Part and Parcel of the Cardiac Autonomic Nerve System: Unravelling Its Cellular Building Blocks during Development
Abstract
:1. Development of the Heart and Cardiac Innervation
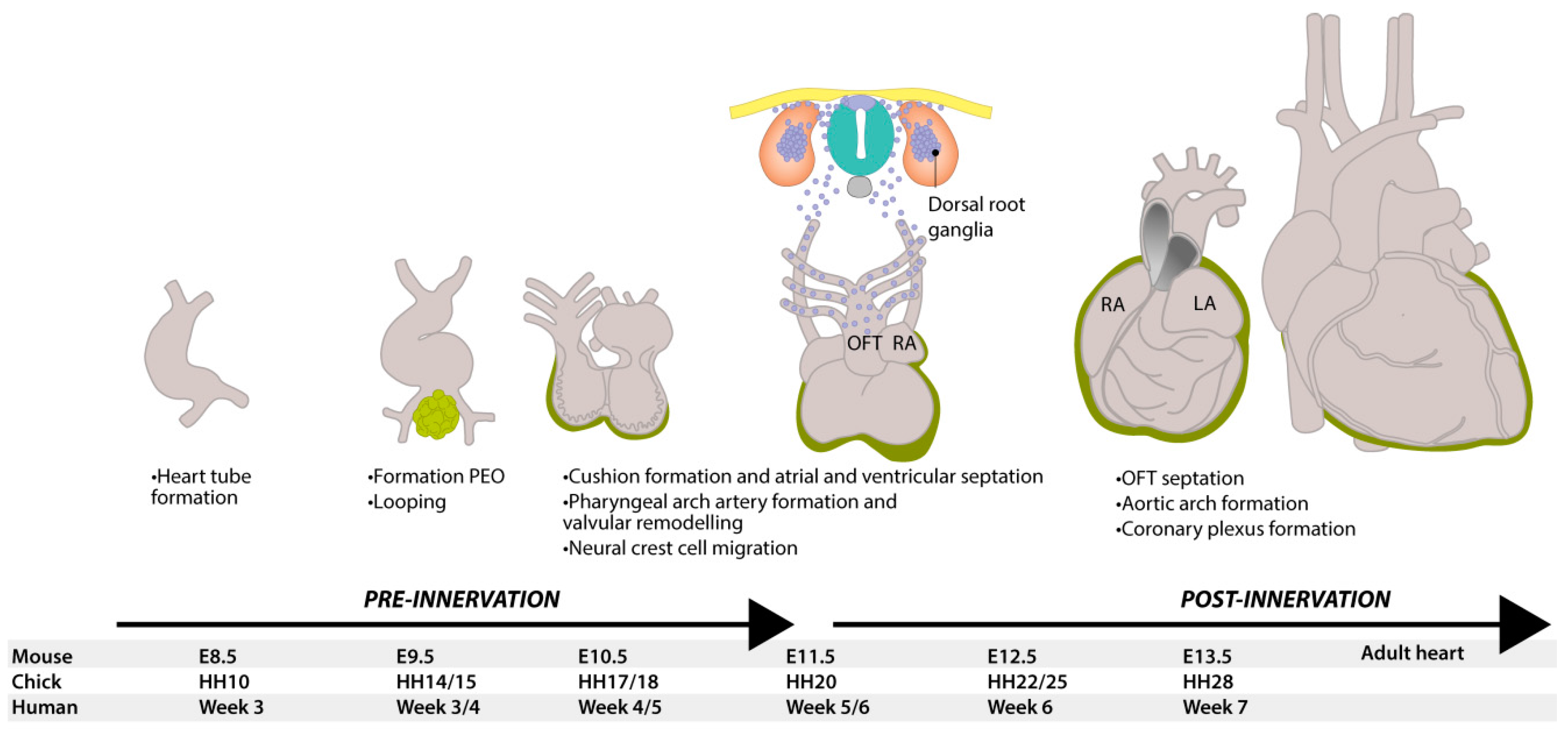
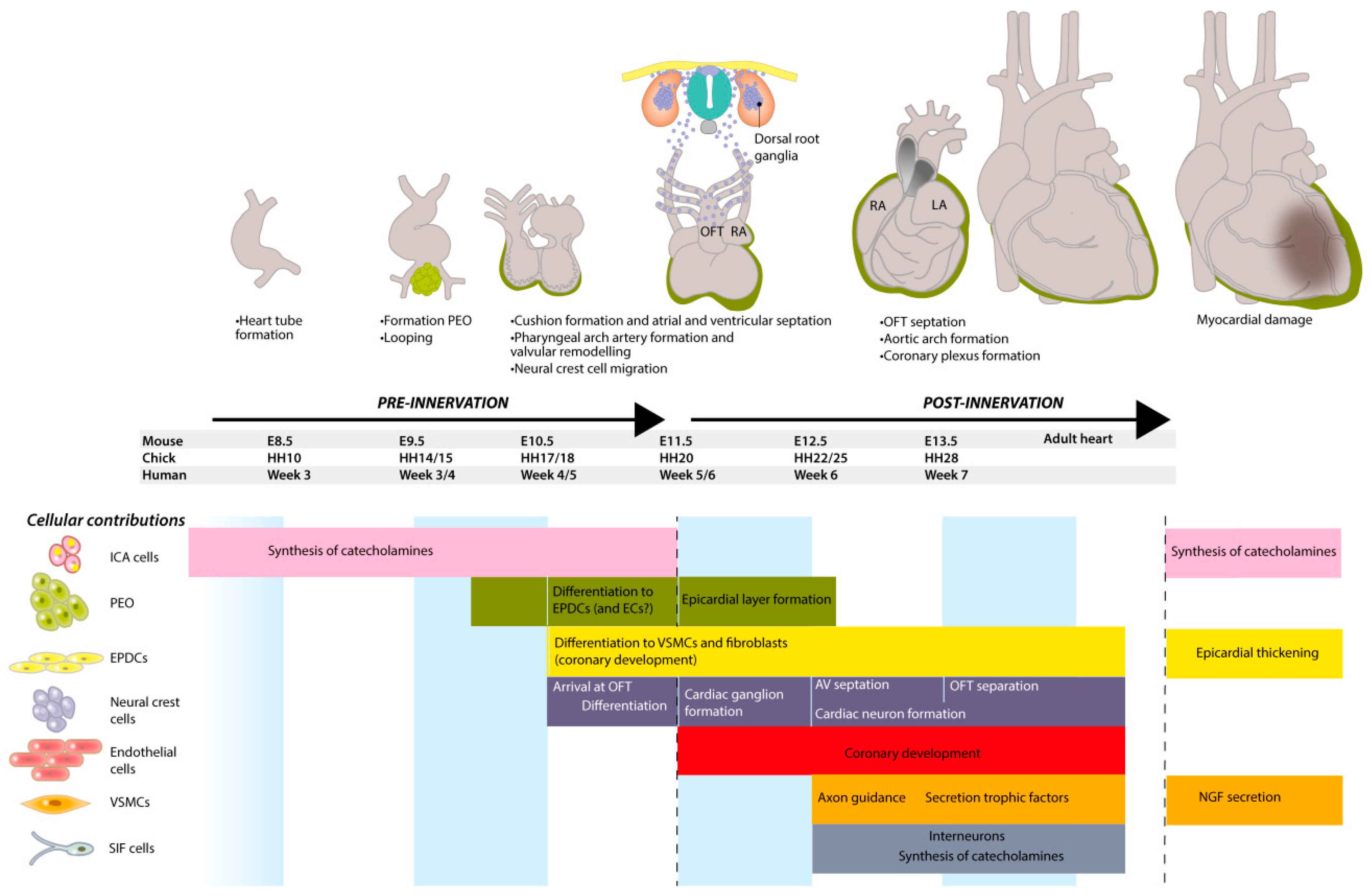
1.1. Embryonic Development of the Heart
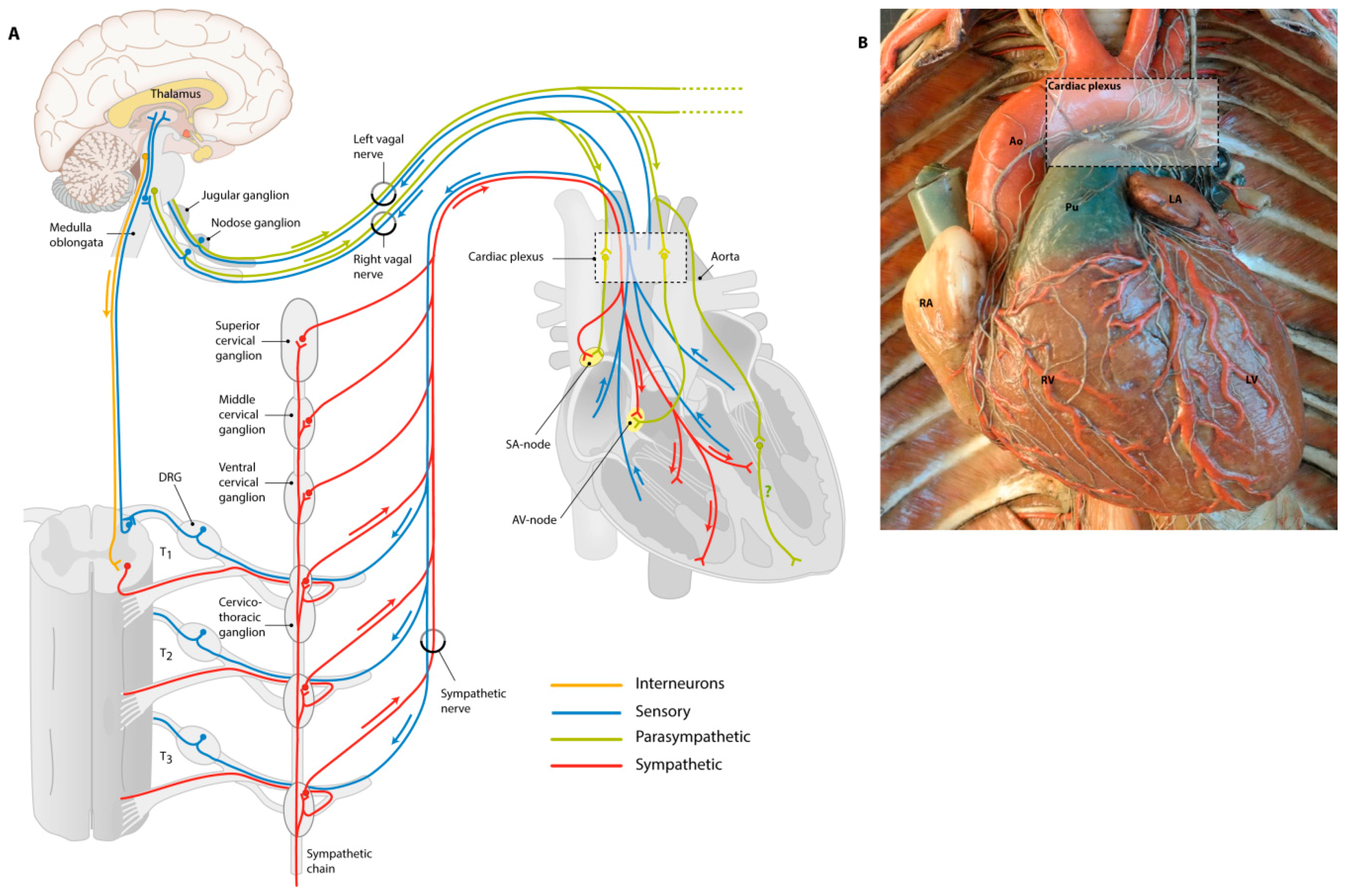
1.2. Anatomy of the Cardiac Nervous System
1.2.1. Sensory Nerves Give Feedback
1.2.2. Sympathetic Nerves Have a Stimulating Effect
1.2.3. Parasympathetic Nerves for Relaxation
2. Catecholamines in the Pre-Innervation Phase of Cardiac Development
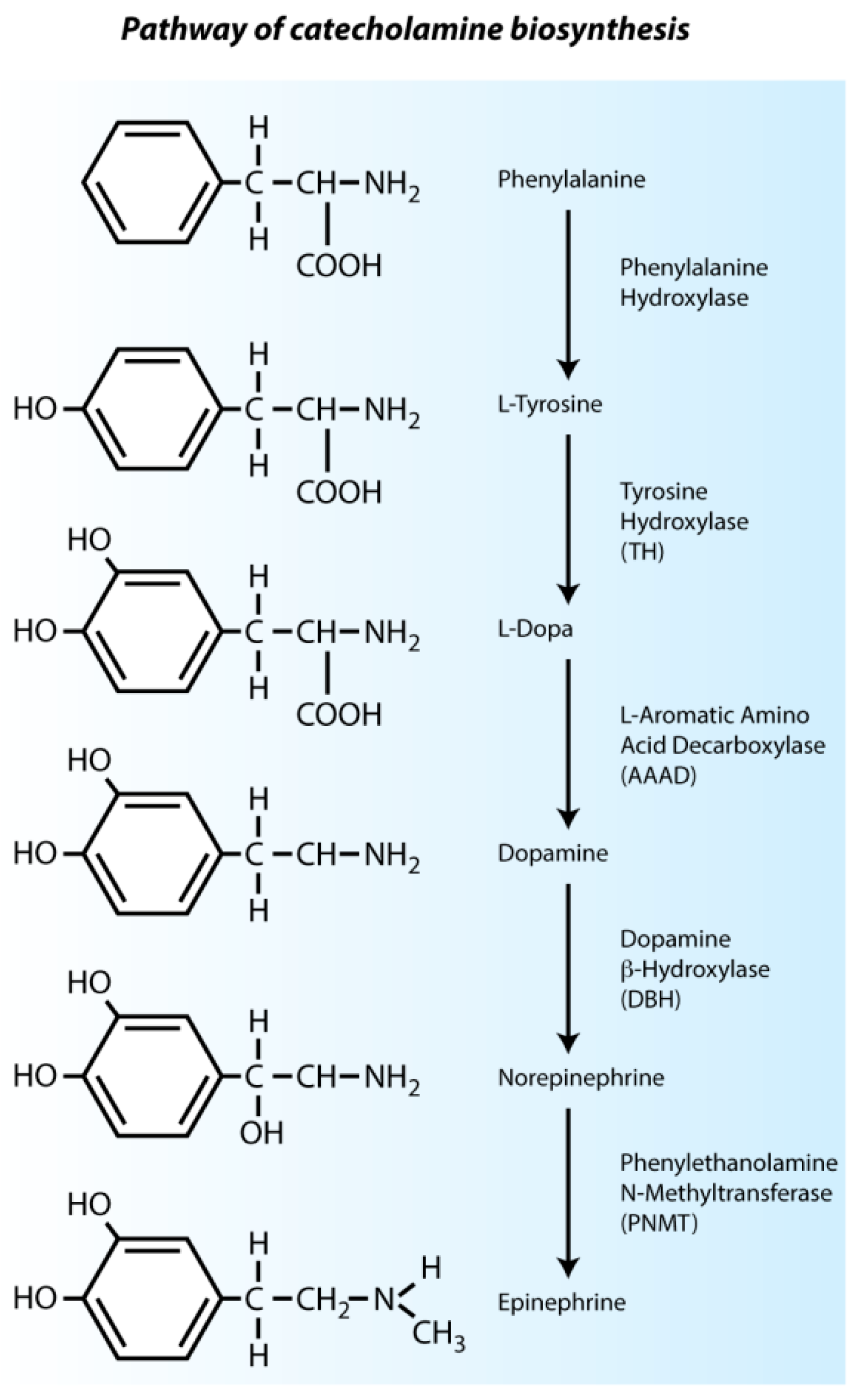
2.1. Intrinsic Cardiac Adrenergic Cells Are an Early Source of Catecholamines
2.2. Response to Epinephrine Administration Is Epicardial Cell Dependent
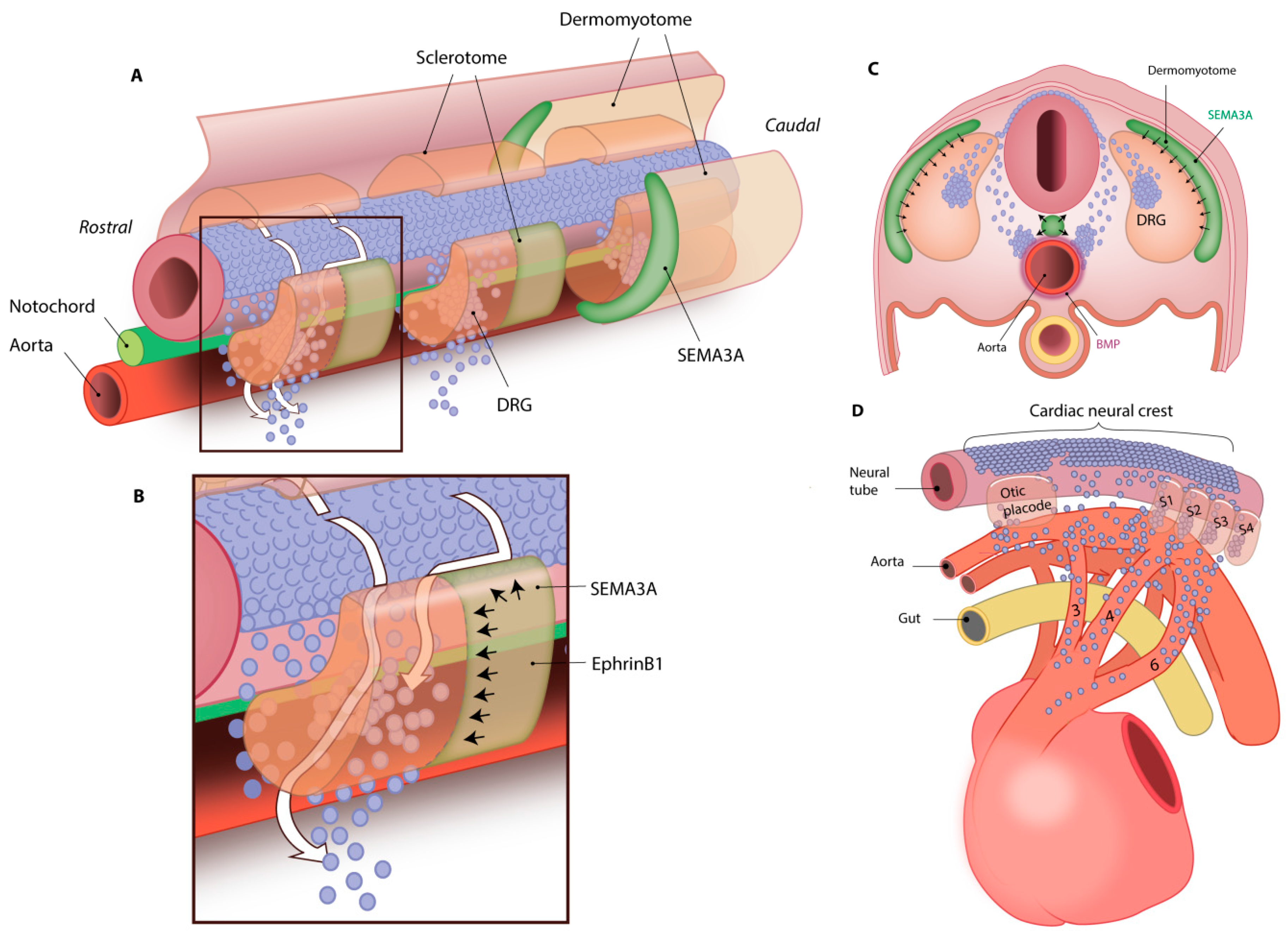
3. Neural Crest Cell (NCC) Migration towards the Heart
3.1. NCC Migration to Sympathetic Ganglia Is Regulated by Trophic Factors
3.2. NCC Migration to Future Parasympathetic Ganglia Locations
4. Differentiation of Neural Crest-Derived Cells
4.1. Do Sympathetic and Parasympathetic Neurons Share a Common Precursor?
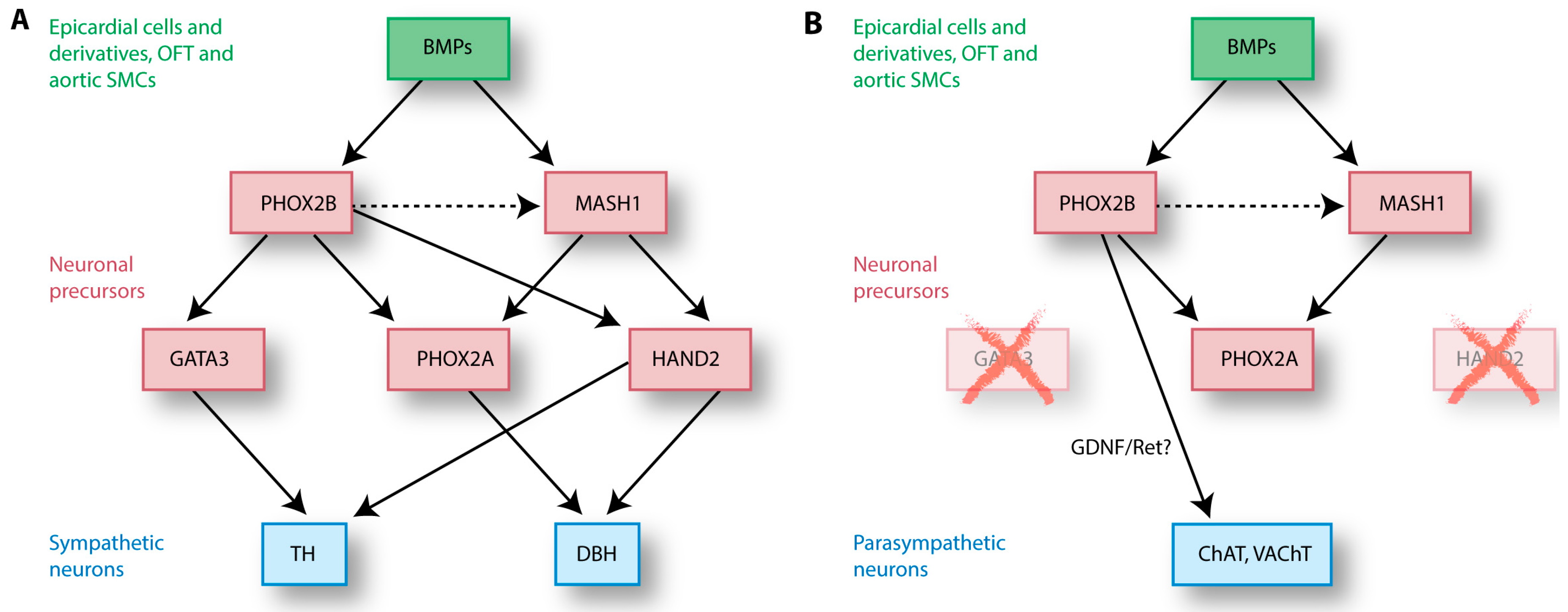
4.2. NCC Differentiation into Sympathetic Neurons
4.3. NCC Differentiation into Parasympathetic Neurons
4.4. Small Intensely Fluorescent (SIF) Cells Act as Interneurons in Autonomic Ganglia
5. Neuronal Survival and Patterning
5.1. Nerve Growth Factor Is Important for Sympathetic Neuron Survival
5.2. Neurotrophin-3 is an Alternative Sympathetic Survival Factor
5.3. Parasympathetic Neuronal Survival by Glial Cell-Derived Neurotrophic Factor and Family Members
6. Parasympathetic Cardiac Innervation Precedes Sympathetic Cardiac Innervation
6.1. The Parasympathetic Pre-Ganglionic Nerve System Is Established by the Vagal Nerve
6.2. Parasympathetic Ganglia Are Located Near the Heart and Connect to Short Postganglionic Nerves
6.3. Developing Sympathetic Innervation Follows Parasympathetic Tracts
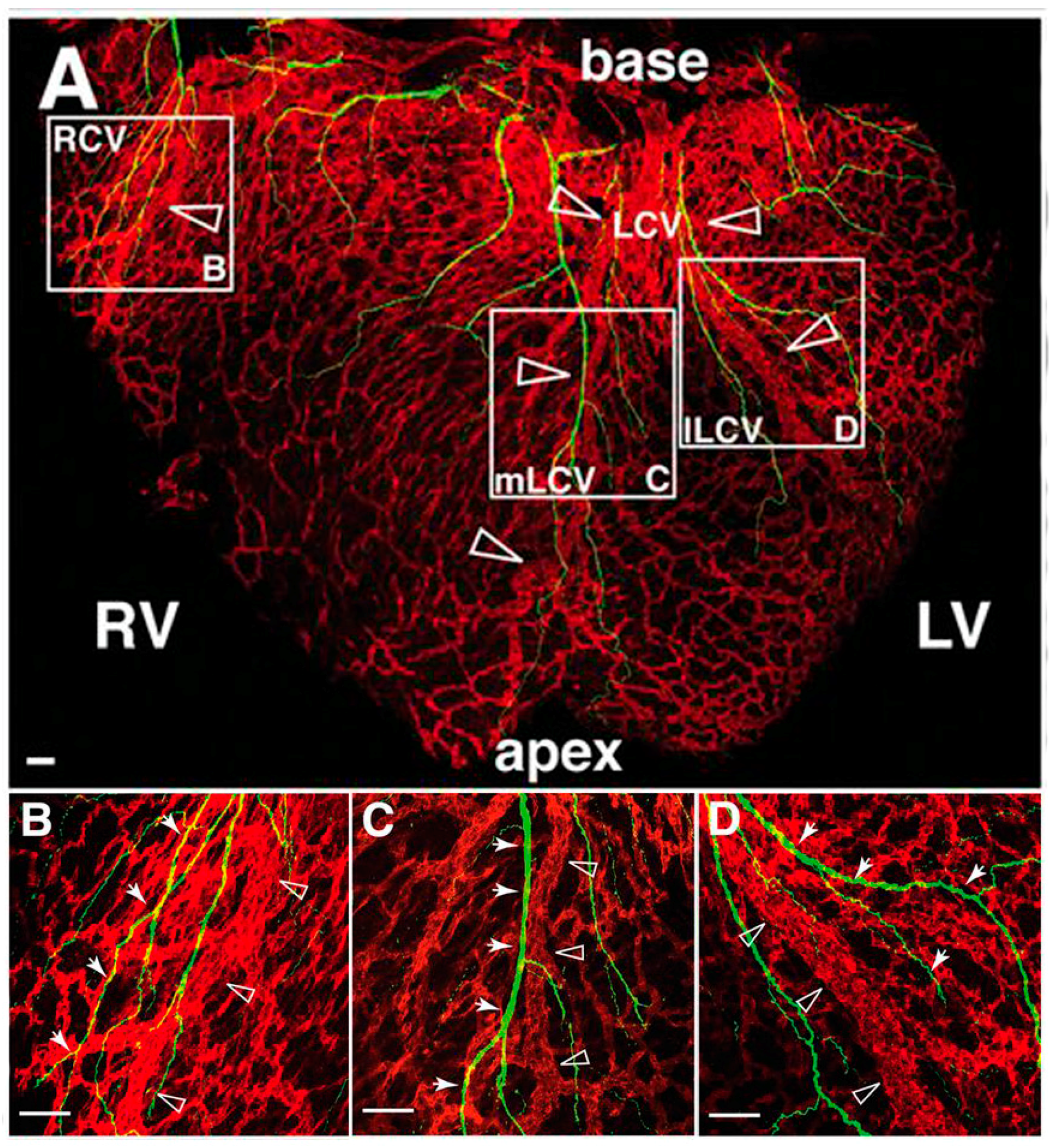
7. Sympathetic Cardiac Axons Follow Coronary Vessels through the Heart
7.1. Sympathetic Axon Guidance over Coronary Vessels
7.2. Parasympathetic and Sensory Axon Guidance
8. Conclusions and Clinical Implications
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Atrioventricular |
| BMP | Bone morphogenetic protein |
| cANS | Cardiac autonomic nervous system |
| CGRP | Calcitonin gene-related peptide |
| ChAT | Choline acetyltransferase |
| CNS | Central nervous system |
| DBH | Dopamine-β-hydroxylase |
| EMT | Epithelial-to-mesenchymal transition |
| EPDC | Epicardium-derived cell |
| GDNF | Glial cell line-derived neurotrophic factor |
| GFRα | α-GDNF family receptors |
| HAND2 | Heart and neural crest derivatives expressed 2 |
| HH | Hamburger and Hamilton stage |
| ICA | Intrinsic cardiac adrenergic |
| MASH | Mammalian achaete-scute homolog |
| MI | Myocardial infarction |
| MMP | Matrix metalloproteinase |
| NCC | Neural crest cells |
| NGF | Nerve growth factor |
| NRP | Neuropilin |
| PEO | Proepicardial organ |
| PHOX | Paired-like homeobox |
| PNMT | Phenylethanolamine-N-methyltransferase |
| PNS | Peripheral nervous system |
| SA | Sinoatrial |
| SEMASIF | Semaphorinsmall intensely fluorescent |
| SMC | Smooth muscle cell |
| TH | Tyrosine hydroxylase |
| VAChT | Vesicular acetylcholine transporter |
| VSMC | Vascular smooth muscle cell |
| WT1 | Wilms’ tumor 1 |
References
- Kimura, K.; Ieda, M.; Kanazawa, H.; Yagi, T.; Tsunoda, M.; Ninomiya, S.; Kurosawa, H.; Yoshimi, K.; Mochizuki, H.; Yamazaki, K.; et al. Cardiac sympathetic rejuvenation: A link between nerve function and cardiac hypertrophy. Circ. Res. 2007, 100, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Mancia, G.; Grassi, G. The autonomic nervous system and hypertension. Circ. Res. 2014, 114, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ. Res. 2014, 114, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Diller, G.-P.; Dimopoulos, K.; Okonko, D.; Uebing, A.; Broberg, C.S.; Babu-Narayan, S.; Bayne, S.; Poole-Wilson, P.A.; Sutton, R.; Francis, D.P.; et al. Heart rate response during exercise predicts survival in adults with congenital heart disease. J. Am. Coll. Cardiol. 2006, 48, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.S.; Reinier, K.; Teodorescu, C.; Evanado, A.; Kehr, E.; Al Samara, M.; Mariani, R.; Gunson, K.; Jui, J. Epidemiology of sudden cardiac death: Clinical and research implications. Prog. Cardiovasc. Dis. 2008, 51, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Ohuchi, H.; Negishi, J.; Miyake, A.; Sakaguchi, H.; Miyazaki, A.; Yamada, O. Long-term prognostic value of cardiac autonomic nervous activity in postoperative patients with congenital heart disease. Int. J. Cardiol. 2011, 151, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Mortara, A.; La Rovere, M.T.; Signorini, M.G.; Pantaleo, P.; Pinna, G.; Martinelli, L.; Ceconi, C.; Cerutti, S.; Tavazzi, L. Can power spectral analysis of heart rate variability identify a high risk subgroup of congestive heart failure patients with excessive sympathetic activation? A pilot study before and after heart transplantation. Br. Hear. J. 1994, 71, 422–430. [Google Scholar] [CrossRef]
- Fishman, M.C.; Chien, K.R. Fashioning the vertebrate heart: Earliest embryonic decisions. Development 1997, 124, 2099–2117. [Google Scholar] [PubMed]
- De Boer, B.A.; van den Berg, G.; de Boer, P.A.J.; Moorman, A.F.M.; Ruijter, J.M. Growth of the developing mouse heart: An interactive qualitative and quantitative 3D atlas. Dev. Biol. 2012, 368, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Männer, J. Cardiac looping in the chick embryo: A morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat. Rec. 2000, 259, 248–262. [Google Scholar] [CrossRef]
- Männer, J.; Wessel, A.; Yelbuz, T.M. How does the tubular embryonic heart work? Looking for the physical mechanism generating unidirectional blood flow in the valveless embryonic heart tube. Dev. Dyn. 2010, 239, 1035–1046. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K.; Hirota, A.; Fujii, S. Localization of pacemaking activity in early embryonic heart monitored using voltage-sensitive dye. Nature 1981, 290, 595–597. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, M.; Männer, J. Cardiac looping may be driven by compressive loads resulting from unequal growth of the heart and pericardial cavity. Observations on a physical simulation model. Front. Physiol. 2014, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Kruithof, B.P.T.; Duim, S.N.; Moerkamp, A.T.; Goumans, M.-J. TGFβ and BMP signaling in cardiac cushion formation: Lessons from mice and chicken. Differentiation 2012, 84, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; van den Hoff, M.J.B.; Adamo, R.F.; Phelps, A.L.; Lockhart, M.M.; Sauls, K.; Briggs, L.E.; Norris, R.A.; van Wijk, B.; Perez-Pomares, J.M.; et al. Epicardially derived fibroblasts preferentially contribute to the parietal leaflets of the atrioventricular valves in the murine heart. Dev. Biol. 2012, 366, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.M.; Mahendran, P.; Singh, E.; Anderson, R.H.; Chaudhry, B.; Henderson, D.J. Neural crest cells are required for correct positioning of the developing outflow cushions and pattern the arterial valve leaflets. Cardiovasc. Res. 2013, 99, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 2012, 22, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Poelmann, R.E.; Gittenberger-de Groot, A.C.; Mentink, M.M.; Bökenkamp, R.; Hogers, B. Development of the cardiac coronary vascular endothelium, studied with antiendothelial antibodies, in chicken-quail chimeras. Circ. Res. 1993, 73, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Hirakow, R. Epicardial formation in staged human embryos. Kaibogaku Zasshi 1992, 67, 616–622. [Google Scholar] [PubMed]
- Duim, S.N.; Kurakula, K.; Goumans, M.-J.; Kruithof, B.P.T. Cardiac endothelial cells express Wilms’ tumor-1: Wt1 expression in the developing, adult and infarcted heart. J. Mol. Cell. Cardiol. 2015, 81, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Steijn, R.; Scherptong, R.W.C.; Kruithof, B.P.T.; Duim, S.N.; Goumans, M.J.T.H.; Wisse, L.J.; Zhou, B.; Pu, W.T.; Poelmann, R.E.; Schalij, M.J.; et al. Regional differences in WT-1 and Tcf21 expression during ventricular development: Implications for myocardial compaction. PLoS ONE 2015, 10, e0136025. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Risebro, C.A.; Melville, A.A.D.; Moses, K.; Schwartz, R.J.; Chien, K.R.; Riley, P.R. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007, 445, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-L.; Martin, J.C.; Sun, Y.; Cui, L.; Wang, L.; Ouyang, K.; Yang, L.; Bu, L.; Liang, X.; Zhang, X.; et al. A myocardial lineage derives from Tbx18 epicardial cells. Nature 2008, 454, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Eralp, I.; Lie-Venema, H.; Bax, N.A.M.; Wijffels, M.C.E.F.; van der Laarse, A.; Deruiter, M.C.; Bogers, A.J.J.C.; van den Akker, N.M.S.; Gourdie, R.G.; Schalij, M.J.; et al. Epicardium-derived cells are important for correct development of the Purkinje fibers in the avian heart. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2006, 288, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Creazzo, T.L.; Godt, R.E.; Leatherbury, L.; Conway, S.J.; Kirby, M.L. Role of cardiac neural crest cells in cardiovascular development. Annu. Rev. Physiol. 1998, 60, 267–286. [Google Scholar] [CrossRef] [PubMed]
- Kolditz, D.P.; Wijffels, M.C.E.F.; Blom, N.A.; van der Laarse, A.; Markwald, R.R.; Schalij, M.J.; Gittenberger-de Groot, A.C. De Persistence of functional atrioventricular accessory pathways in postseptated embryonic avian hearts: Implications for morphogenesis and functional maturation of the cardiac conduction system. Circulation 2007, 115, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, V.; Webb, S.; Bradshaw, L.; Brown, N.A.; Anderson, R.H.; Henderson, D.J. Cells migrating from the neural crest contribute to the innervation of the venous pole of the heart. J. Anat. 2008, 212, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.B. Autonomic receptor—Effector coupling during post-natal development. Cardiovasc. Res. 1996, 31, E68–E76. [Google Scholar] [CrossRef]
- Freedman, N.J.; Liggett, S.B.; Drachman, D.E.; Pei, G.; Caron, M.G.; Lefkowitz, R.J. Phosphorylation and desensitization of the human beta 1-adrenergic receptor. Involvement of G protein-coupled receptor kinases and cAMP-dependent protein kinase. J. Biol. Chem. 1995, 270, 17953–17961. [Google Scholar] [PubMed]
- Billman, G.E. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: Effect of endurance exercise training. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1171–H1193. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.; Koch, W.J.; Rockman, H.; Smith, B.; Bond, R.A.; Sulik, K.K.; Ross, J.; Lefkowitz, R.J.; Caron, M.G.; Giros, B. Essential role of β-adrenergic receptor kinase 1 in cardiac development and function. Proc. Natl. Acad. Sci. USA 1996, 93, 12974–12979. [Google Scholar] [CrossRef] [PubMed]
- Pauza, D.H.; Skripka, V.; Pauziene, N.; Stropus, R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat. Rec. 2000, 259, 353–382. [Google Scholar] [CrossRef]
- Ulphani, J.S.; Cain, J.H.; Inderyas, F.; Gordon, D.; Gikas, P.V.; Shade, G.; Mayor, D.; Arora, R.; Kadish, A.H.; Goldberger, J.J. Quantitative analysis of parasympathetic innervation of the porcine heart. Heart Rhythm 2010, 7, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- Pauza, D.H.; Saburkina, I.; Rysevaite, K.; Inokaitis, H.; Jokubauskas, M.; Jalife, J.; Pauziene, N. Neuroanatomy of the murine cardiac conduction system: A combined stereomicroscopic and fluorescence immunohistochemical study. Auton. Neurosci. 2013, 176, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Casadei, B. Vagal control of myocardial contractility in humans. Exp. Physiol. 2001, 86, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Coote, J.H. Myths and realities of the cardiac vagus. J. Physiol. 2013, 591, 4073–4085. [Google Scholar] [CrossRef] [PubMed]
- Kroese, J.M.; Broekhuizen, M.L.A.A.; Poelmann, R.E.; Mulder, P.G.H.H.; Wladimiroff, J.W. Epinephrine affects hemodynamics of noninnervated normal and all-trans retinoic acid-treated embryonic chick hearts. Fetal Diagn. Ther. 2004, 19, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Kelder, T.P.; Duim, S.N.; Vicente-Steijn, R.; Végh, A.M.D.; Kruithof, B.P.T.; Smits, A.M.; van Bavel, T.C.; Bax, N.A.M.; Schalij, M.J.; Gittenberger-de Groot, A.C.; et al. The epicardium as modulator of the cardiac autonomic response during early development. J. Mol. Cell. Cardiol. 2015, 89, 251–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thomas, S.A.; Matsumoto, A.M.; Palmiter, R.D. Noradrenaline is essential for mouse fetal development. Nature 1995, 374, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.-Y.Y.; Quaife, C.J.; Palmiter, R.D. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature 1995, 374, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.C.; Lakshmanan, G.; Crawford, S.E.; Gu, Y.; Grosveld, F.; Engel, J.D. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat. Genet. 2000, 25, 209–212. [Google Scholar] [PubMed]
- Ebert, S.N.; Rong, Q.; Boe, S.; Pfeifer, K. Catecholamine-synthesizing cells in the embryonic mouse heart. Ann. N. Y. Acad. Sci. 2008, 1148, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Ignarro, L.J.; Shideman, F.E. Norepinephrine and epinephrine in the embryo and embryonic heart of the chick: Uptake and subcellular distribution. J. Pharmacol. Exp. Ther. 1968, 159, 49–58. [Google Scholar] [PubMed]
- Ebert, S.N.; Thompson, R.P. Embryonic epinephrine synthesis in the rat heart before innervation: Association with pacemaking and conduction tissue development. Circ. Res. 2001, 88, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Verberne, M.E.; Gittenberger-De Groot, A.C.; Poelmann, R.E. Distribution of antigen epitopes shared by nerves and the myocardium of the embryonic chick heart using different neuronal markers. Anat. Rec. 2000, 260, 335–350. [Google Scholar] [CrossRef]
- Huang, M.H.; Friend, D.S.; Sunday, M.E.; Singh, K.; Haley, K.; Austen, K.F.; Kelly, R.A.; Smith, T.W. An intrinsic adrenergic system in mammalian heart. J. Clin. Investig. 1996, 98, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.R.; Rong, Q.; Katchman, A.N.; Ebert, S.N. Intrinsic cardiac catecholamines help maintain beating activity in neonatal rat cardiomyocyte cultures. Pediatr. Res. 2004, 56, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Elayan, H.H.; Kennedy, B.P.; Ziegler, M.G. Cardiac atria and ventricles contain different inducible adrenaline synthesising enzymes. Cardiovasc. Res. 1990, 24, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Virágh, S.; Challice, C.E. The development of the conduction system in the mouse embryo heart. Dev. Biol. 1982, 89, 25–40. [Google Scholar] [CrossRef]
- Ebert, S.N.; Rong, Q.; Boe, S.; Thompson, R.P.; Grinberg, A.; Pfeifer, K. Targeted insertion of the Cre-recombinase gene at the phenylethanolamine n-methyltransferase locus: A new model for studying the developmental distribution of adrenergic cells. Dev. Dyn. 2004, 231, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Hutson, M.R.; Kirby, M.L. Neural crest and cardiovascular development: A 20-year perspective. Birth Defects Res. C Embryo Today 2003, 69, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Sano, M.; Nakamura, H.; Ito, K.; Sato, Y.; Shinmura, K.; Ieda, M.; Fujita, J.; Kurosawa, H.; Ogawa, S.; et al. Neural crest-derived resident cardiac cells contribute to the restoration of adrenergic function of transplanted heart in rodent. Cardiovasc. Res. 2016, 109, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.H.; Nguyen, V.; Wu, Y.; Rastogi, S.; Lui, C.Y.; Birnbaum, Y.; Wang, H.Q.; Ware, D.L.; Chauhan, M.; Garg, N.; et al. Reducing ischaemia/reperfusion injury through delta-opioid-regulated intrinsic cardiac adrenergic cells: Adrenopeptidergic co-signalling. Cardiovasc. Res. 2009, 84, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Brain, S.D.; Grant, A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004, 84, 903–934. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; Hutson, M.R. Factors controlling cardiac neural crest cell migration. Cell Adhes. Migr. 2010, 4, 609–621. [Google Scholar] [CrossRef]
- Loring, J.F.; Erickson, C.A. Neural crest cell migratory pathways in the trunk of the chick embryo. Dev. Biol. 1987, 121, 220–236. [Google Scholar] [CrossRef]
- Kuo, B.R.; Erickson, C.A. Regional differences in neural crest morphogenesis. Cell Adhes. Migr. 2010, 4, 567–585. [Google Scholar] [CrossRef]
- Santagati, F.; Rijli, F.M. Cranial neural crest and the building of the vertebrate head. Nat. Rev. Neurosci. 2003, 4, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; Turnage, K.L.; Hays, B.M. Characterization of conotruncal malformations following ablation of “cardiac” neural crest. Anat. Rec. 1985, 213, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; Stewart, D.E. Neural crest origin of cardiac ganglion cells in the chick embryo: Identification and extirpation. Dev. Biol. 1983, 97, 433–443. [Google Scholar] [CrossRef]
- Verberne, M.E.; Gittenberger-de Groot, A.C.; Poelmann, R.E. Lineage and development of the parasympathetic nervous system of the embryonic chick heart. Anat. Embryol. (Berl.) 1998, 198, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L.; Gale, T.F.; Stewart, D.E. Neural crest cells contribute to normal aorticopulmonary septation. Science 1983, 220, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M. Investigations on the neural crest. Methodological aspects and recent advances. Ann. N. Y. Acad. Sci. 1986, 486, 66–86. [Google Scholar] [CrossRef] [PubMed]
- Rickmann, M.; Fawcett, J.W.; Keynes, R.J. The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J. Embryol. Exp. Morphol. 1985, 90, 437–455. [Google Scholar] [PubMed]
- Kasemeier-Kulesa, J.C.; Bradley, R.; Pasquale, E.B.; Lefcort, F.; Kulesa, P.M. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development 2006, 133, 4839–4847. [Google Scholar] [CrossRef] [PubMed]
- Toyofuku, T.; Yoshida, J.; Sugimoto, T.; Yamamoto, M.; Makino, N.; Takamatsu, H.; Takegahara, N.; Suto, F.; Hori, M.; Fujisawa, H.; et al. Repulsive and attractive semaphorins cooperate to direct the navigation of cardiac neural crest cells. Dev. Biol. 2008, 321, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Plein, A.; Calmont, A.; Fantin, A.; Denti, L.; Anderson, N.A.; Scambler, P.J.; Ruhrberg, C. Neural crest-derived SEMA3C activates endothelial NRP1 for cardiac outflow tract septation. J. Clin. Investig. 2015, 125, 2661–2676. [Google Scholar] [CrossRef] [PubMed]
- Fantin, A.; Maden, C.H.; Ruhrberg, C. Neuropilin ligands in vascular and neuronal patterning. Biochem. Soc. Trans. 2009, 37, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.; Farinas, I.; Brennan, C.; Rivas-Plata, K.; Backus, C.; Reichardt, L.; Landis, S. NT-3, like NGF, is required for survival of sympathetic neurons, but not their precursors. Dev. Biol. 1999, 210, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y.; Wessels, A.; Smith, B.R.; Linask, K.K.; Ewart, J.L.; Lo, C.W. Alteration in connexin 43 gap junction gene dosage impairs conotruncal heart development. Dev. Biol. 1998, 198, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Bekku, Y.; Suto, F.; Kitsukawa, T.; Taniguchi, M.; Nagatsu, I.; Nagatsu, T.; Itoh, K.; Yagi, T.; Fujisawa, H. Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development 2002, 129, 671–680. [Google Scholar] [PubMed]
- Young, H.M.; Anderson, R.B.; Anderson, C.R. Guidance cues involved in the development of the peripheral autonomic nervous system. Auton. Neurosci. 2004, 112, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.N.G.; Ohta, K.; Quick, M.M.; Fleming, A.; Keynes, R.; Tannahill, D. Molecular analysis of axon repulsion by the notochord. Development 2003, 130, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Kanazawa, H.; Kimura, K.; Hattori, F.; Ieda, Y.; Taniguchi, M.; Lee, J.-K.; Matsumura, K.; Tomita, Y.; Miyoshi, S.; et al. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat. Med. 2007, 13, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Maden, C.H.; Gomes, J.; Schwarz, Q.; Davidson, K.; Tinker, A.; Ruhrberg, C. NRP1 and NRP2 cooperate to regulate gangliogenesis, axon guidance and target innervation in the sympathetic nervous system. Dev. Biol. 2012, 369, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Le Douarin, N.M.; Teillet, M.A. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J. Embryol. Exp. Morphol. 1973, 30, 31–48. [Google Scholar] [PubMed]
- Kirby, M.L. Nodose placode contributes autonomic neurons to the heart in the absence of cardiac neural crest. J. Neurosci. 1988, 8, 1089–1095. [Google Scholar] [PubMed]
- Jain, R.; Engleka, K.A.; Rentschler, S.L.; Manderfield, L.J.; Li, L.; Yuan, L.; Epstein, J.A. Cardiac neural crest orchestrates remodeling and functional maturation of mouse semilunar valves. J. Clin. Investig. 2011, 121, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607–1616. [Google Scholar] [PubMed]
- Bhatt, S.; Diaz, R.; Trainor, P.A.; Wu, D.K.; Kelley, M.W.; Tam, P.L.; Nichols, J.; Smith, A. Signals and Switches in Mammalian Neural Crest Cell Differentiation Signals and Switches in Mammalian Neural Crest Cell Differentiation. Cold Spring Harb. Perspect. Biol. 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Stemple, D.L.; Anderson, D.J. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell 1992, 71, 973–985. [Google Scholar] [CrossRef]
- Shah, N.M.; Groves, A.K.; Anderson, D.J. Alternative neural crest cell fates are instructively promoted by TGFbeta superfamily members. Cell 1996, 85, 331–343. [Google Scholar] [CrossRef]
- Reissmann, E.; Ernsberger, U.; Francis-West, P.H.; Rueger, D.; Brickell, P.M.; Rohrer, H. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development 1996, 122, 2079–2088. [Google Scholar] [PubMed]
- Kruithof, B.P.T.; Xu, J.; Fritz, D.T.; Cabral, C.S.; Gaussin, V.; Rogers, M.B. An in vivo map of bone morphogenetic protein 2 post-transcriptional repression in the heart. Genesis 2011, 49, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.M.; Hogan, B.L.; Robertson, E.J. Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech. Dev. 1995, 50, 71–83. [Google Scholar] [CrossRef]
- Guillemot, F.; Lo, L.C.; Johnson, J.E.; Auerbach, A.; Anderson, D.J.; Joyner, A.L. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 1993, 75, 463–476. [Google Scholar] [CrossRef]
- Shoba, T.; Dheen, S.T.; Tay, S.S.W. Retinoic acid influences the expression of the neuronal regulatory genes Mash-1 and c-ret in the developing rat heart. Neurosci. Lett. 2002, 318, 129–132. [Google Scholar] [CrossRef]
- Lo, L.C.; Johnson, J.E.; Wuenschell, C.W.; Saito, T.; Anderson, D.J. Mammalian achaete-scute homolog 1 is transiently expressed by spatially restricted subsets of early neuroepithelial and neural crest cells. Genes Dev. 1991, 5, 1524–1537. [Google Scholar] [CrossRef] [PubMed]
- Gradwohl, G.; Fode, C.; Guillemot, F. Restricted expression of a novel murine atonal-related bHLH protein in undifferentiated neural precursors. Dev. Biol. 1996, 180, 227–241. [Google Scholar] [CrossRef] [PubMed]
- White, P.M.; Morrison, S.J.; Orimoto, K.; Kubu, C.J.; Verdi, J.M.; Anderson, D.J. Neural crest stem cells undergo cell-intrinsic developmental changes in sensitivity to instructive differentiation signals. Neuron 2001, 29, 57–71. [Google Scholar] [CrossRef]
- Müller, F.; Rohrer, H. Molecular control of ciliary neuron development: BMPs and downstream transcriptional control in the parasympathetic lineage. Development 2002, 129, 5707–5717. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.R.; Tiveron, M.C.; Guillemot, F.; Brunet, J.F.; Goridis, C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development 1998, 125, 599–608. [Google Scholar] [PubMed]
- Yang, C.; Kim, H.S.; Seo, H.; Kim, C.H.; Brunet, J.F.; Kim, K.S. Paired-like homeodomain proteins, Phox2a and Phox2b, are responsible for noradrenergic cell-specific transcription of the dopamine beta-hydroxylase gene. J. Neurochem. 1998, 71, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, A.; Morin, X.; Cremer, H.; Goridis, C.; Brunet, J.F. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 1999, 399, 366–370. [Google Scholar] [PubMed]
- Enomoto, H.; Heuckeroth, R.O.; Golden, J.P.; Johnson, E.M.; Milbrandt, J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development 2000, 127, 4877–4889. [Google Scholar] [PubMed]
- Hendershot, T.J.; Liu, H.; Clouthier, D.E.; Shepherd, I.T.; Coppola, E.; Studer, M.; Firulli, A.B.; Pittman, D.L.; Howard, M.J. Conditional deletion of Hand2 reveals critical functions in neurogenesis and cell type-specific gene expression for development of neural crest-derived noradrenergic sympathetic ganglion neurons. Dev. Biol. 2008, 319, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; D’Autréaux, F.; Gershon, M.D.; Cserjesi, P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev. Biol. 2007, 307, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Lin, S.; Pape, M.; Ernsberger, U.; Stanke, M.; Kobayashi, K.; Howard, M.J.; Rohrer, H. The bHLH transcription factor Hand2 is essential for the maintenance of noradrenergic properties in differentiated sympathetic neurons. Dev. Biol. 2009, 329, 191–200. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morin, X.; Cremer, H.; Hirsch, M.R.; Kapur, R.P.; Goridis, C.; Brunet, J.F. Defects in sensory and autonomic ganglia and absence of locus coeruleus in mice deficient for the homeobox gene Phox2a. Neuron 1997, 18, 411–423. [Google Scholar] [CrossRef]
- Mekhail, N.A.; Nawar, N.N.Y.; Khairallah, P.A. New synapses associated with the granule-containing cells of rat sympathetic ganglia. Acta Anat. 1988, 133, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Shtukmaster, S.; Schier, M.C.; Huber, K.; Krispin, S.; Kalcheim, C.; Unsicker, K. Sympathetic neurons and chromaffin cells share a common progenitor in the neural crest in vivo. Neural Dev. 2013, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Powley, T.L.; Schwaber, J.S.; Doyle, F.J. Vagal afferent innervation of the atria of the rat heart reconstructed with confocal microscopy. J. Comp. Neurol. 1997, 381, 1–17. [Google Scholar] [CrossRef]
- Prud’homme, M.J.; Houdeau, E.; Serghini, R.; Tillet, Y.; Schemann, M.; Rousseau, J.P. Small intensely fluorescent cells of the rat paracervical ganglion synthesize adrenaline, receive afferent innervation from postganglionic cholinergic neurones, and contain muscarinic receptors. Brain Res. 1999, 821, 141–149. [Google Scholar] [CrossRef]
- Matthews, M.R. Small, intensely fluorescent cells and the paraneuron concept. J. Electron. Microsc. Tech. 1989, 12, 408–416. [Google Scholar] [CrossRef] [PubMed]
- McKeon, T.W.; Parsons, R.L. Galanin immunoreactivity in the mudpuppy cardiac ganglion. J. Auton. Nerv. Syst. 1990, 31, 135–140. [Google Scholar] [CrossRef]
- Soinila, S. Clustering of intensely fluorescent sympathetic cells in embryonal and postnatal rats. J. Auton. Nerv. Syst. 1984, 11, 207–222. [Google Scholar] [CrossRef]
- Jacobowitz, D. Histochemical studies of the relationship of chromaffin cells and adrenergic nerve fibers to the cardiac ganglia of several species. J. Pharmacol. Exp. Ther. 1967, 158, 227–240. [Google Scholar] [PubMed]
- Chiba, T.; Williams, T.H. Histofluorescence characteristics and quantification of small intensely fluorescent (SIF) cells in sympathetic ganglia of several species. Cell. Tissue Res. 1975, 162, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Doupe, A.J.; Patterson, P.H.; Landis, S.C. Small intensely fluorescent cells in culture: Role of glucocorticoids and growth factors in their development and interconversions with other neural crest derivatives. J. Neurosci. 1985, 5, 2143–2160. [Google Scholar] [PubMed][Green Version]
- Ciaranello, R.D.; Wooten, G.F.; Axwlrod, J. Regulation of dopamine beta-hydroxylase in rat adrenal glands. J. Biol. Chem. 1975, 250, 3204–3211. [Google Scholar] [PubMed]
- Otten, U.; Thoenen, H. Modulatory role of glucocorticoids on NGF-mediated enzyme induction in organ cultures of sympathetic ganglia. Brain Res. 1976, 111, 438–441. [Google Scholar] [CrossRef]
- Heathcote, R.D.; Chen, A. Morphogenesis of adrenergic cells in a frog parasympathetic ganglion. J. Comp. Neurol. 1991, 308, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, O.; Matsuo, A.; Bellier, J.-P.; Aimi, Y. Demonstration of choline acetyltransferase of a peripheral type in the rat heart. J. Histochem. Cytochem. 2007, 55, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Hoard, J.L.; Hoover, D.B.; Mabe, A.M.; Blakely, R.D.; Feng, N.; Paolocci, N. Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norepinephrine transporters, and the neurotrophin receptors tropomyosin-related kinase A and p75. Neuroscience 2008, 156, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W.; Smith, P.G. Modulation of rat parasympathetic cardiac ganglion phenotype and NGF synthesis by adrenergic nerves. Auton. Neurosci. 2009, 145, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Kermani, P.; Teng, K.K.; Hempstead, B.L. Regulation of cell survival by secreted proneurotrophins. Science 2001, 294, 1945–1948. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.A.; Cuello, A.C. Activity-dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc. Natl. Acad. Sci. USA 2006, 103, 6735–6740. [Google Scholar] [CrossRef] [PubMed]
- Lobos, E.; Gebhardt, C.; Kluge, A.; Spanel-Borowski, K. Expression of nerve growth factor (NGF) isoforms in the rat uterus during pregnancy: Accumulation of precursor proNGF. Endocrinology 2005, 146, 1922–1929. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bierl, M.A.; Isaacson, L.G. Increased NGF proforms in aged sympathetic neurons and their targets. Neurobiol. Aging 2007, 28, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Korsching, S.; Thoenen, H. Developmental changes of nerve growth factor levels in sympathetic ganglia and their target organs. Dev. Biol. 1988, 126, 40–46. [Google Scholar] [CrossRef]
- Korsching, S.; Thoenen, H. Nerve growth factor in sympathetic ganglia and corresponding target organs of the rat: Correlation with density of sympathetic innervation. Proc. Natl. Acad. Sci. USA 1983, 80, 3513–3516. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Fukuda, K.; Hisaka, Y.; Kimura, K.; Kawaguchi, H.; Fujita, J.; Shimoda, K.; Takeshita, E.; Okano, H.; Kurihara, Y.; et al. Endothelin-1 regulates cardiac sympathetic innervation in the rodent heart by controlling nerve growth factor expression. J. Clin. Investig. 2004, 113, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W.; Pedchenko, T.; Krizsan-Agbas, D.; Baum, L.; Smith, P.G. Sympathetic neurons synthesize and secrete pro-nerve growth factor protein. J. Neurobiol. 2003, 57, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Kuruvilla, R.; Zweifel, L.S.; Ginty, D.D. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron 2003, 39, 57–68. [Google Scholar] [CrossRef]
- Hendry, I.A.; Stöckel, K.; Thoenen, H.; Iversen, L.L. The retrograde axonal transport of nerve growth factor. Brain Res. 1974, 68, 103–121. [Google Scholar] [CrossRef]
- Hasan, W.; Smith, P.G. Nerve growth factor expression in parasympathetic neurons: Regulation by sympathetic innervation. Eur. J. Neurosci. 2000, 12, 4391–4397. [Google Scholar] [CrossRef] [PubMed]
- Crowley, C.; Spencer, S.D.; Nishimura, M.C.; Chen, K.S.; Pitts-Meek, S.; Armanini, M.P.; Ling, L.H.; McMahon, S.B.; Shelton, D.L.; Levinson, A.D.; et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 1994, 76, 1001–1011. [Google Scholar] [CrossRef]
- Levi-Montalcini, R.; Booker, B. Destruction of the Sympathetic Ganglia in Mammals by an Antiserum to a Nerve-Growth Protein. Proc. Natl. Acad. Sci. USA 1960, 46, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.S.; Nishimura, M.C.; Armanini, M.P.; Crowley, C.; Spencer, S.D.; Phillips, H.S. Disruption of a single allele of the nerve growth factor gene results in atrophy of basal forebrain cholinergic neurons and memory deficits. J. Neurosci. 1997, 17, 7288–7296. [Google Scholar] [PubMed]
- Birren, S.J.; Lo, L.; Anderson, D.J. Sympathetic neuroblasts undergo a developmental switch in trophic dependence. Development 1993, 119, 597–610. [Google Scholar] [PubMed]
- Tessarollo, L.; Tsoulfas, P.; Donovan, M.J.; Palko, M.E.; Blair-Flynn, J.; Hempstead, B.L.; Parada, L.F. Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 14776–14781. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, P.M.; Camargos, E.R.S.; Morel, G.; Tavares, C.A.P.; Nagib, P.R.A.; Machado, C.R.S. Rat heart GDNF: Effect of chemical sympathectomy. Histochem. Cell Biol. 2002, 118, 337–343. [Google Scholar] [PubMed]
- Airaksinen, M.S.; Saarma, M. The Gdnf Family: Signalling, Biological Functions and Therapeutic Value. Nat. Rev. Neurosci. 2002, 3, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Coulpier, M.; Ibáñez, C.F. Retrograde propagation of GDNF-mediated signals in sympathetic neurons. Mol. Cell. Neurosci. 2004, 27, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lavandeira, M.; Diaz-Rodriguez, E.; Garcia-Rendueles, M.E.R.; Rodrigues, J.S.; Perez-Romero, S.; Bravo, S.B.; Alvarez, C.V. Functional role of the RET Dependence receptor, GFRa co-receptors and ligands in the pituitary. Pituit. Today II New Mol. Physiol. Clin. Asp. 2010, 38, 127–138. [Google Scholar]
- Hiltunen, J.O.; Laurikainen, A.; Airaksinen, M.S.; Saarma, M. GDNF family receptors in the embryonic and postnatal rat heart and reduced cholinergic innervation in mice hearts lacking ret or GFRalpha2. Dev. Dyn. 2000, 219, 28–39. [Google Scholar] [CrossRef]
- Moore, M.W.; Klein, R.D.; Fariñas, I.; Sauer, H.; Armanini, M.; Phillips, H.; Reichardt, L.F.; Ryan, A.M.; Carver-Moore, K.; Rosenthal, A. Renal and neuronal abnormalities in mice lacking GDNF. Nature 1996, 382, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Pichel, J.G.; Shen, L.; Sheng, H.Z.; Granholm, A.C.; Drago, J.; Grinberg, A.; Lee, E.J.; Huang, S.P.; Saarma, M.; Hoffer, B.J.; et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 1996, 382, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Cacalano, G.; Fariñas, I.; Wang, L.C.; Hagler, K.; Forgie, A.; Moore, M.; Armanini, M.; Phillips, H.; Ryan, A.M.; Reichardt, L.F.; et al. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 1998, 21, 53–62. [Google Scholar] [CrossRef]
- Heuckeroth, R.O.; Enomoto, H.; Grider, J.R.; Golden, J.P.; Hanke, J.A.; Jackman, A.; Molliver, D.C.; Bardgett, M.E.; Snider, W.D.; Johnson, E.M.; et al. Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 1999, 22, 253–263. [Google Scholar] [CrossRef]
- Rossi, J.; Luukko, K.; Poteryaev, D.; Laurikainen, A.; Sun, Y.F.; Laakso, T.; Eerikäinen, S.; Tuominen, R.; Lakso, M.; Rauvala, H.; et al. Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFRα2, a functional neurturin receptor. Neuron 1999, 22, 243–252. [Google Scholar] [CrossRef]
- Golden, J.P.; Milbrandt, J.; Johnson, E.M. Neurturin and persephin promote the survival of embryonic basal forebrain cholinergic neurons in vitro. Exp. Neurol. 2003, 184, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Hashino, E.; Shero, M.; Junghans, D.; Rohrer, H.; Milbrandt, J.; Johnson, E.M. GDNF and neurturin are target-derived factors essential for cranial parasympathetic neuron development. Development 2001, 128, 3773–3782. [Google Scholar] [PubMed]
- Poelmann, R.E.; Gittenberger-de Groot, A.C. A subpopulation of apoptosis-prone cardiac neural crest cells targets to the venous pole: Multiple functions in heart development? Dev. Biol. 1999, 207, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Shoba, T.; Tay, S.S. Nitrergic and peptidergic innervation in the developing rat heart. Anat. Embryol. (Berl.) 2000, 201, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, V.; Anderson, R.H.; Henderson, D.J. Autonomic innervation of the developing heart: Origins and function. Clin. Anat. 2009, 22, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Verberne, M.E.; Gittenberger-De Groot, A.C.; van Iperen, L.; Poelmann, R.E. Contribution of the cervical sympathetic ganglia to the innervation of the pharyngeal arch arteries and the heart in the chick embryo. Anat. Rec. 1999, 255, 407–419. [Google Scholar] [CrossRef]
- Nam, J.; Onitsuka, I.; Hatch, J.; Uchida, Y.; Ray, S.; Huang, S.; Li, W.; Zang, H.; Ruiz-Lozano, P.; Mukouyama, Y.-S. Coronary veins determine the pattern of sympathetic innervation in the developing heart. Development 2013, 140, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Mikawa, T.; Fischman, D.A. Retroviral analysis of cardiac morphogenesis: Discontinuous formation of coronary vessels. Proc. Natl. Acad. Sci. USA 1992, 89, 9504–9508. [Google Scholar] [CrossRef] [PubMed]
- Mukouyama, Y.; Shin, D.; Britsch, S.; Taniguchi, M.; Anderson, D.J. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell 2002, 109, 693–705. [Google Scholar] [CrossRef]
- Fregoso, S.P.; Hoover, D.B. Development of cardiac parasympathetic neurons, glial cells, and regional cholinergic innervation of the mouse heart. Neuroscience 2012, 221, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Tollet, J.; Everett, A.W.; Sparrow, M.P. Development of neural tissue and airway smooth muscle in fetal mouse lung explants: A role for glial-derived neurotrophic factor in lung innervation. Am. J. Respir. Cell Mol. Biol. 2002, 26, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Young, H.M.; Hearn, C.J.; Farlie, P.G.; Canty, A.J.; Thomas, P.Q.; Newgreen, D.F. GDNF is a chemoattractant for enteric neural cells. Dev. Biol. 2001, 229, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.; Serpooshan, V.; Hurtado, C.; Diez-Cuñado, M.; Zhao, M.; Maruyama, S.; Zhu, W.; Fajardo, G.; Noseda, M.; Nakamura, K.; et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature 2015, 525, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Gueret, A.; Harouki, N.; Favre, J.; Galmiche, G.; Nicol, L.; Henry, J.-P.; Besnier, M.; Thuillez, C.; Richard, V.; Kolkhof, P.; et al. Vascular smooth muscle mineralocorticoid receptor contributes to coronary and left ventricular dysfunction after myocardial infarction. Hypertension 2016, 67, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Hasan, W.; Jama, A.; Donohue, T.; Wernli, G.; Onyszchuk, G.; Al-Hafez, B.; Bilgen, M.; Smith, P.G. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res. 2006, 1124, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Hassankhani, A.; Steinhelper, M.E.; Soonpaa, M.H.; Katz, E.B.; Taylor, D.A.; Andrade-Rozental, A.; Factor, S.M.; Steinberg, J.J.; Field, L.J.; Federoff, H.J. Overexpression of NGF within the heart of transgenic mice causes hyperinnervation, cardiac enlargement, and hyperplasia of ectopic cells. Dev. Biol. 1995, 169, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Wernli, G.; Hasan, W.; Bhattacherjee, A.; van Rooijen, N.; Smith, P.G. Macrophage depletion suppresses sympathetic hyperinnervation following myocardial infarction. Basic Res. Cardiol. 2009, 104, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Meloni, M.; Caporali, A.; Graiani, G.; Lagrasta, C.; Katare, R.; van Linthout, S.; Spillmann, F.; Campesi, I.; Madeddu, P.; Quaini, F.; et al. Nerve growth factor promotes cardiac repair following myocardial infarction. Circ. Res. 2010, 106, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- White, I.A.; Gordon, J.; Balkan, W.; Hare, J.M. Sympathetic reinnervation is required for mammalian cardiac regeneration. Circ. Res. 2015, 117, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Dzimiri, N.; Muiya, P.; Andres, E.; Al-Halees, Z. Differential functional expression of human myocardial G protein receptor kinases in left ventricular cardiac diseases. Eur. J. Pharmacol. 2004, 489, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ungerer, M.; Bohm, M.; Elce, J.S.; Erdmann, E.; Lohse, M.J. Altered expression of beta-adrenergic receptor kinase and beta 1-adrenergic receptors in the failing human heart. Circulation 1993, 87, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Rengo, G.; Lymperopoulos, A.; Leosco, D.; Koch, W.J. GRK2 as a novel gene therapy target in heart failure. J. Mol. Cell. Cardiol. 2011, 50, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Rengo, G.; Perrone-Filardi, P.; Femminella, G.D.; Liccardo, D.; Zincarelli, C.; de Lucia, C.; Pagano, G.; Marsico, F.; Lymperopoulos, A.; Leosco, D. Targeting the -Adrenergic Receptor System Through G-Protein-Coupled Receptor Kinase 2: A New Paradigm for Therapy and Prognostic Evaluation in Heart Failure: From Bench to Bedside. Circ. Hear. Fail. 2012, 5, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-H.; Jiang, P.; Yang, J.; Ma, D.-F.; Lin, H.-Q.; Su, W.; Wang, Z.; Li, X. Cardiac dysregulation and myocardial injury in a 6-hydroxydopamine-induced rat model of sympathetic denervation. PLoS ONE 2015, 10, e0133971. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Shibata, S.; Okamoto, Y.; Sato, S.; Fujisawa, S.; Ohba, T.; Ono, K. The mechanism of increased postnatal heart rate and sinoatrial node pacemaker activity in mice. J. Physiol. Sci. 2013, 63, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Sun, Y.; Li, C.Z.; Wang, H.W.; Wang, X.J.; Zheng, Y.Q.; Liu, K.; Liu, Y.M. Reduced sinoatrial cAMP content plays a role in postnatal heart rate slowing in the rabbit. Clin. Exp. Pharmacol. Physiol. 2006, 33, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Hewett, K.W.; Rosen, M.R. Developmental changes in the rabbit sinus node action potential and its response to adrenergic agonists. J. Pharmacol. Exp. Ther. 1985, 235, 308–312. [Google Scholar] [PubMed]
- Yiallourou, S.R.; Witcombe, N.B.; Sands, S.A.; Walker, A.M.; Horne, R.S.C. The development of autonomic cardiovascular control is altered by preterm birth. Early Hum. Dev. 2013, 89, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Harper, R.M.; Hoppenbrouwers, T.; Sterman, M.B.; McGinty, D.J.; Hodgman, J. Polygraphic studies of normal infants during the first six months of life. I. Heart rate and variability as a function of state. Pediatr. Res. 1976, 10, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Sato, S. Quantitative evaluation of ontogenetic change in heart rate and its autonomic regulation in newborn mice with the use of a noninvasive piezoelectric sensor. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1708–H1715. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.C. Components of functional sympathetic control of heart rate in neonatal rats. Am. J. Physiol. 1985, 248, R601–R610. [Google Scholar] [PubMed]
- Chow, L.T.; Chow, S.S.; Anderson, R.H.; Gosling, J.A. Autonomic innervation of the human cardiac conduction system: Changes from infancy to senility—An immunohistochemical and histochemical analysis. Anat. Rec. 2001, 264, 169–182. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Végh, A.M.D.; Duim, S.N.; Smits, A.M.; Poelmann, R.E.; Ten Harkel, A.D.J.; DeRuiter, M.C.; Goumans, M.J.; Jongbloed, M.R.M. Part and Parcel of the Cardiac Autonomic Nerve System: Unravelling Its Cellular Building Blocks during Development. J. Cardiovasc. Dev. Dis. 2016, 3, 28. https://doi.org/10.3390/jcdd3030028
Végh AMD, Duim SN, Smits AM, Poelmann RE, Ten Harkel ADJ, DeRuiter MC, Goumans MJ, Jongbloed MRM. Part and Parcel of the Cardiac Autonomic Nerve System: Unravelling Its Cellular Building Blocks during Development. Journal of Cardiovascular Development and Disease. 2016; 3(3):28. https://doi.org/10.3390/jcdd3030028
Chicago/Turabian StyleVégh, Anna M. D., Sjoerd N. Duim, Anke M. Smits, Robert E. Poelmann, Arend D. J. Ten Harkel, Marco C. DeRuiter, Marie José Goumans, and Monique R. M. Jongbloed. 2016. "Part and Parcel of the Cardiac Autonomic Nerve System: Unravelling Its Cellular Building Blocks during Development" Journal of Cardiovascular Development and Disease 3, no. 3: 28. https://doi.org/10.3390/jcdd3030028
APA StyleVégh, A. M. D., Duim, S. N., Smits, A. M., Poelmann, R. E., Ten Harkel, A. D. J., DeRuiter, M. C., Goumans, M. J., & Jongbloed, M. R. M. (2016). Part and Parcel of the Cardiac Autonomic Nerve System: Unravelling Its Cellular Building Blocks during Development. Journal of Cardiovascular Development and Disease, 3(3), 28. https://doi.org/10.3390/jcdd3030028







