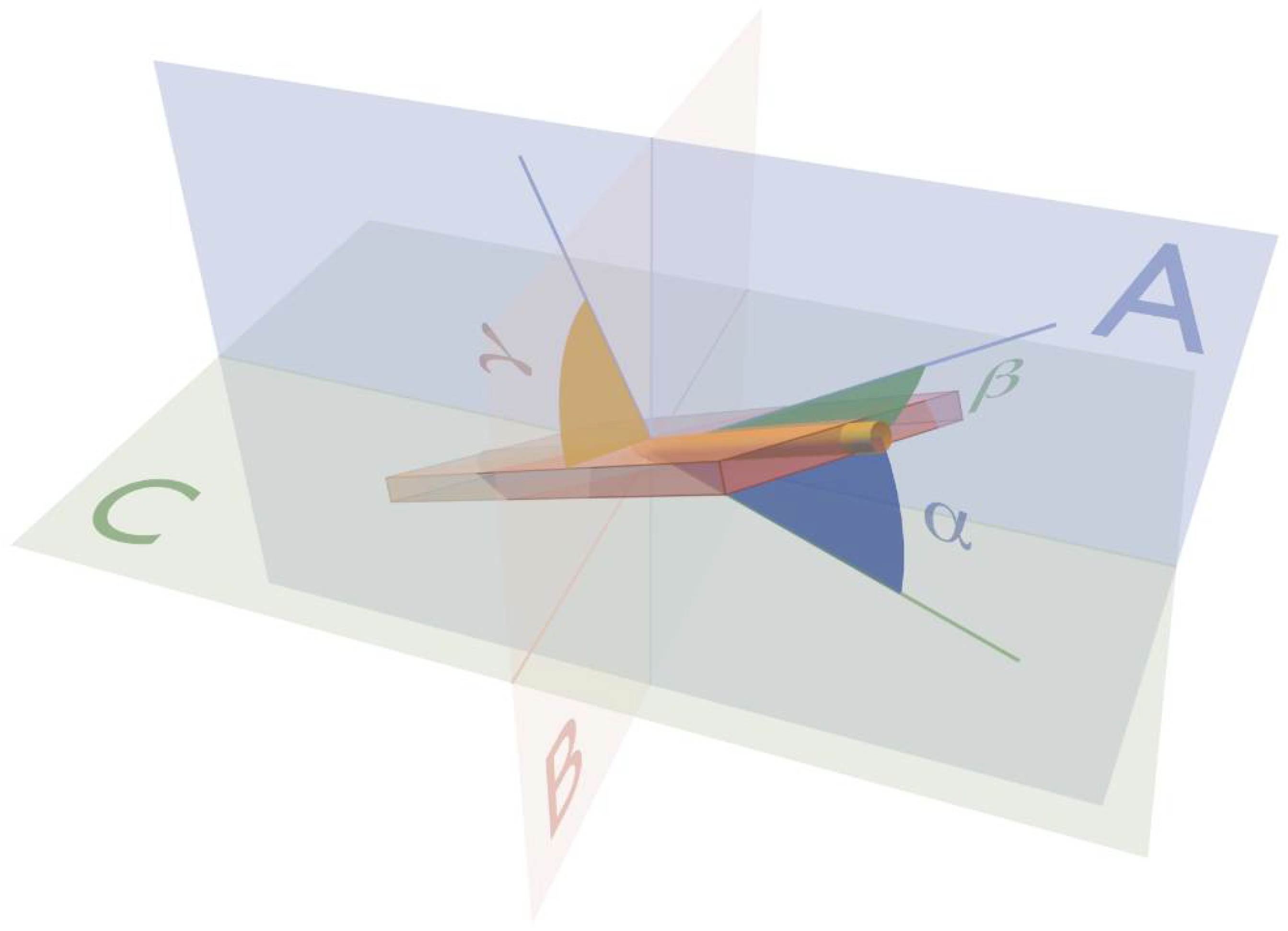
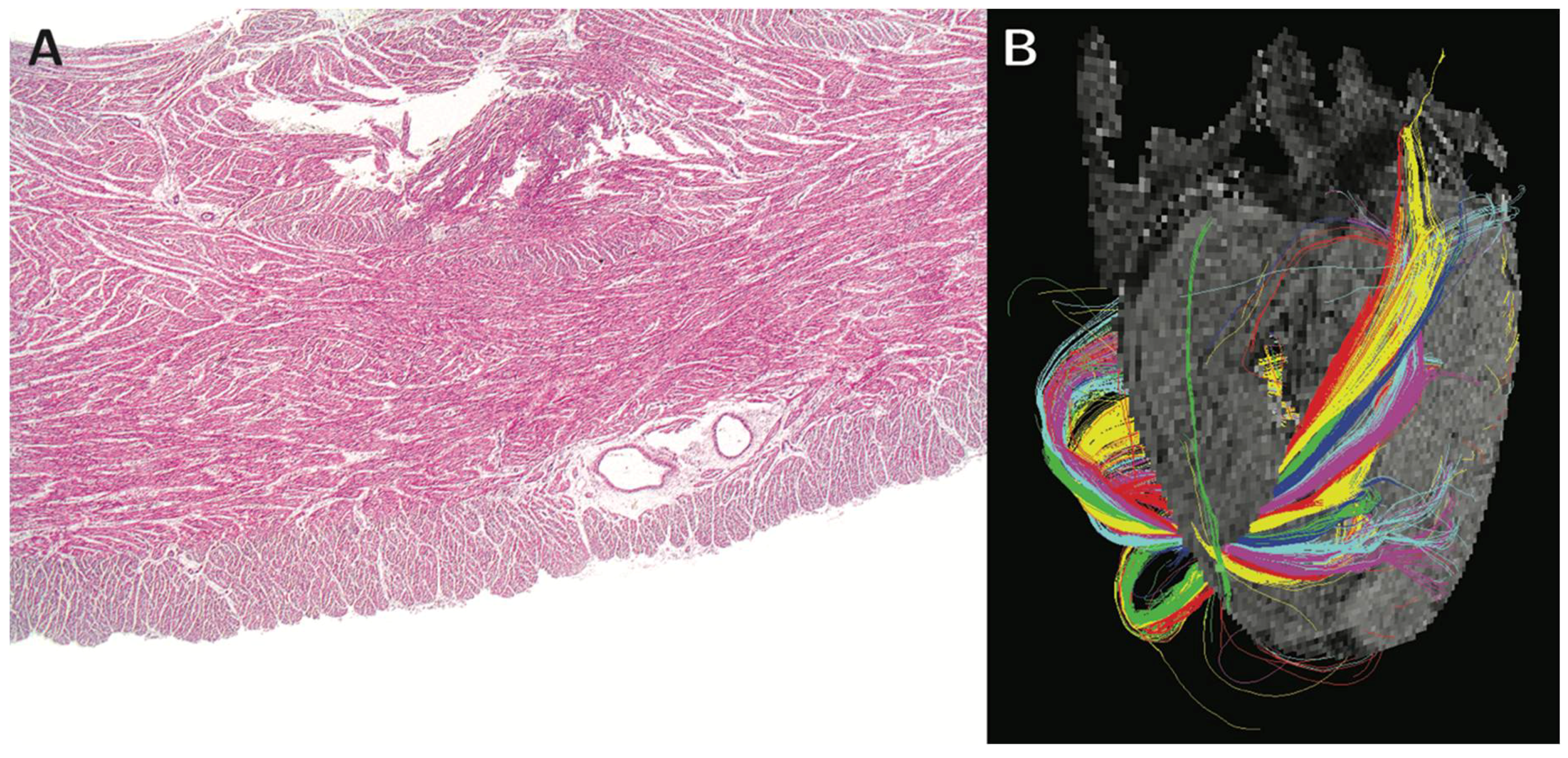
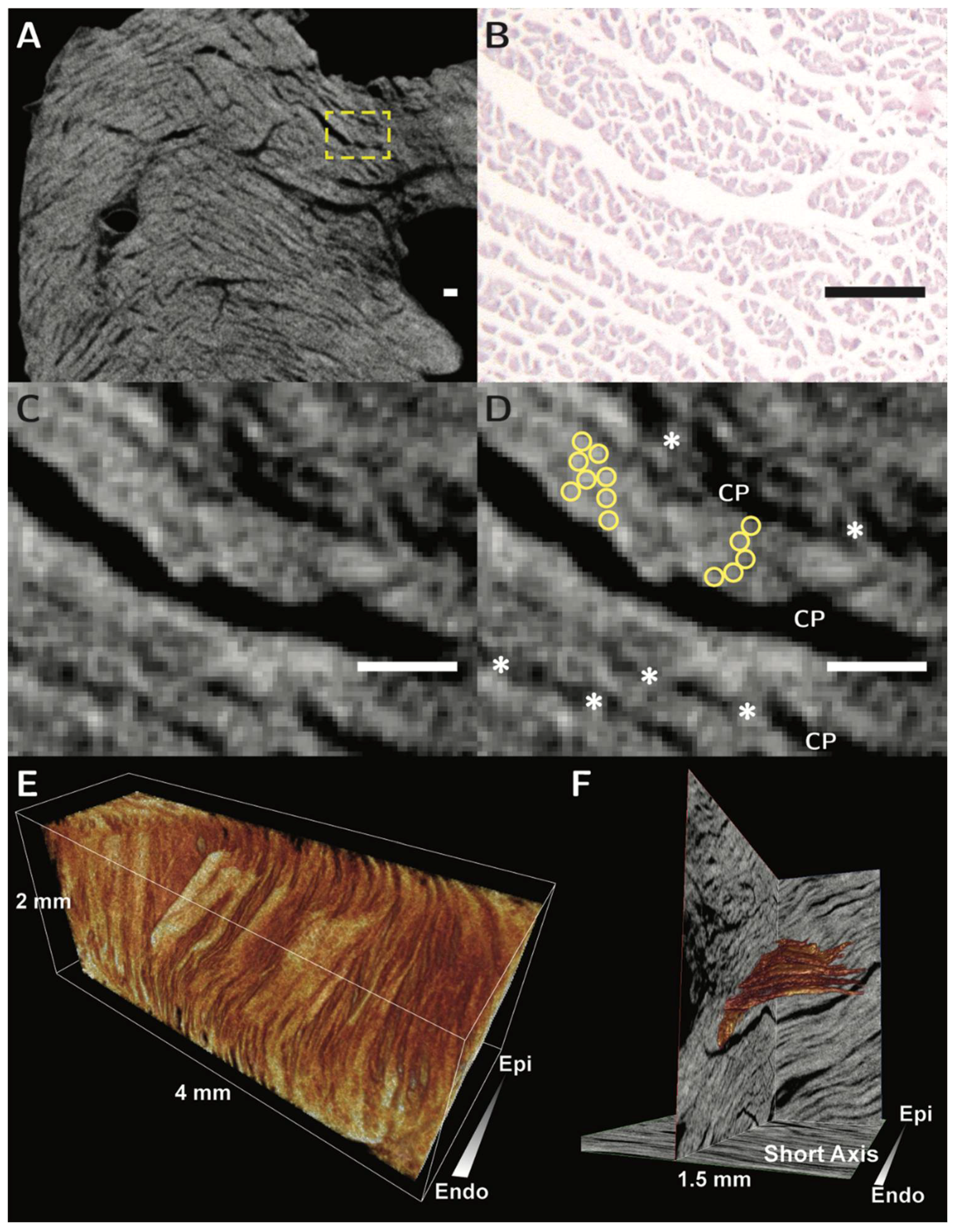
3. Ventricular Mural Architecture as Revealed by 3-Dimensional Imaging
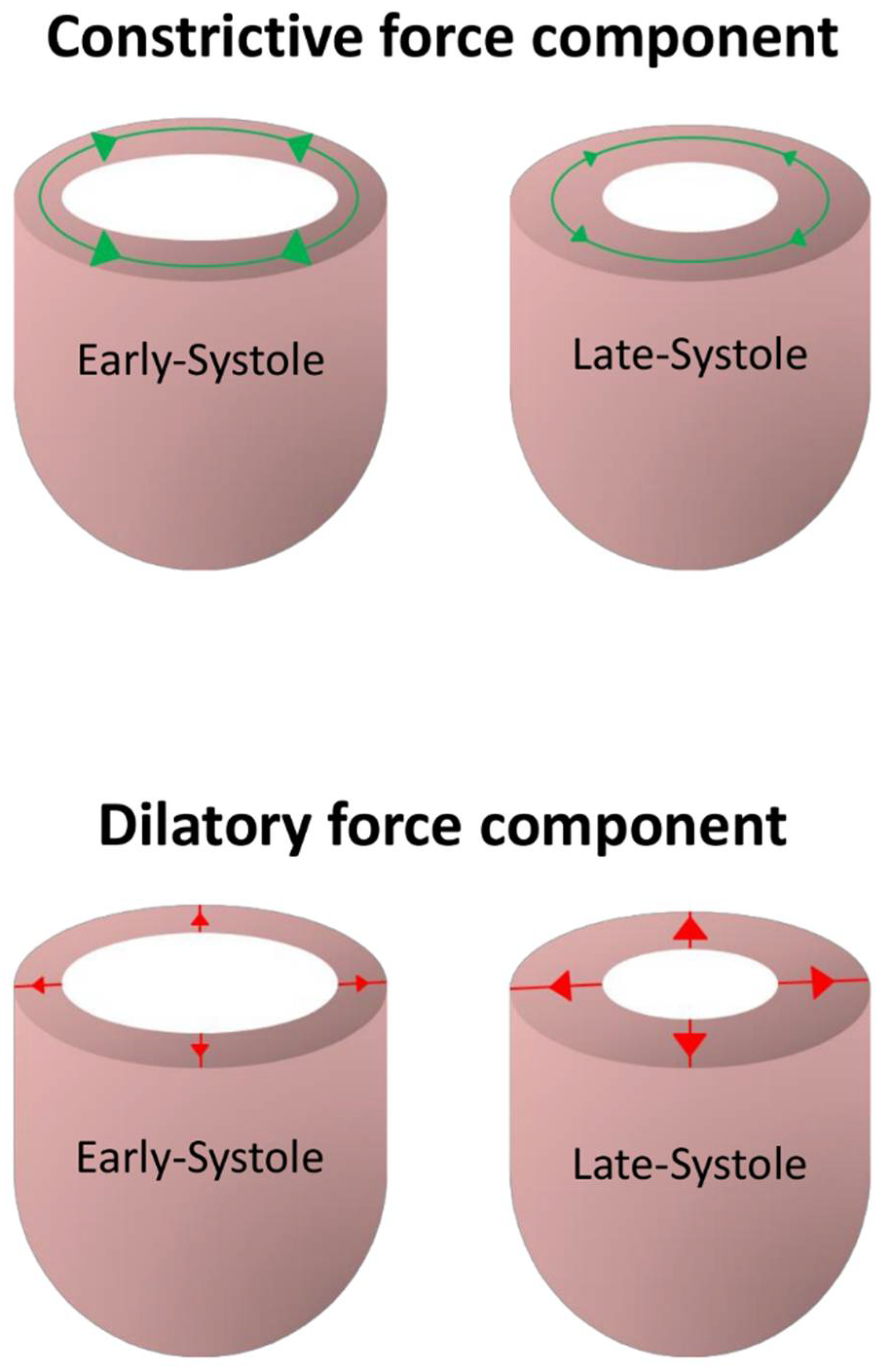
4. Functional Considerations
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, R.H.; Ho, S.Y.; Redmann, K.; Sanchez-Quintana, D.; Lunkenheimer, P.P. The anatomical arrangement of the myocardial cells making up the ventricular mass. Eur. J. Cardio-Thorac. Surg. 2005, 28, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Lunkenheimer, P.P.; Redmann, K.; Kling, N.; Jiang, X.; Rothaus, K.; Cryer, C.W.; Wübbeling, F.; Niederer, P.; Heitz, P.U.; Yen Ho, S.; et al. Three-dimensional architecture of the left ventricular myocardium. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288A, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Lunkenheimer, P.P.; Redmann, K.; Westermann, P.; Rothaus, K.; Cryer, C.W.; Niederer, P.; Anderson, R.H. The myocardium and its fibrous matrix working in concert as a spatially netted mesh: A critical review of the purported tertiary structure of the ventricular mass. Eur. J. Cardio-Thorac. Surg. 2006, 29, S41–S49. [Google Scholar] [CrossRef] [PubMed]
- Torrent-Guasp, F. La estructuracion macroscopica del miocardio ventricular. Rev. Esp. Cardiol. 1980, 33, 265–298. [Google Scholar] [PubMed]
- Torrent-Guasp, F.; Kocica, M.J.; Corno, A.F.; Komeda, M.; Carreras-Costa, F.; Flotats, A.; Cosin-Aguillar, J.; Wen, H. Towards new understanding of the heart structure and function. Eur. J. Cardio-Thorac. Surg. 2005, 27, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.D.; Coghlan, H.C.; Torrent-Guasp, F. The structure and function of the helical heart and its buttress wrapping. v. anatomic and physiologic considerations in the healthy and failing heart. Semin. Thorac. Cardiovasc. Surg. 2001, 13, 358–385. [Google Scholar] [CrossRef] [PubMed]
- LeGrice, I.J.; Smaill, B.H.; Chai, L.Z.; Edgar, S.G.; Gavin, J.B.; Hunter, P.J. Laminar structure of the heart: Ventricular myocyte arrangement and connective tissue architecture in the dog. Am. J. Physiol. 1995, 269, H571–H582. [Google Scholar] [CrossRef] [PubMed]
- Hooks, D.A.; Tomlinson, K.A.; Marsden, S.G.; LeGrice, I.J.; Smaill, B.H.; Pullan, A.J.; Hunter, P.J. Cardiac microstructure. implications for electrical propagation and defibrillation in the heart. Circ. Res. 2002. [Google Scholar] [CrossRef]
- Pope, A.J.; Sands, G.B.; Smaill, B.H.; LeGrice, I.J. Three-dimensional transmural organization of perimysial collagen in the heart. Am. J. Physiol. 2008, 295, H1243–H1252. [Google Scholar] [CrossRef] [PubMed]
- Axel, L.; Wedeen, V.J.; Ennis, D.B. Probing dynamic myocardial microstructure with cardiac magnetic resonance diffusion tensor imaging. J. Cardiovasc. Magn. Reson. 2014, 16, 89. [Google Scholar] [CrossRef] [PubMed]
- Nielles-Vallespin, S.; Khalique, Z.; Ferreira, P.F.; de Silva, R.; Scott, A.D.; Kilner, P.; McGill, L.-A.; Giannakidis, A.; Gatehouse, P.D.; Ennis, D.; et al. Assessment of myocardial microstructural dynamics by in vivo diffusion tensor cardiac magnetic resonance. J. Am. Coll. Cardiol. 2017, 69, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Caulfield, J.B.; Borg, T. The collagen network of the heart. Lab. Investig. 1979, 40, 364–372. [Google Scholar] [PubMed]
- Anderson, R.H.; Ho, S.Y.; Sanchez-Quintana, D.; Redmann, K.; Lunkenheimer, P.P. Heuristic problems in defining the three-dimensional arrangement of the ventricular myocytes. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288A, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Dorri, F.; Niederer, P.F.; Redmann, K.; Lunkenheimer, P.P.; Cryer, C.W.; Anderson, R.H. An analysis of the spatial arrangement of the myocardial aggregates making up the wall of the left ventricle. Eur. J. Cardio-Thorac. Surg. 2007, 31, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Kung, G.L.; Nguyen, T.C.; Itoh, A.; Skare, S.; Ingels, N.B.; Miller, D.C.; Ennis, D.B. The presence of two local myocardial sheet populations confirmed by diffusion tensor MRI and histological validation. J. Magn. Reson. Imaging 2011, 34, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Lunkenheimer, P.P.; Niederer, P.; Stephenson, R.S.; Redmann, K.; Batista, R.V.; Smerup, M.; Anderson, R.H. What is the clinical significance of ventricular mural antagonism? Eur. J. Cardio-Thorac. Surg. 2018, 53, 714–723. [Google Scholar] [CrossRef] [PubMed]
- MacIver, D.H.; Stephenson, R.S.; Jensen, B.; Agger, P.; Sánchez-Quintana, D.; Jarvis, J.C.; Partridge, J.B.; Anderson, R.H. The end of the unique myocardial band: Part I. Anatomical considerations. Eur. J. Cardio-Thorac. Surg. 2018, 53, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.S.; Agger, P.; Lunkenheimer, P.P.; Zhao, J.; Smerup, M.; Niederer, P.; Anderson, R.H.; Jarvis, J.C. The functional architecture of skeletal compared to cardiac musculature: Myocyte orientation, lamellar unit morphology, and the helical ventricular myocardial band. Clin. Anat. 2016, 29, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Agger, P.; Ilkjær, C.; Laustsen, C.; Smerup, M.; Frandsen, J.R.; Ringgaard, S.; Pedersen, M.; Partridge, J.B.; Anderson, R.H.; Hjortdal, V. Changes in overall ventricular myocardial architecture in the setting of a porcine animal model of right ventricular dilation. J. Cardiovasc. Magn. Reson. 2017, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Lunkenheimer, P.P.; Redmann, K.; Florek, J.; Fassnacht, U.; Cryer, C.W.; Wubbeling, F.; Niederer, P.; Anderson, R.H. The forces generated within the musculature of the left ventricular wall. Heart 2004, 90, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Wells, F. The Heart of Leonardo; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Keele, K.D. Leonardo da Vinci, and the Movement of the Heart and Blood; Harvey and Blythe Ltd.: London, UK, 1952. [Google Scholar]
- Galen, C. De usu Partium Corporis Humani: Magna cura ad Exemplaris Graeci Veritatem Castigatum, Universo Hominum Generi Apprime Necessarium; Exofficina Simonis Colinaei, 1528; p. 484. [Google Scholar]
- Lower, R. Tractatus de Corde; Early Science in Oxford: London, UK, 1669. [Google Scholar]
- Sénac, J.-B. Traité de la Structure du Coeur, de son Action, et de ses Maladies; Briasson, 1749; Volume 2. [Google Scholar]
- Pettigrew, J.B. The croonian lecture: on the arrangement of the muscular fibres of the ventricular portion of the heart of the mammal. Proc. R. Soc. Lond. 1860, 10, 433–440. [Google Scholar] [CrossRef]
- Pettigrew, J.B. On the arrangement of the muscular fibres in the ventricles of the vertebrate heart, with physiological remarks. Philos. Trans. R. Soc. Lond. 1864, 154, 445–500. [Google Scholar] [CrossRef]
- Pettigrew, J.B. Design in Nature; Longmans, Green & Co.: London, UK; New York NY, USA; Bombay, India; Calcutta, India, 1908. [Google Scholar]
- MacCallum, J.B. On the muscular architecture and growth of the ventricles of the heart. Bull. Johns Hopkins Hosp. 1900, 9, 307–335. [Google Scholar]
- Mall, F.P. On the muscular architecture of the ventricles of the human heart. Am. J. Anat. 1911, 11, 211–266. [Google Scholar] [CrossRef]
- Lev, M.; Simkins, C.S. Architecture of the human ventricular myocardium; technic for study using a modification of the Mall-MacCallum method. Lab. Investig. 1956, 5, 396–409. [Google Scholar] [PubMed]
- Grant, R.P. Architectonics of the heart. Am. Heart J. 1953, 46, 405–430. [Google Scholar] [CrossRef]
- Streeter, D.D.; Bassett, D.L. An engineering analysis of myocardial fiber orientation in pig’s left ventricle in systole. Anat. Rec. 1966, 155, 503–511. [Google Scholar] [CrossRef]
- Streeter, D.D.; Torrent-Guasp, F. Geodesic paths in the left ventricle of the mammalian heart. Circ. Suppl. 1973, 48, 4–14. [Google Scholar]
- Streeter, D.D.; Powers, W.E.; Ross, M.A.; Torrent-Guasp, F. Three dimensional fiber orientation in the mammalian left venticular wall. In Cardiovascular System Dynamics; Barn, J., Noordegraf, A., Raines, J., Eds.; MIT Press: Cambridge, MA, USA, 1978; pp. 73–84. [Google Scholar]
- Streeter, D.D. Gross morphology and fiber geometry of the heart. In Handbook of Physiology; American Physiological Society, 1979; pp. 61–112. [Google Scholar]
- Krehl, L. Beiträge zur kenntniss der füllung und entleerung des herzens. Abh. Math. Phys. Kl. Saechs. Akad. Wiss. 1891, 17, 341–362. [Google Scholar]
- Fox, C.C.; Hutchins, G.M. The architecture of the human ventricular myocardium. Johns Hopkins Med. J. 1972, 130, 289–299. [Google Scholar] [PubMed]
- Frank, O. Isometrie und Isotonie des Herzmuskels. Z. Biol. 1901, 41, 14–34. [Google Scholar]
- Feneis, H. Das gefüge des herzmuskels bei systole und diastole. Morphol. Jahrb. 1943, 89, 371–406. [Google Scholar]
- Hort, W. Makroskopische und mikrometrische Untersuchungen am Myokard verschieden stark gefüllter linker Kammern. Virchows Arch. Pathol. Anat. Physiol. Klin. Med. 1960, 333, 523–564. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Lunkenheimer, P.P.; Redmann, K.; Rothaus, K.; Jiang, X.; Cryer, C.W.; Jaermann, T.; Niederer, P.; Boesiger, P.; Anderson, R.H. Statistical analysis of the angle of intrusion of porcine ventricular myocytes from epicardium to endocardium using diffusion tensor magnetic resonance imaging. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2007, 290, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Teh, I.; McClymont, D.; Zdora, M.-C.; Whittington, H.J.; Davidoiu, V.; Lee, J.; Lygate, C.A.; Rau, C.; Zanette, I.; Schneider, J.E. Validation of diffusion tensor MRI measurements of cardiac microstructure with structure tensor synchrotron radiation imaging. J. Cardiovasc. Magn. Reson. 2017, 19, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Butters, T.D.; Zhang, H.; Pullan, A.J.; LeGrice, I.J.; Sands, G.B.; Smaill, B.H. An image-based model of atrial muscular architecture: effects of structural anisotropy on electrical activation. Circ. Arrhythm. Electrophysiol. 2012, 5, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Agger, P.; Stephenson, R.S.; Dobrzynski, H.; Atkinson, A.; Iaizzo, P.A.; Anderson, R.H.; Jarvis, J.C.; Allan, S.L.; Partridge, J.B.; Zhao, J.; et al. Insights from echocardiography, magnetic resonance imaging, and microcomputed tomography relative to the mid-myocardial left ventricular echogenic zone. Echocardiography 2016, 33, 1546–1556. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.I.E. Will the real ventricular architecture please stand up? Physiol. Rep. 2017, 5, e13404. [Google Scholar] [CrossRef] [PubMed]
- Hayabuchi, Y.; Sakata, M.; Kagami, S. Assessment of the helical ventricular myocardial band using standard echocardiography. Echocardiography 2015, 32, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Agger, P.; Lakshminrusimha, S.; Laustsen, C.; Gugino, S.; Frandsen, J.R.; Smerup, M.; Anderson, R.H.; Hjortdal, V.; Steinhorn, R.H. The myocardial architecture changes in persistent pulmonary hypertension of the newborn in an ovine animal model. Pediatr. Res. 2015, 79, 565. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.Y.; Nihoyannopoulos, P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006, 92, i2–i13. [Google Scholar] [CrossRef] [PubMed]
- Buckberg, G.D. Reply to Anderson et al. Eur. J. Cardio-Thorac. Surg. 2018. [Google Scholar] [CrossRef] [PubMed]
- Smerup, M.; Agger, P.; Nielsen, E.A.; Ringgaard, S.; Pedersen, M.; Niederer, P.; Anderson, R.H.; Lunkenheimer, P.P. Regional and epi- to endocardial differences in transmural angles of left ventricular cardiomyocytes measured in ex vivo pig hearts: functional implications. Anat. Rec. 2013, 296, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Brachet, J. Sur la Cause du Mouvement de Dilatiation du Coeur; Faculté de Médecine de Paris: Paris, France, 1813. [Google Scholar]
- Lunkenheimer, P.P.; Redmann, K.; Cryer, C.W.; Batista, R.V.; Stanton, J.J.; Niederer, P.; Anderson, R.H. Beta-blockade at low doses restoring the physiological balance in myocytic antagonism. Eur. J. Cardio-Thorac. Surg. 2007, 32, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Kier, W.; Smith, K. Tongues, tentacles and trunks: The biomechanics of movement in muscular-hydrostats. Zool. J. Linnean Soc. 1985, 83, 307–324. [Google Scholar] [CrossRef]
- Carlsson, M.; Cain, P.; Holmqvist, C.; Stahlberg, F.; Lundback, S.; Arheden, H. Total heart volume variation throughout the cardiac cycle in humans. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H243–H250. [Google Scholar] [CrossRef] [PubMed]
- Smerup, M.; Partridge, J.; Agger, P.; Ringgaard, S.; Pedersen, M.; Petersen, S.; Hasenkam, J.M.; Niederer, P.; Lunkenheimer, P.P.; Anderson, R.H. A mathematical model of the mechanical link between shortening of the cardiomyocytes and systolic deformation of the left ventricular myocardium. Technol. Health Care 2013, 21, 63–79. [Google Scholar] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephenson, R.S.; Agger, P.; Omann, C.; Sanchez-Quintana, D.; Jarvis, J.C.; Anderson, R.H. Resolving the True Ventricular Mural Architecture. J. Cardiovasc. Dev. Dis. 2018, 5, 34. https://doi.org/10.3390/jcdd5020034
Stephenson RS, Agger P, Omann C, Sanchez-Quintana D, Jarvis JC, Anderson RH. Resolving the True Ventricular Mural Architecture. Journal of Cardiovascular Development and Disease. 2018; 5(2):34. https://doi.org/10.3390/jcdd5020034
Chicago/Turabian StyleStephenson, Robert S., Peter Agger, Camilla Omann, Damian Sanchez-Quintana, Jonathan C. Jarvis, and Robert H. Anderson. 2018. "Resolving the True Ventricular Mural Architecture" Journal of Cardiovascular Development and Disease 5, no. 2: 34. https://doi.org/10.3390/jcdd5020034
APA StyleStephenson, R. S., Agger, P., Omann, C., Sanchez-Quintana, D., Jarvis, J. C., & Anderson, R. H. (2018). Resolving the True Ventricular Mural Architecture. Journal of Cardiovascular Development and Disease, 5(2), 34. https://doi.org/10.3390/jcdd5020034