The Role of Cerl2 in the Establishment of Left-Right Asymmetries during Axis Formation and Heart Development
Abstract
:1. The Development and Evolution of Left-Right Asymmetry
2. Left-Right Establishment
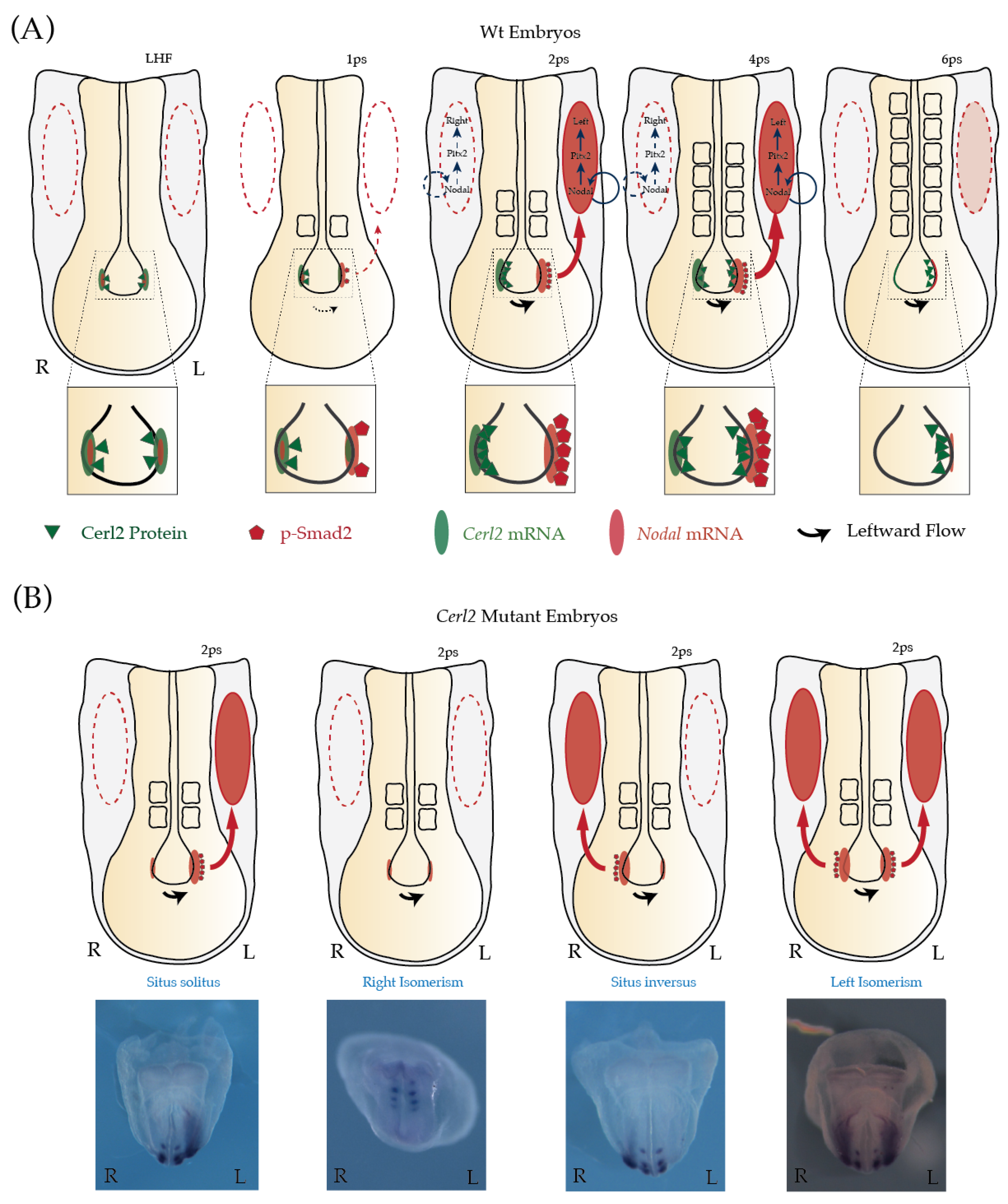
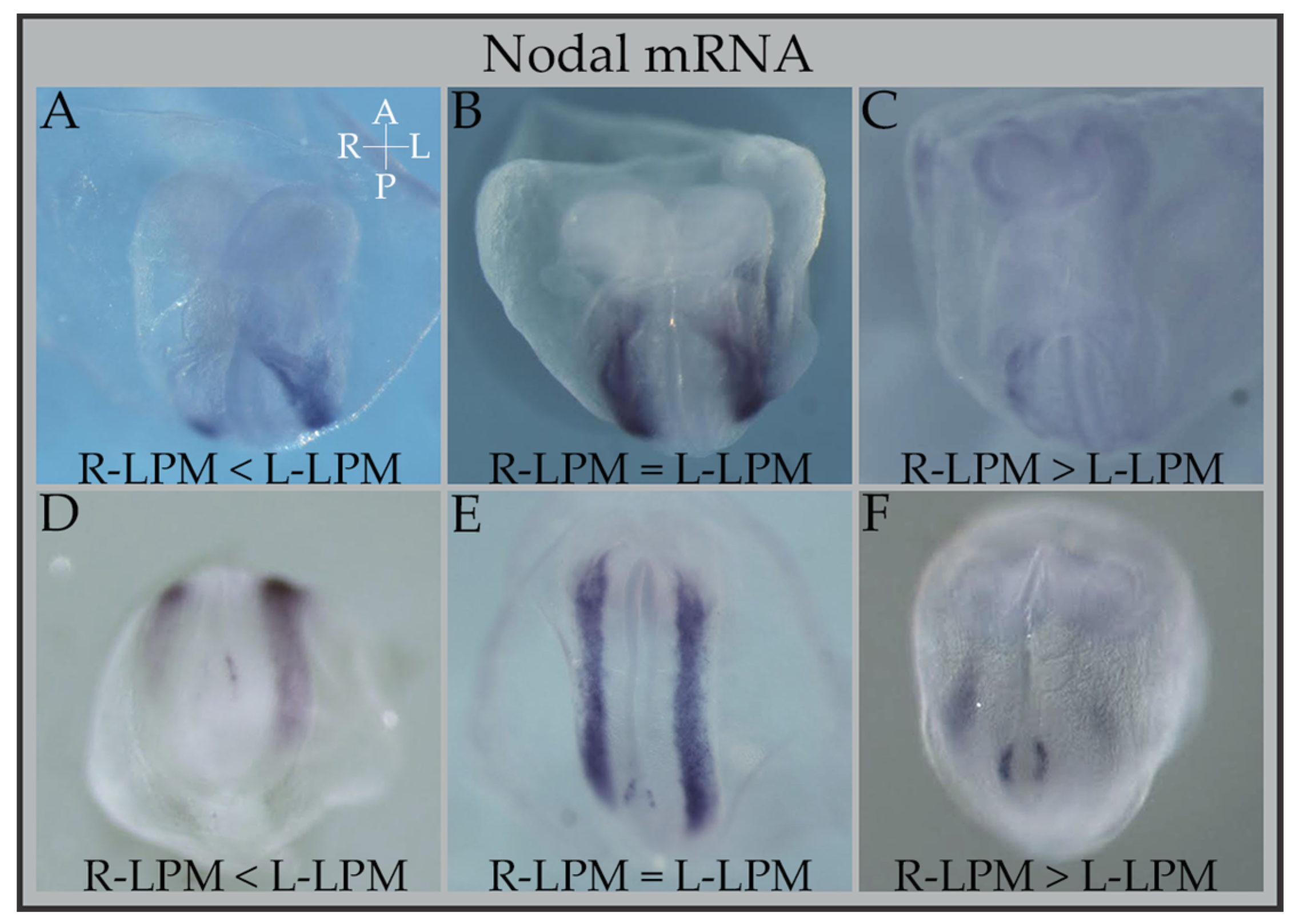
3. Cerl2 Is Crucial to Tune Nodal’s Bioavailability to the Embryo
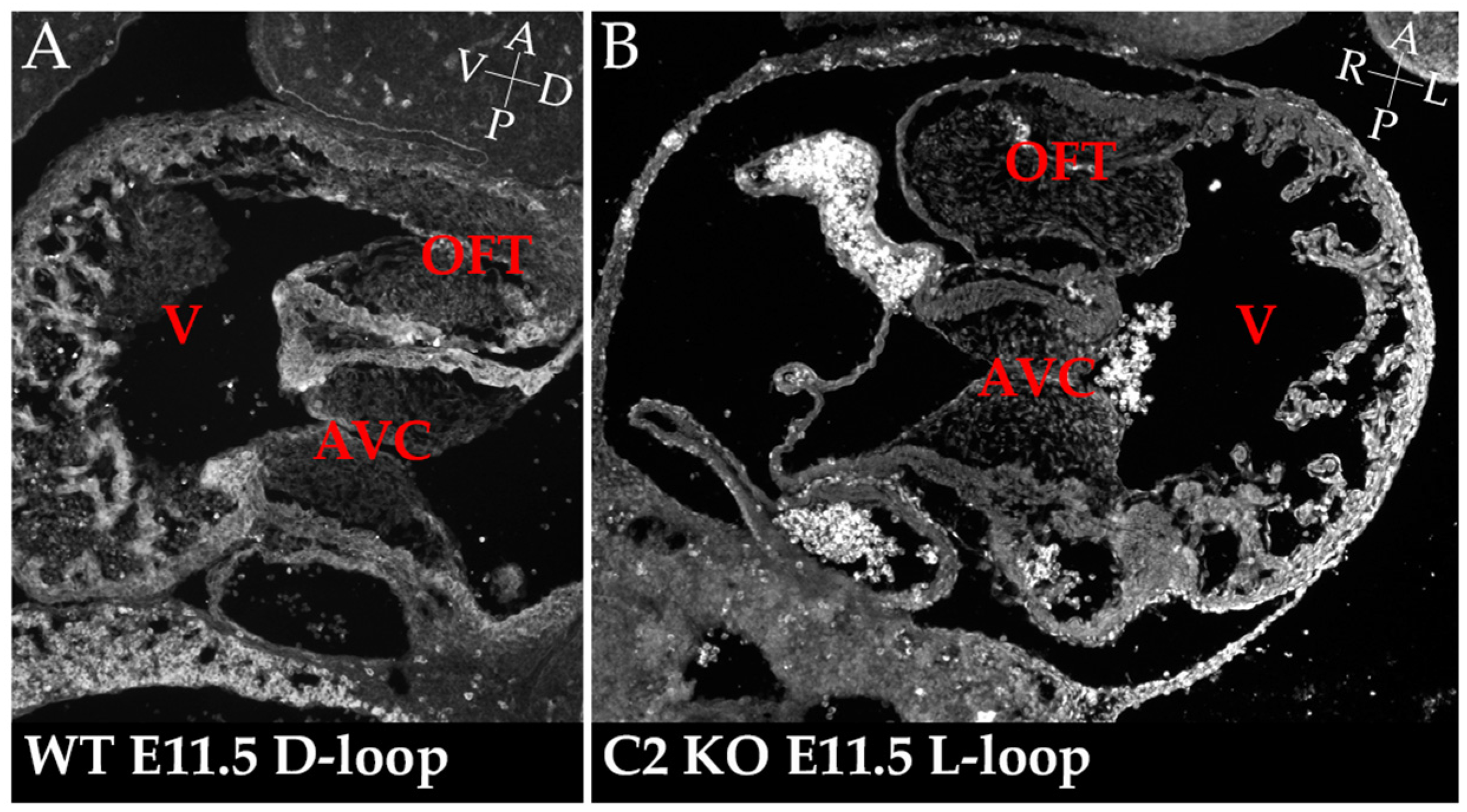
4. Cerl2 and Cardiac Left-Right Development
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cooke, J. Developmental mechanism and evolutionary origin of vertebrate left/right asymmetries. Biol. Rev. 2004, 79, 377–407. [Google Scholar] [CrossRef] [PubMed]
- Kilner, P.J.; Yang, G.Z.; Wilkes, A.J.; Mohiaddin, R.H.; Firmin, D.N.; Yacoub, M.H. Asymmetric redirection of flow through the heart. Nature 2000, 404, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Ramsdell, A.F. Left-right asymmetry and congenital cardiac defects: Getting to the heart of the matter in vertebrate left-right axis determination. Dev. Biol. 2005, 288, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Tam, P.P. Mechanisms of left-right asymmetry and patterning: Driver, mediator and responder. F1000Prime Rep. 2014, 6, 110. [Google Scholar] [CrossRef] [PubMed]
- Levin, M. Left-right asymmetry in embryonic development: A comprehensive review. Mech. Dev. 2005, 122, 3–25. [Google Scholar] [CrossRef] [PubMed]
- Burdine, R.D.; Schier, A.F. Conserved and divergent mechanisms in left-right axis formation. Genes Dev. 2000, 14, 763–776. [Google Scholar] [PubMed]
- Capdevila, J.; Vogan, K.J.; Tabin, C.J.; Izpisua Belmonte, J.C. Mechanisms of left-right determination in vertebrates. Cell 2000, 101, 9–21. [Google Scholar] [CrossRef]
- Grimes, D.T.; Burdine, R.D. Left-right patterning: Breaking symmetry to asymmetric morphogenesis. Trends Genet. 2017, 33, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Raya, A.; Izpisua Belmonte, J.C. Left-right asymmetry in the vertebrate embryo: From early information to higher-level integration. Nat. Rev. Genet. 2006, 7, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Tabin, C. Do we know anything about how left-right asymmetry is first established in the vertebrate embryo? J. Mol. Histol. 2005, 36, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.; Norris, D.P.; Robertson, E.J. Nodal activity in the node governs left-right asymmetry. Genes Dev. 2002, 16, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.D.; Anderson, K.V. Morphogenesis of the node and notochord: The cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Dev. Dyn. 2008, 237, 3464–3476. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, D.; Papaioannou, V.E. Nature and extent of left/right axis defects in TWis/TWis mutant mouse embryos. Dev. Dyn. 2014, 243, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y. The multiple roles of notch signaling during left-right patterning. Cell. Mol. Life Sci. 2011, 68, 2555–2567. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, H.; Hamada, H. The left-right axis in the mouse: From origin to morphology. Development 2006, 133, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Hu, J.; Chen, W.; Elliott, G.; Andre, P.; Gao, B.; Yang, Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature 2010, 466, 378–382. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.; Somlo, S.; Makova, S.; Tian, X.; Brueckner, M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 2003, 114, 61–73. [Google Scholar] [CrossRef]
- Tabin, C.J.; Vogan, K.J. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yoshiba, S.; Shiratori, H.; Kuo, I.Y.; Kawasumi, A.; Shinohara, K.; Nonaka, S.; Asai, Y.; Sasaki, G.; Belo, J.A.; Sasaki, H.; et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 2012, 338, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Belo, J.A.; Silva, A.C.; Borges, A.C.; Filipe, M.; Bento, M.; Goncalves, L.; Vitorino, M.; Salgueiro, A.M.; Texeira, V.; Tavares, A.T.; et al. Generating asymmetries in the early vertebrate embryo: The role of the cerberus-like family. Int. J. Dev. Biol. 2009, 53, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Inacio, J.M.; Marques, S.; Nakamura, T.; Shinohara, K.; Meno, C.; Hamada, H.; Belo, J.A. The dynamic right-to-left translocation of Cerl2 is involved in the regulation and termination of Nodal activity in the mouse node. PLoS ONE 2013, 8, e60406. [Google Scholar] [CrossRef] [PubMed]
- Marques, S.; Borges, A.C.; Silva, A.C.; Freitas, S.; Cordenonsi, M.; Belo, J.A. The activity of the nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes Dev. 2004, 18, 2342–2347. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, K.; Kawasumi, A.; Takamatsu, A.; Yoshiba, S.; Botilde, Y.; Motoyama, N.; Reith, W.; Durand, B.; Shiratori, H.; Hamada, H. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat. Commun. 2012, 3, 622. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Kitajima, K.; Marques, S.; Belo, J.A.; Yokoyama, T.; Hamada, H.; Meno, C. Reversal of left-right asymmetry induced by aberrant nodal signaling in the node of mouse embryos. Development 2009, 136, 3917–3925. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Saito, D.; Kawasumi, A.; Shinohara, K.; Asai, Y.; Takaoka, K.; Dong, F.; Takamatsu, A.; Belo, J.A.; Mochizuki, A.; et al. Fluid flow and interlinked feedback loops establish left-right asymmetric decay of Cerl2 mRNA. Nat. Commun. 2012, 3, 1322. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Oki, S.; Ohkawa, Y.; Sumi, T.; Meno, C. Wnt signaling regulates left-right axis formation in the node of mouse embryos. Dev. Biol. 2013, 380, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Kawasumi, A.; Nakamura, T.; Iwai, N.; Yashiro, K.; Saijoh, Y.; Belo, J.A.; Shiratori, H.; Hamada, H. Left-right asymmetry in the level of active nodal protein produced in the node is translated into left-right asymmetry in the lateral plate of mouse embryos. Dev. Biol. 2011, 353, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Hashimoto, R.; Okui, Y.; Shen, M.M.; Mekada, E.; Otani, H.; Saijoh, Y.; Hamada, H. Sulfated glycosaminoglycans are necessary for nodal signal transmission from the node to the left lateral plate in the mouse embryo. Development 2007, 134, 3893–3904. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.K.; Olale, F.; Brivanlou, A.H.; Schier, A.F. Lefty blocks a subset of TGFβ signals by antagonizing EGF-CFC coreceptors. PLoS Biol. 2004, 2, E30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, L.A.; Supp, D.M.; Sampath, K.; Yokoyama, T.; Wright, C.V.; Potter, S.S.; Overbeek, P.; Kuehn, M.R. Conserved left-right asymmetry of Nodal expression and alterations in murine situs inversus. Nature 1996, 381, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Meno, C.; Saijoh, Y.; Fujii, H.; Ikeda, M.; Yokoyama, T.; Yokoyama, M.; Toyoda, Y.; Hamada, H. Left-right asymmetric expression of the TGF β-family member lefty in mouse embryos. Nature 1996, 381, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, R.; Ohnishi Yi, Y.; Meno, C.; Fujii, H.; Juan, H.; Takeuchi, J.; Ogura, T.; Li, E.; Miyazono, K.; Hamada, H. Inhibition of nodal signalling by lefty mediated through interaction with common receptors and efficient diffusion. Genes Cells 2002, 7, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, H.; Gierer, A. Pattern formation by local self-activation and lateral inhibition. Bioessays 2000, 22, 753–760. [Google Scholar] [CrossRef]
- Muller, P.; Rogers, K.W.; Jordan, B.M.; Lee, J.S.; Robson, D.; Ramanathan, S.; Schier, A.F. Differential diffusivity of nodal and lefty underlies a reaction-diffusion patterning system. Science 2012, 336, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Mine, N.; Nakaguchi, E.; Mochizuki, A.; Yamamoto, M.; Yashiro, K.; Meno, C.; Hamada, H. Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev. Cell 2006, 11, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Norris, D.; Ventikos, Y. The active and passive ciliary motion in the embryo node: A computational fluid dynamics model. J. Biomech. 2009, 42, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Van Mierop, L.H.; Gessner, I.H. Pathogenetic mechanisms in congenital cardiovascular malformations. Prog. Cardiovasc. Dis. 1972, 15, 67–85. [Google Scholar] [CrossRef]
- Gage, P.J.; Suh, H.; Camper, S.A. Dosage requirement of Pitx2 for development of multiple organs. Development 1999, 126, 4643–4651. [Google Scholar] [PubMed]
- Kitamura, K.; Miura, H.; Miyagawa-Tomita, S.; Yanazawa, M.; Katoh-Fukui, Y.; Suzuki, R.; Ohuchi, H.; Suehiro, A.; Motegi, Y.; Nakahara, Y.; et al. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development 1999, 126, 5749–5758. [Google Scholar] [PubMed]
- Liu, C.; Liu, W.; Lu, M.F.; Brown, N.A.; Martin, J.F. Regulation of left-right asymmetry by thresholds of Pitx2c activity. Development 2001, 128, 2039–2048. [Google Scholar] [PubMed]
- Liu, C.; Liu, W.; Palie, J.; Lu, M.F.; Brown, N.A.; Martin, J.F. Pitx2c patterns anterior myocardium and aortic arch vessels and is required for local cell movement into atrioventricular cushions. Development 2002, 129, 5081–5091. [Google Scholar] [PubMed]
- Patterson, K.D.; Drysdale, T.A.; Krieg, P.A. Embryonic origins of spleen asymmetry. Development 2000, 127, 167–175. [Google Scholar] [PubMed]
- Taber, L.A. Morphomechanics: Transforming tubes into organs. Curr. Opin. Genet. Dev. 2014, 27, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ocana, O.H.; Coskun, H.; Minguillon, C.; Murawala, P.; Tanaka, E.M.; Galceran, J.; Munoz-Chapuli, R.; Nieto, M.A. A right-handed signalling pathway drives heart looping in vertebrates. Nature 2017, 549, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.A.; Gridley, T. Snail family genes are required for left-right asymmetry determination, but not neural crest formation, in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 10300–10304. [Google Scholar] [CrossRef] [PubMed]
- Noel, E.S.; Verhoeven, M.; Lagendijk, A.K.; Tessadori, F.; Smith, K.; Choorapoikayil, S.; den Hertog, J.; Bakkers, J. A nodal-independent and tissue-intrinsic mechanism controls heart-looping chirality. Nat. Commun. 2013, 4, 2754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristo, F.; Inacio, J.M.; de Almeida, S.; Mendes, P.; Martins, D.S.; Maio, J.; Anjos, R.; Belo, J.A. Functional study of DAND5 variant in patients with congenital heart disease and laterality defects. BMC Med. Genet. 2017, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.C.; Marques, S.; Belo, J.A. Targeted inactivation of cerberus like-2 leads to left ventricular cardiac hyperplasia and systolic dysfunction in the mouse. PLoS ONE 2014, 9, e102716. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Hota, S.; Zhou, Y.-Q.; Novak, S.; Miguel-Perez, D.; Christodoulou, D.; Seidman, C.; Seidman, J.; Gregorio, C.; Henkelman, M.; et al. Cardiac enriched baf chromatin remodeling complex subunit BAF60c regulates gene expression programs essential for heart development and function. Biol. Open 2017. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Rebagliati, M.; Ahmad, N.; Muraoka, O.; Kurokawa, T.; Hibi, M.; Suzuki, T. The Cerberus/Dan-family protein Charon is a negative regulator of Nodal signaling during left-right patterning in zebrafish. Development 2004, 131, 1741–1753. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Esteban, C.; Capdevila, J.; Economides, A.N.; Pascual, J.; Ortiz, A.; Izpisua Belmonte, J.C. The novel cer-like protein caronte mediates the establishment of embryonic left-right asymmetry. Nature 1999, 401, 243–251. [Google Scholar] [PubMed]
- Vonica, A.; Brivanlou, A.H. The left-right axis is regulated by the interplay of Coco, Xnr1 and derriere in Xenopus embryos. Dev. Biol. 2007, 303, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Hojo, M.; Takashima, S.; Kobayashi, D.; Sumeragi, A.; Shimada, A.; Tsukahara, T.; Yokoi, H.; Narita, T.; Jindo, T.; Kage, T.; et al. Right-elevated expression of charon is regulated by fluid flow in medaka kupffer’s vesicle. Dev. Growth Differ. 2007, 49, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Schweickert, A.; Vick, P.; Getwan, M.; Weber, T.; Schneider, I.; Eberhardt, M.; Beyer, T.; Pachur, A.; Blum, M. The nodal inhibitor Coco is a critical target of leftward flow in xenopus. Curr. Biol. 2010, 20, 738–743. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belo, J.A.; Marques, S.; Inácio, J.M. The Role of Cerl2 in the Establishment of Left-Right Asymmetries during Axis Formation and Heart Development. J. Cardiovasc. Dev. Dis. 2017, 4, 23. https://doi.org/10.3390/jcdd4040023
Belo JA, Marques S, Inácio JM. The Role of Cerl2 in the Establishment of Left-Right Asymmetries during Axis Formation and Heart Development. Journal of Cardiovascular Development and Disease. 2017; 4(4):23. https://doi.org/10.3390/jcdd4040023
Chicago/Turabian StyleBelo, José A., Sara Marques, and José M. Inácio. 2017. "The Role of Cerl2 in the Establishment of Left-Right Asymmetries during Axis Formation and Heart Development" Journal of Cardiovascular Development and Disease 4, no. 4: 23. https://doi.org/10.3390/jcdd4040023
APA StyleBelo, J. A., Marques, S., & Inácio, J. M. (2017). The Role of Cerl2 in the Establishment of Left-Right Asymmetries during Axis Formation and Heart Development. Journal of Cardiovascular Development and Disease, 4(4), 23. https://doi.org/10.3390/jcdd4040023




