Ciona as a Simple Chordate Model for Heart Development and Regeneration
Abstract
:1. Introduction
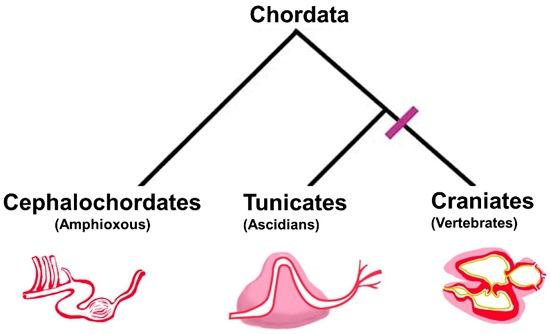
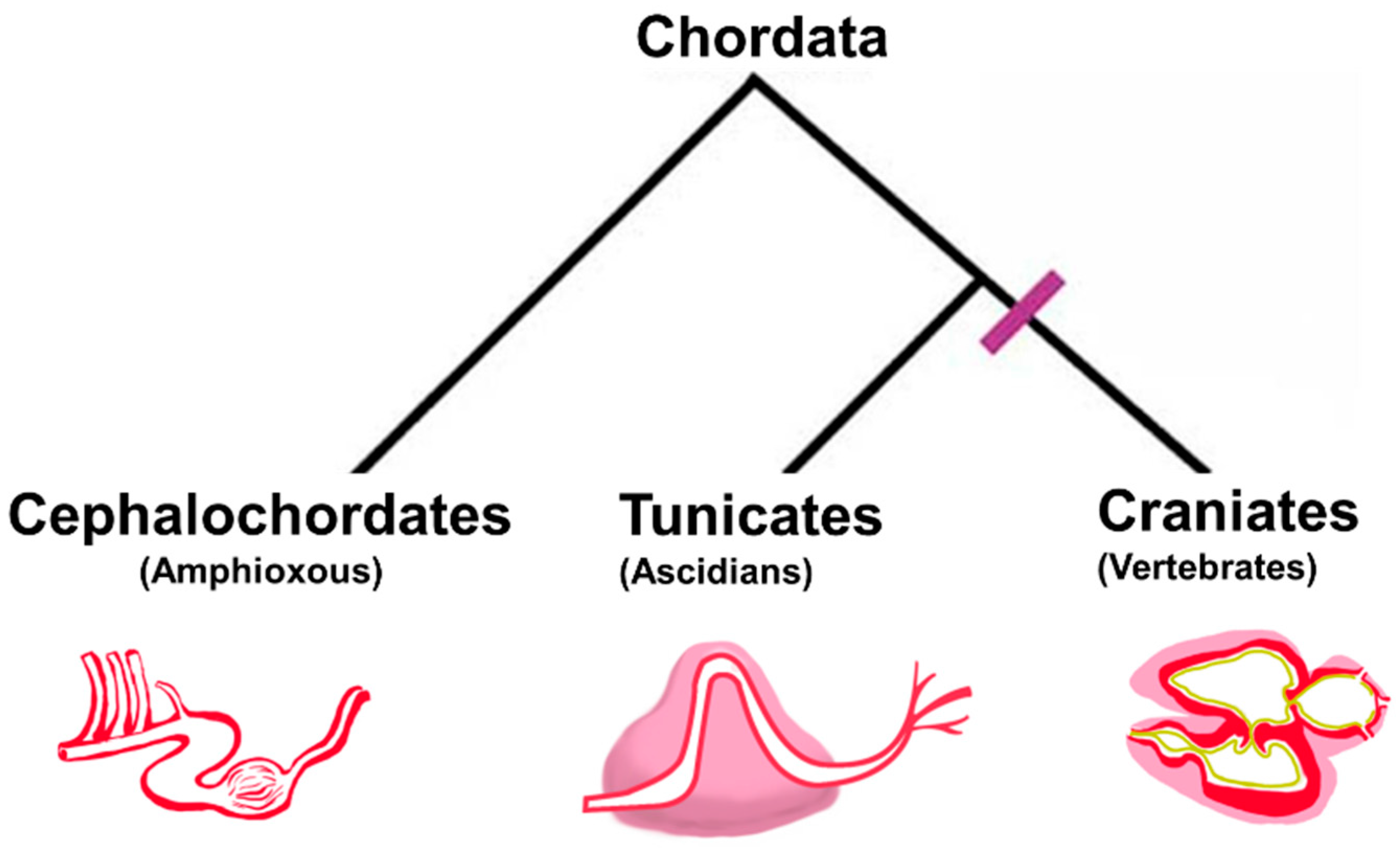
2. Chordate Circulatory Systems: From Divergent Anatomies to Conserved Cytology
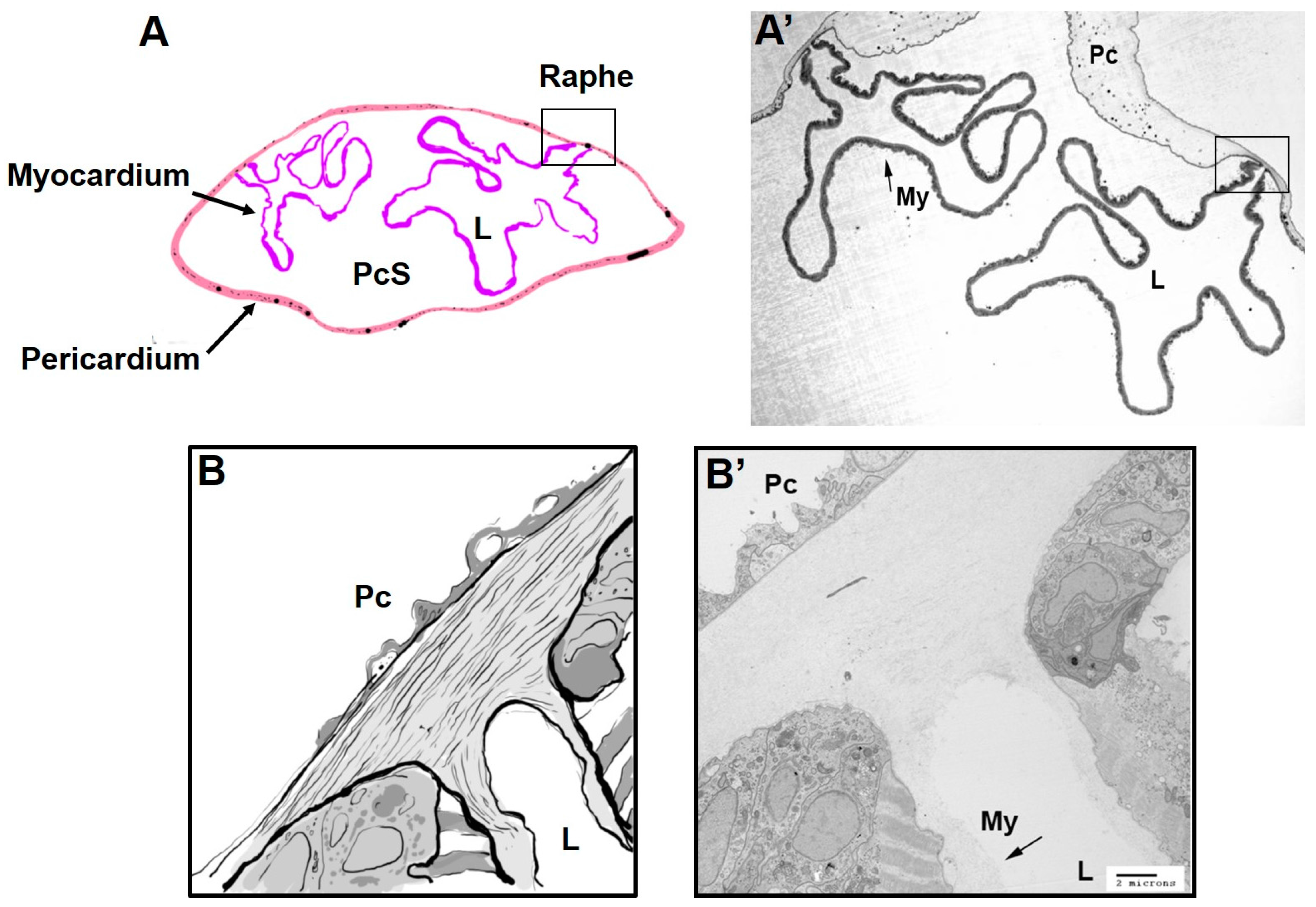
2.1. Divergent Cardiac Anatomy and Histology
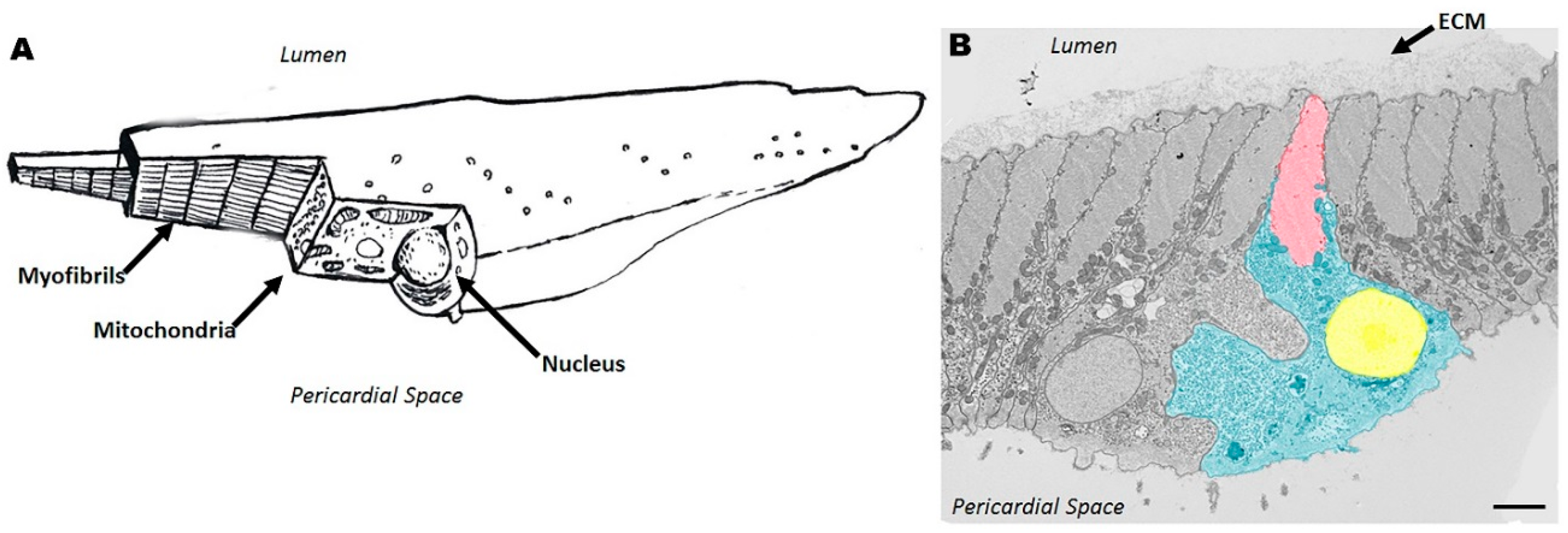
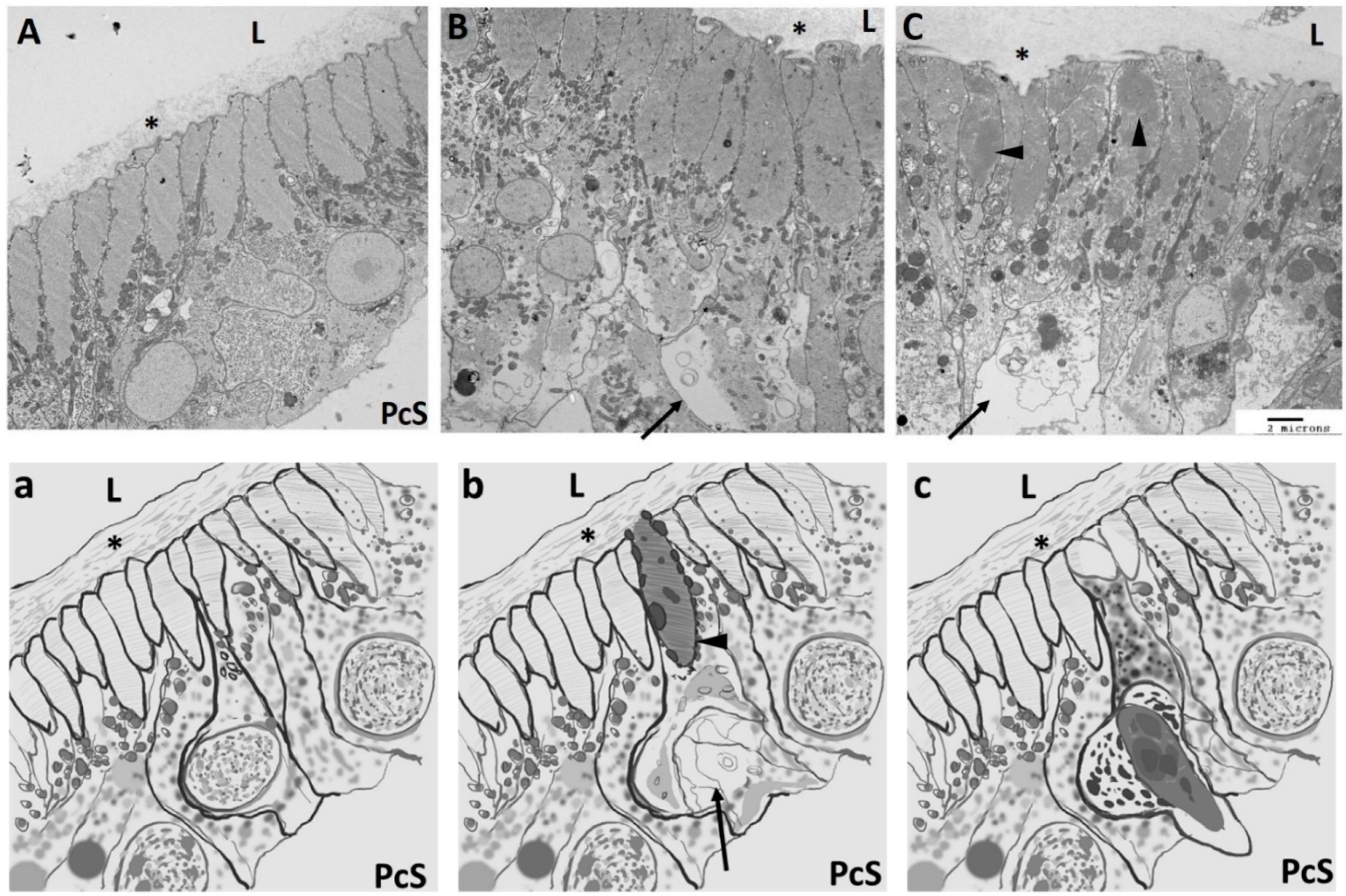
2.2. Cellular Structure of Ciona Heart
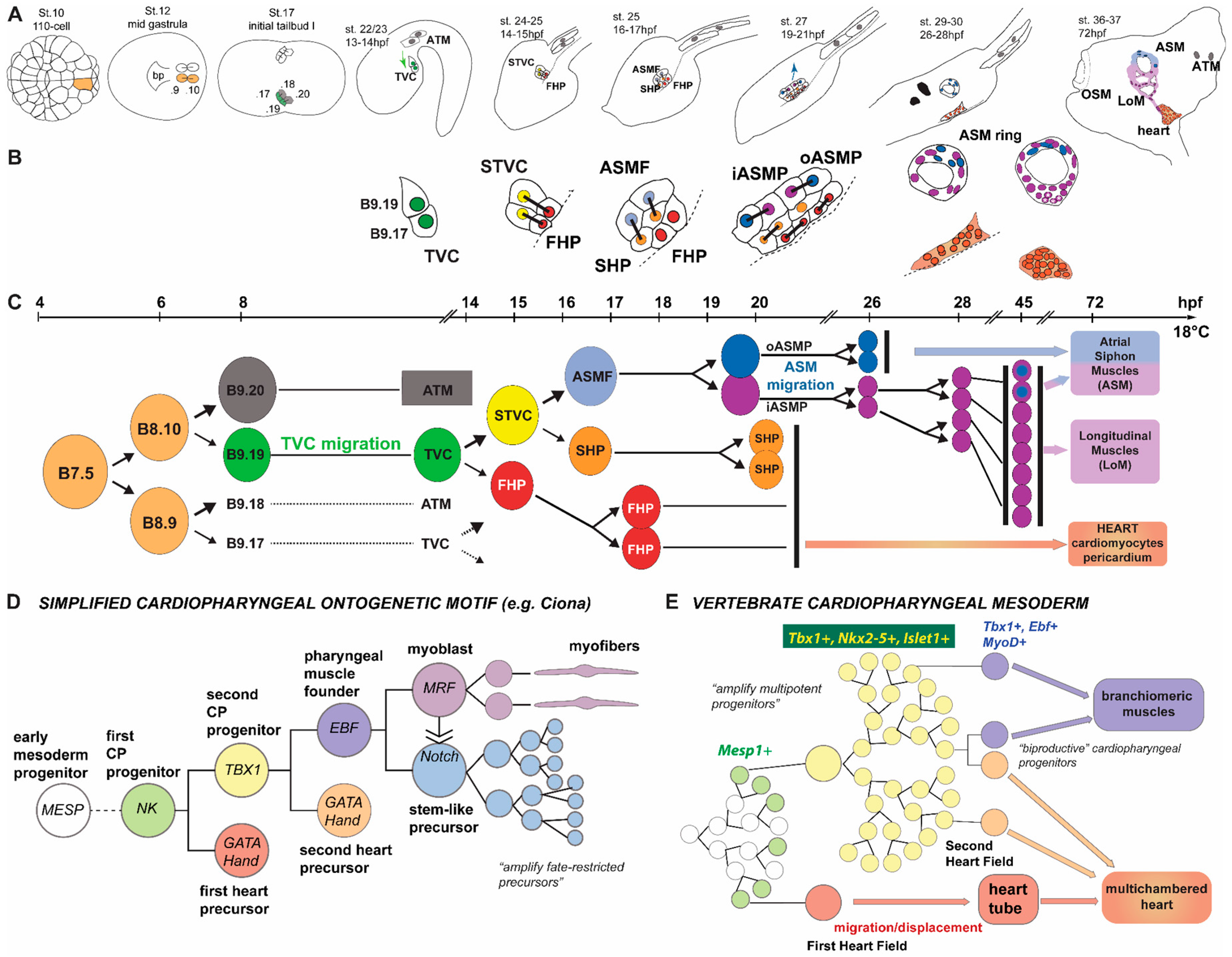
3. Development of the Ciona Heart and Parallels with the Vertebrates
4. Ciona as a Model for Basic Cellular and Molecular Aspects of Congenital Heart Defects
5. Ciona as an Experimental Model for Cardiac Regeneration
6. Concluding Remarks
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CHD | congenital heart defects/disease |
| Myo | myocardium |
| Pc | pericardium |
| PcB | pericardial body |
| PcS | pericardial space |
| L | lumen |
| TEM | transmission electron microscopy |
| ATMs | anterior tail muscle precursors |
| ATM | anterior tail muscles |
| TVCs | trunk ventral cells |
| FHPs | first heart precursors |
| STVCs | second trunk ventral cells |
| SHPs | second heart precursors |
| ASMFs | atrial siphon muscle founder cells |
| ASMPs | atrial siphon muscle precursors |
| LoM | longitudinal body wall muscles |
| iASMP | inner atrial siphon muscle precursor |
| oASMP | outer atrial siphon |
| OSM | oral siphon muscles |
| ECM | extracellular matrix |
References
- Davidson, E.H. Gene Regulatory Networks and the Evolution of Animal Body Plans. Science 2006, 311, 796–800. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.N. Gene regulatory networks in the evolution and development of the heart. Science 2006, 313, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development 1993, 118, 719–729. [Google Scholar] [PubMed]
- Imai, K.S.; Levine, M.; Satoh, N.; Satou, Y. Regulatory blueprint for a chordate embryo. Science 2006, 312, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.; Olson, E.N. A genetic blueprint for cardiac development. Nature 2000, 407, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Rokhsar, D.; Nishikawa, T. Chordate evolution and the three-phylum system. Proc. Biol. Sci. 2014, 281, 20141729. [Google Scholar] [CrossRef] [PubMed]
- Delsuc, F.; Brinkmann, H.; Chourrout, D.; Philippe, H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature 2006, 439, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Putnam, N.H.; Butts, T.; Ferrier, D.E.K.; Furlong, R.F.; Hellsten, U.; Kawashima, T.; Robinson-Rechavi, M.; Shoguchi, E.; Terry, A.; Yu, K., Jr.; et al. The amphioxus genome and the evolution of the chordate karyotype. Nature 2008, 453, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B. Ciona intestinalis as a model for cardiac development. Semin. Cell Dev. Biol. 2007, 18, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pomares, J.M.; González-Rosa, J.M.; Muñoz-Chápuli, R. Building the vertebrate heart—An evolutionary approach to cardiac development. Int. J. Dev. Biol. 2009, 53, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D.; Thompson, R.P. Myocyte proliferation in the developing heart. Dev. Dyn. 2011, 240, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Wang, T.; Christoffels, V.M.; Moorman, A.F.M. Evolution and development of the building plan of the vertebrate heart. Biochim. Biophys. Acta 2013, 1833, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.P.; Jaffe, D.B.; O’Neill, K.; Karlsson, E.K.; Stange-Thomann, N.; Anderson, S.; Mesirov, J.P.; Satoh, N.; Satou, Y.; Nusbaum, C.; et al. Assembly of polymorphic genomes: Algorithms and application to Ciona savignyi. Genome Res. 2005, 15, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Cañestro, C.; Bassham, S.; Postlethwait, J.H. Seeing chordate evolution through the Ciona genome sequence. Genome Biol. 2003, 4, 208. [Google Scholar] [CrossRef] [PubMed]
- Jong, H.K.; Waterman, M.S.; Li, L.M. Diploid genome reconstruction of Ciona intestinalis and comparative analysis with Ciona savignyi. Genome Res. 2007, 17, 1101–1110. [Google Scholar]
- Dehal, P.; Satou, Y.; Campbell, R.K.; Chapman, J.; Degnan, B.; De Tomaso, A.; Davidson, B.; Di Gregorio, A.; Gelpke, M.; Goodstein, D.M.; et al. The Draft Genome of Ciona intestinalis: Insights into Chordate and Vertebrate Origins. Science 2002, 298, 2157–2167. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N.; Satou, Y.; Davidson, B.; Levine, M. Ciona intestinalis: An emerging model for whole-genome analyses. Trends Genet. 2003, 19, 376–381. [Google Scholar] [CrossRef]
- Pennati, R.; Ficetola, G.F.; Brunetti, R.; Caicci, F.; Gasparini, F.; Griggio, F.; Sato, A.; Stach, T.; Kaul-Strehlow, S.; Gissi, C.; et al. Morphological differences between larvae of the Ciona intestinalis species complex: Hints for a valid taxonomic definition of distinct species. PLoS ONE 2015, 10, e0122879. [Google Scholar] [CrossRef] [PubMed]
- Berrill, N.J. The development and growth of Ciona. J. Mar. Biol. Assoc. UK 1947, 26, 616–625. [Google Scholar] [CrossRef]
- Millar, R.H. Ciona. In L.M.B.C. Memoirs on Typical British Marine Plants and Animals; Liverpool University Press: Liverpool, UK, 1953. [Google Scholar]
- Dybern, B.I. The Life Cycle of Ciona intestinalis (L.) f. typica in Relation to the Environmental Temperature. Oikos 1965, 16, 109–131. [Google Scholar] [CrossRef]
- Petersen, J.K.; Svane, I. Larval dispersal in the ascidian Ciona intestinalis (L.). Evidence for a closed population. J. Exp. Mar. Biol. Ecol. 1995, 186, 89–102. [Google Scholar] [CrossRef]
- Jeffery, W.R. Siphon regeneration capacity is compromised during aging in the ascidian Ciona intestinalis. Mech. Ageing Dev. 2012, 133, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H. Specification of developmental fates in ascidian embryos: Molecular approach to maternal determinants and signaling molecules. Int. Rev. Cytol. 2002, 217, 227–276. [Google Scholar] [PubMed]
- Yamada, L.; Kobayashi, K.; Satou, Y.; Satoh, N. Microarray analysis of localization of maternal transcripts in eggs and early embryos of the ascidian, Ciona intestinalis. Dev. Biol. 2005, 284, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, W.R. Determinants of cell and positional fate in ascidian embryos. Int. Rev. Cytol. 2001, 203, 3–62. [Google Scholar] [PubMed]
- Nishida, H. Cell lineage and timing of fate restriction, determination and gene expression in ascidian embryos. Semin. Cell Dev. Biol. 1997, 8, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Imai, K.S.; Satoh, N. The ascidian Mesp gene specifies heart precursor cells. Development 2004, 131, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Levine, M. Evolutionary origins of the vertebrate heart: Specification of the cardiac lineage in Ciona intestinalis. Proc. Natl. Acad. Sci. USA 2003, 100, 11469–11473. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, A.; Gainous, T.B.; Young, J.J.; Mori, A.; Levine, M.; Christiaen, L. Early chordate origins of the vertebrate second heart field. Science 2010, 329, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Razy-Krajka, F.; Lam, K.; Wang, W.; Stolfi, A.; Joly, M.; Bonneau, R.; Christiaen, L. Collier/OLF/EBF-Dependent Transcriptional Dynamics Control Pharyngeal Muscle Specification from Primed Cardiopharyngeal Progenitors. Dev. Cell 2014, 29, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Woznica, A.; Haeussler, M.; Starobinska, E.; Jemmett, J.; Li, Y.; Mount, D.; Davidson, B. Initial deployment of the cardiogenic gene regulatory network in the basal chordate, Ciona intestinalis. Dev. Biol. 2012, 368, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, N.; Razy-Krajka, F.; Christiaen, L. Regulation and evolution of cardiopharyngeal cell identity and behavior: Insights from simple chordates. Curr. Opin. Genet. Dev. 2015, 32, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Cota, C.D.; Segade, F.; Davidson, B. Heart genetics in a small package, exploiting the condensed genome of Ciona intestinalis. Brief. Funct. Genom. 2014, 13, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.; Cooley, J.; Islam, A.F.M.T.; Cota, C.D.; Davidson, B. Matrix adhesion polarizes heart progenitor induction in the invertebrate chordate Ciona intestinalis. Development 2013, 140, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Razy-Krajka, F.; Siu, E.; Ketcham, A.; Christiaen, L. NK4 Antagonizes Tbx1/10 to Promote Cardiac versus Pharyngeal Muscle Fate in the Ascidian Second Heart Field. PLoS Biol. 2013, 11, e1001725. [Google Scholar] [CrossRef] [PubMed]
- Lescroart, F.; Chabab, S.; Lin, X.; Rulands, S.; Paulissen, C.; Rodolosse, A.; Auer, H.; Achouri, Y.; Dubois, C.; Bondue, A.; et al. Early lineage restriction in temporally distinct populations of Mesp1 progenitors during mammalian heart development. Nat. Cell Biol. 2014, 16, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R.; Kelly, R.G.; Christiaen, L.; Levine, M.; Ziermann, J.M.; Molnar, J.L.; Noden, D.M.; Tzahor, E. A new heart for a new head in vertebrate cardiopharyngeal evolution. Nature 2015, 520, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Sasaki, A.; Nakayama, A.; Takamura, K.; Satoh, N. Development of Ciona intestinalis juveniles (through 2nd ascidian stage). Zool. Sci. 2004, 21, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, L.W. Ultrastructure and Mechanism of Contraction of the Ascidian Heart. Ph.D. Thesis, University of Washington, Seattle, WA, USA, 1972. [Google Scholar]
- Satou, Y.; Imai, K.S.; Levine, M.; Kohara, Y.; Rokhsar, D.; Satoh, N. A genomewide survey of developmentally relevant genes in Ciona intestinalis. I. Genes for bHLH transcription factors. Dev. Genes Evol. 2003, 213, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.; Tokuoka, M.; Shoguchi, E.; Kobayashi, K.; Di Gregorio, A.; Spagnuolo, A.; Branno, M.; Kohara, Y.; Rokhsar, D.; Levine, M.; et al. A genomewide survey of developmentally relevant genes in Ciona intestinalis. II. Genes for homeobox transcription factors. Dev. Genes Evol. 2003, 213, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Yamada, L.; Kobayashi, K.; Degnan, B.; Satoh, N.; Satou, Y. A genomewide survey of developmentally relevant genes in Ciona intestinalis. IV. Genes for HMG transcriptional regulators, bZip and GATA/Gli/Zic/Snail. Dev. Genes Evol. 2003, 213, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Satou, Y.; Mineta, K.; Ogasawara, M.; Sasakura, Y.; Shoguchi, E.; Ueno, K.; Yamada, L.; Matsumoto, J.; Wasserscheid, J.; Dewar, K.; et al. Improved genome assembly and evidence-based global gene model set for the chordate Ciona intestinalis: New insight into intron and operon populations. Genome Biol. 2008, 9. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Nishida, H. Developmental fates of larval tissues after metamorphosis in ascidian Halocynthia roretzi. I. Origin of mesodermal tissues of the juvenile. Dev. Biol. 1997, 192, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Shi, W.; Levine, M. Uncoupling heart cell specification and migration in the simple chordate Ciona intestinalis. Development 2005, 132, 4811–4818. [Google Scholar] [CrossRef] [PubMed]
- Saga, Y.; Miyagawa-Tomita, S.; Takagi, A.; Kitajima, S.; Miyazaki, J.I.; Inoue, T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development 1999, 126, 3437–3447. [Google Scholar] [PubMed]
- Saga, Y.; Kitajima, S.; Miyagawa-Tomita, S. Mesp1 expression is the earliest sign of cardiovascular development. Trends Cardiovasc. Med. 2000, 10, 345–352. [Google Scholar] [CrossRef]
- Devine, W.P.; Wythe, J.D.; George, M.; Koshiba-Takeuchi, K.; Bruneau, B.G. Early patterning and specification of cardiac progenitors in gastrulating mesoderm. Elife 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Cooley, J.; Whitaker, S.; Sweeney, S.; Fraser, S.; Davidson, B. Cytoskeletal polarity mediates localized induction of the heart progenitor lineage. Nat. Cell Biol. 2011, 13, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Shi, W.; Beh, J.; Christiaen, L.; Levine, M. FGF signaling delineates the cardiac progenitor field in the simple chordate, Ciona intestinalis. Genes Dev. 2006, 20, 2728–2738. [Google Scholar] [CrossRef] [PubMed]
- Beh, J.; Shi, W.; Levine, M.; Davidson, B.; Christiaen, L. FoxF is essential for FGF-induced migration of heart progenitor cells in the ascidian Ciona intestinalis. Development 2007, 134, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Znosko, W.A.; Yu, S.; Thomas, K.; Molina, G.A.; Li, C.; Tsang, W.; Dawid, I.B.; Moon, A.M.; Tsang, M. Overlapping functions of Pea3 ETS transcription factors in FGF signaling during zebrafish development. Dev. Biol. 2010, 342, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Christiaen, L.; Davidson, B.; Kawashima, T.; Powell, W.; Nolla, H.; Vranizan, K.; Levine, M. The transcription/migration interface in heart precursors of Ciona intestinalis. Science 2008, 320, 1349–1352. [Google Scholar] [CrossRef] [PubMed]
- Ragkousi, K.; Beh, J.; Sweeney, S.; Starobinska, E.; Davidson, B. A single GATA factor plays discrete, lineage specific roles in ascidian heart development. Dev. Biol. 2011, 352, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.-L.; Liang, X.; Shi, Y.; Chu, P.-H.; Pfaff, S.L.; Chen, J.; Evans, S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell 2003, 5, 877–889. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, X.; Najafi, N.; Cass, M.; Lin, L.; Cai, C.-L.; Chen, J.; Evans, S.M. Islet 1 is expressed in distinct cardiovascular lineages, including pacemaker and coronary vascular cells. Dev. Biol. 2007, 304, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Nathan, E.; Monovich, A.; Tirosh-Finkel, L.; Harrelson, Z.; Rousso, T.; Rinon, A.; Harel, I.; Evans, S.M.; Tzahor, E. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development 2008, 135, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Mitsuhara, K.; Takahashi, H.; Inaba, K.; Oka, K.; Gojobori, T.; Ikeo, K. A web-based interactive developmental table for the ascidian Ciona intestinalis, including 3D real-image embryo reconstructions: I. From fertilized egg to hatching larva. Dev. Dyn. 2007, 236, 1790–1805. [Google Scholar] [CrossRef] [PubMed]
- Tokuoka, M.; Satoh, N.; Satou, Y. A bHLH transcription factor gene, Twist-like 1, is essential for the formation of mesodermal tissues of Ciona juveniles. Dev. Biol. 2005, 288, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Tirosh-Finkel, L.; Elhanany, H.; Rinon, A.; Tzahor, E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development 2006, 133, 1943–1953. [Google Scholar] [CrossRef] [PubMed]
- Lescroart, F.; Kelly, R.G.; Le Garrec, J.-F.; Nicolas, J.-F.; Meilhac, S.M.; Buckingham, M. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development 2010, 137, 3269–3279. [Google Scholar] [CrossRef] [PubMed]
- Lescroart, F.; Hamou, W.; Francou, A.; Théveniau-Ruissy, M.; Kelly, R.G.; Buckingham, M. Clonal analysis reveals a common origin between nonsomite-derived neck muscles and heart myocardium. Proc. Natl. Acad. Sci. USA 2015, 112, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Chabab, S.; Lescroart, F.; Rulands, S.; Mathiah, N.; Simons, B.D.; Blanpain, C. Uncovering the Number and Clonal Dynamics of Mesp1 Progenitors during Heart Morphogenesis. Cell Rep. 2016, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meilhac, S.M.; Esner, M.; Kelly, R.G.; Nicolas, J.-F.; Buckingham, M.E. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Dev. Cell 2004, 6, 685–698. [Google Scholar] [CrossRef]
- Schultheiss, T.M.; Xydas, S.; Lassar, A.B. Induction of avian cardiac myogenesis by anterior endoderm. Development 1995, 121, 4203–4214. [Google Scholar] [PubMed]
- Schultheiss, T.M.; Burch, J.B.; Lassar, A.B. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997, 11, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Tzahor, E.; Lassar, A.B. Wnt signals from the neural tube block ectopic cardiogenesis. Genes Dev. 2001, 15, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Tzahor, E.; Kempf, H.; Mootoosamy, R.C.; Poon, A.C.; Abzhanov, A.; Tabin, C.J.; Dietrich, S.; Lassar, A.B. Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev. 2003, 17, 3087–3099. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.-K.; Shi, X.; Toyama, A.; Arpke, R.W.; Dandapat, A.; Iacovino, M.; Kang, J.; Gengyun, L.; Hagen, H.R.; Garry, D.J.; et al. Mesp1 patterns mesoderm into cardiac, hematopoietic, or skeletal myogenic progenitors in a context-dependent manner. Cell Stem Cell 2013, 12, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.-K.; Hagen, H.R.; Swanson, S.A.; Stewart, R.; Boll, K.A.A.; Aho, J.; Thomson, J.A.A.; Kyba, M. Development of Bipotent Cardiac/Skeletal Myogenic Progenitors from MESP1+ Mesoderm. Stem Cell Rep. 2016, 6, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, A.; Lowe, E.K.; Racioppi, C.; Ristoratore, F.; Brown, C.T.; Swalla, B.J.; Christiaen, L. Divergent mechanisms regulate conserved cardiopharyngeal development and gene expression in distantly related ascidians. Elife 2014, 3, e03728. [Google Scholar] [CrossRef] [PubMed]
- Tolkin, T.; Christiaen, L. Development and Evolution of the Ascidian Cardiogenic Mesoderm. Curr. Top. Dev. Biol. 2012, 100, 107–142. [Google Scholar]
- Rickert-Sperling, S.; Kelly, R.G. Congenital Heart Diseases: The Broken Heart; Driscoll, D.J., Ed.; Springer: Vienna, Austria, 2016. [Google Scholar]
- Yagi, H.; Furutani, Y.; Hamada, H.; Sasaki, T.; Asakawa, S.; Minoshima, S.; Ichida, F.; Joo, K.; Kimura, M.; Imamura, S.I.; et al. Role of TBX1 in human del22q11.2 syndrome. Lancet 2003, 362, 1366–1373. [Google Scholar] [CrossRef]
- Lindsay, E.A.; Vitelli, F.; Su, H.; Morishima, M.; Huynh, T.; Pramparo, T.; Jurecic, V.; Ogunrinu, G.; Sutherland, H.F.; Scambler, P.J.; et al. Tbx1 haploinsufficieny in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 2001, 410, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Merscher, S.; Funke, B.; Epstein, J.A.; Heyer, J.; Puech, A.; Lu, M.M.; Xavier, R.J.; Demay, M.B.; Russell, R.G.; Factor, S.; et al. TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 2001, 104, 619–629. [Google Scholar] [CrossRef]
- Jerome, L.A.; Papaioannou, V.E. DiGeorge syndrome phenotype in mice mutant for the T-box gene, Tbx1. Nat. Genet. 2001, 27, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huynh, T.; Baldini, A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development 2006, 133, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Prall, O.W.J.; Menon, M.K.; Solloway, M.J.; Watanabe, Y.; Zaffran, S.; Bajolle, F.; Biben, C.; McBride, J.J.; Robertson, B.R.; Chaulet, H.; et al. An Nkx2–5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell 2007, 128, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fulcoli, F.G.; Tang, S.; Baldini, A. Tbx1 regulates proliferation and differentiation of multipotent heart progenitors. Circ. Res. 2009, 105, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Morishima, M.; Wylie, J.N.; Schwartz, R.J.; Bruneau, B.G.; Lindsay, E.A.; Baldini, A. Tbx1 has a dual role in the morphogenesis of the cardiac outflow tract. Development 2004, 131, 3217–3227. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.G.; Jerome-Majewska, L.A.; Papaioannou, V.E. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum. Mol. Genet. 2004, 13, 2829–2840. [Google Scholar] [CrossRef] [PubMed]
- Grifone, R.; Jarry, T.; Dandonneau, M.; Grenier, J.; Duprez, D.; Kelly, R.G. Properties of branchiomeric and somite-derived muscle development in Tbx1 mutant embryos. Dev. Dyn. 2008, 237, 3071–3078. [Google Scholar] [CrossRef] [PubMed]
- Tolkin, T.; Christiaen, L. Rewiring of an Ancestral Tbx1/10-Ebf-Mrf Network for Pharyngeal Muscle Specification in Distinct Embryonic Lineages. bioRxiv 2016. [Google Scholar] [CrossRef]
- Green, Y.S.; Vetter, M.L. EBF proteins participate in transcriptional regulation of Xenopus muscle development. Dev. Biol. 2011, 358, 240–250. [Google Scholar] [CrossRef] [PubMed]
- El-Magd, M.A.; Saleh, A.A.; Farrag, F.; Abd El-Aziz, R.M.; Ali, H.A.; Salama, M.F. Regulation of chick Ebf1–3 gene expression in the pharyngeal arches, cranial sensory ganglia and placodes. Cells Tissues Organs 2014, 199, 278–293. [Google Scholar] [CrossRef] [PubMed]
- El-Magd, M.A.; Allen, S.; McGonnell, I.; Otto, A.; Patel, K. Bmp4 regulates chick Ebf2 and Ebf3 gene expression in somite development. Dev. Growth Differ. 2013, 55, 710–722. [Google Scholar] [PubMed]
- Nevis, K.; Obregon, P.; Walsh, C.; Guner-Ataman, B.; Burns, C.G.; Burns, C.E. Tbx1 is required for second heart field proliferation in zebrafish. Dev. Dyn. 2013, 242, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Fulcoli, F.G.; Huynh, T.; Scambler, P.J.; Baldini, A. Tbx1 regulates the BMP-Smad1 pathway in a transcription independent manner. PLoS ONE 2009, 4, e6049. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Aggarwal, V.S.; Nowotschin, S.; Bondarev, A.; Lipner, S.; Morrow, B.E. Identification of downstream genetic pathways of Tbx1 in the second heart field. Dev. Biol. 2008, 316, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Schott, J.J.; Benson, D.W.; Basson, C.T.; Pease, W.; Silberbach, G.M.; Moak, J.P.; Maron, B.J.; Seidman, C.E.; Seidman, J.G. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science 1998, 281, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.W.; Silberbach, G.M.; Kavanaugh-McHugh, A.; Cottrill, C.; Zhang, Y.; Riggs, S.; Smalls, O.; Johnson, M.C.; Watson, M.S.; Seidman, J.G.; et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J. Clin. Investig. 1999, 104, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Lyons, I.; Parsons, L.M.; Hartley, L.; Li, R.; Andrews, J.E.; Robb, L.; Harvey, R.P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2–5. Genes Dev. 1995, 9, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Nomura-Kitabayashi, A.; Sultana, N.; Cai, W.; Cai, X.; Moon, A.M.; Cai, C.-L. Mesodermal Nkx2.5 is necessary and sufficient for early second heart field development. Dev. Biol. 2014, 390, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Zaffran, S.; Kuroiwa, A.; Higuchi, H.; Ogura, T.; Harvey, R.P.; Kelly, R.G.; Buckingham, M. Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2-5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proc. Natl. Acad. Sci. USA. 2012, 109, 18273–18280. [Google Scholar] [CrossRef] [PubMed]
- Nowotschin, S.; Liao, J.; Gage, P.J.; Epstein, J.A.; Campione, M.; Morrow, B.E. Tbx1 affects asymmetric cardiac morphogenesis by regulating Pitx2 in the secondary heart field. Development 2006, 133, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Azpiazu, N.; Frasch, M. Tinman and bagpipe: Two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993, 7, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Oberpriller, J.O.; Oberpriller, J.C. Response of the adult newt ventricle to injury. J. Exp. Zool. 1974, 187, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Witman, N.; Murtuza, B.; Davis, B.; Arner, A.; Morrison, J.I. Recapitulation of developmental cardiogenesis governs the morphological and functional regeneration of adult newt hearts following injury. Dev. Biol. 2011, 354, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Rumyantsev, P.P. Autoradiographic study on the synthesis of DNA, RNA, and proteins in normal cardiac muscle cells and those changed by experimental injury. Folia Histochem. Cytochem. 1966, 4, 397–424. [Google Scholar]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart regeneration in zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- González-Rosa, J.M.; Martín, V.; Peralta, M.; Torres, M.; Mercader, N. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 2011, 138, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Panáková, D.; Kikuchi, K.; Holdway, J.E.; Gemberling, M.; Burris, J.S.; Singh, S.P.; Dickson, A.L.; Lin, Y.F.; Sabeh, M.K.; et al. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 2011, 138, 3421–3430. [Google Scholar] [CrossRef] [PubMed]
- Jopling, C.; Sleep, E.; Raya, M.; Martí, M.; Raya, A.; Belmonte, J.C.I.; Izpisúa Belmonte, J.C. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 2010, 464, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Holdway, J.E.; Werdich, A.A.; Anderson, R.M.; Fang, Y.; Egnaczyk, G.F.; Evans, T.; Macrae, C.A.; Stainier, D.Y.R.; Poss, K.D. Primary contribution to zebrafish heart regeneration by gata4+ cardiomyocytes. Nature 2010, 464, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Smart, N.; Bollini, S.; Dubé, K.N.; Vieira, J.M.; Zhou, B.; Davidson, S.; Yellon, D.; Riegler, J.; Price, A.N.; Lythgoe, M.F.; et al. De novo cardiomyocytes from within the activated adult heart after injury. Nature 2011, 474, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Jovinge, S.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Senyo, S.E.; Steinhauser, M.L.; Pizzimenti, C.L.; Yang, V.K.; Cai, L.; Wang, M.; Wu, T.-D.; Guerquin-Kern, J.-L.; Lechene, C.P.; Lee, R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 2012, 493, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, W.R. Closing the wounds: One hundred and twenty five years of regenerative biology in the ascidian Ciona intestinalis. Genesis 2014, 18, 1–18. [Google Scholar]
- Auger, H.; Sasakura, Y.; Joly, J.-S.S.; Jeffery, W.R. Regeneration of oral siphon pigment organs in the ascidian Ciona intestinalis. Dev. Biol. 2010, 339, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Dahlberg, C.; Auger, H.; Dupont, S.; Sasakura, Y.; Thorndyke, M.; Joly, J.-S. Refining the Ciona intestinalis model of central nervous system regeneration. PLoS ONE 2009, 4, e4458. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Goricki, S.; Byerly, M.S.; Satoh, N.; Jeffery, W.R. Evolution of the chordate regeneration blastema: Differential gene expression and conserved role of notch signaling during siphon regeneration in the ascidian Ciona. Dev. Biol. 2015, 405, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Borikova, A.L.; Ben-Yair, R.; Guner-Ataman, B.; MacRae, C.A.; Lee, R.T.; Burns, C.G.; Burns, C.E. Notch signaling regulates cardiomyocyte proliferation during zebrafish heart regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, W.R. Regeneration, Stem Cells, and Aging in the Tunicate Ciona: Insights from the Oral Siphon. Int. Rev. Cell Mol. Biol. 2015, 319, 255–282. [Google Scholar] [PubMed]
- Soonpaa, M.H.; Rubart, M.; Field, L.J. Challenges measuring cardiomyocyte renewal. Biochim. Biophys. Acta 2013, 1833, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Cattaneo, P.; Chen, J.; Evans, S.M. Revisiting Preadolescent Cardiomyocyte Proliferation in Mice. Circ. Res. 2016, 118, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Satoh, N. The ascidian tadpole larva: Comparative molecular development and genomics. Nat. Rev. Genet. 2003, 4, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Brozovic, M.; Martin, C.; Dantec, C.; Dauga, D.; Mendez, M.; Simion, P.; Percher, M.; Laporte, B.; Scornavacca, C.; Di Gregorio, A.; et al. ANISEED 2015: A digital framework for the comparative developmental biology of ascidians. Nucleic Acids Res. 2016, 44, D808–D818. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, A.; Christiaen, L. Genetic and genomic toolbox of the Chordate Ciona intestinalis. Genetics 2012, 192, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, M.J.; Sobral, D.; Khoueiry, P.; Daian, F.; Laporte, B.; Patrushev, I.; Matsumoto, J.; Dewar, K.; Hastings, K.E.M.; Satou, Y.; et al. A pipeline for the systematic identification of non-redundant full-ORF cDNAs for polymorphic and evolutionary divergent genomes: Application to the ascidian Ciona intestinalis. Dev. Biol. 2015, 404, 149–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joly, J.-S.; Kano, S.; Matsuoka, T.; Auger, H.; Hirayama, K.; Satoh, N.; Awazu, S.; Legendre, L.; Sasakura, Y. Culture of Ciona intestinalis in closed systems. Dev. Dyn. 2007, 236, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Sasakura, Y. Germline transgenesis and insertional mutagenesis in the ascidian Ciona intestinalis. Dev. Dyn. 2007, 236, 1758–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouchard-Madrelle, C. Régéneration de l’ovaire chez une Ascidie simple Ciona intestinalis, L. (Prochordé). Bull. Soc. Zool. Fr. 1966, 91, 107–114. [Google Scholar]
- Kawamura, K.; Sunanaga, T. Hemoblasts in colonial tunicates: Are they stem cells or tissue-restricted progenitor cells. Dev. Growth Differ. 2010, 52, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Losick, V.P.; Fox, D.T.; Spradling, A.C. Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr. Biol. 2013, 23, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.; Sancho-Martinez, I.; Izpisua Belmonte, J.C. Reprogramming toward heart regeneration: Stem cells and beyond. Cell Stem Cell 2013, 12, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, X.; Capasso, J.M.; Gerdes, A.M. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J. Mol. Cell. Cardiol. 1996, 28, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Soonpaa, M.H.; Kim, K.K.; Pajak, L.; Franklin, M.; Field, L.J. Cardiomyocyte DNA synthesis and binucleation during murine development. Am. J. Physiol. 1996, 271, H2183–H2189. [Google Scholar] [PubMed]
- Walsh, S.; Pontén, A.; Fleischmann, B.K.; Jovinge, S. Cardiomyocyte cell cycle control and growth estimation in vivo—An analysis based on cardiomyocyte nuclei. Cardiovasc. Res. 2010, 86, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.-Y.; Silberstein, L.E.; dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, P.; Perriard, E.; Perriard, J.-C.; Ehler, E. Sequential myofibrillar breakdown accompanies mitotic division of mammalian cardiomyocytes. J. Cell Sci. 2004, 117, 3295–3306. [Google Scholar] [CrossRef] [PubMed]
- Sasakura, Y.; Shoguchi, E.; Takatori, N.; Wada, S.; Meinertzhagen, I.A.; Satou, Y.; Satoh, N. A genomewide survey of developmentally relevant genes in Ciona intestinalis. X. Genes for cell junctions and extracellular matrix. Dev. Genes Evol. 2003, 213, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Ieda, M.; Tsuchihashi, T.; Ivey, K.N.; Ross, R.S.; Hong, T.-T.; Shaw, R.M.; Srivastava, D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev. Cell 2009, 16, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Han, P.; Yang, H.; Ouyang, K.; Lee, D.; Lin, Y.-F.; Ocorr, K.; Kang, G.; Chen, J.; Stainier, D.Y.R.; et al. In vivo cardiac reprogramming contributes to zebrafish heart regeneration. Nature 2013, 498, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Foglia, M.J.; Poss, K.D. Building and re-building the heart by cardiomyocyte proliferation. Development 2016, 143, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Yoshida, K.; Hozumi, A.; Sasakura, Y. CRISPR/Cas9-mediated gene knockout in the ascidian Ciona intestinalis. Dev. Growth Differ. 2014, 56, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, A.; Gandhi, S.; Salek, F.; Christiaen, L. Tissue-specific genome editing in Ciona embryos by CRISPR/Cas9. Development 2014, 141, 4115–4120. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Christiaen, L.; Stolfi, A. Rational Design and Whole-Genome Predictions of Single Guide RNAs for Efficient CRISPR/Cas9-Mediated Genome Editing in Ciona; Cold Spring Harbor Labs Journals: New York, NY, USA, 2016. [Google Scholar]
- Sasakura, Y.; Oogai, Y.; Matsuoka, T.; Satoh, N.; Awazu, S. Transposon mediated transgenesis in a marine invertebrate chordate: Ciona intestinalis. Genome Biol. 2007, 8 (Suppl. S1), S3. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evans Anderson, H.; Christiaen, L. Ciona as a Simple Chordate Model for Heart Development and Regeneration. J. Cardiovasc. Dev. Dis. 2016, 3, 25. https://doi.org/10.3390/jcdd3030025
Evans Anderson H, Christiaen L. Ciona as a Simple Chordate Model for Heart Development and Regeneration. Journal of Cardiovascular Development and Disease. 2016; 3(3):25. https://doi.org/10.3390/jcdd3030025
Chicago/Turabian StyleEvans Anderson, Heather, and Lionel Christiaen. 2016. "Ciona as a Simple Chordate Model for Heart Development and Regeneration" Journal of Cardiovascular Development and Disease 3, no. 3: 25. https://doi.org/10.3390/jcdd3030025
APA StyleEvans Anderson, H., & Christiaen, L. (2016). Ciona as a Simple Chordate Model for Heart Development and Regeneration. Journal of Cardiovascular Development and Disease, 3(3), 25. https://doi.org/10.3390/jcdd3030025