Prognostic Role of the Endothelial Activation and Stress Index (EASIX) in Functional Outcomes and Mortality After Acute Ischemic Stroke: A Retrospective Pilot Cohort Study
Abstract
1. Introduction
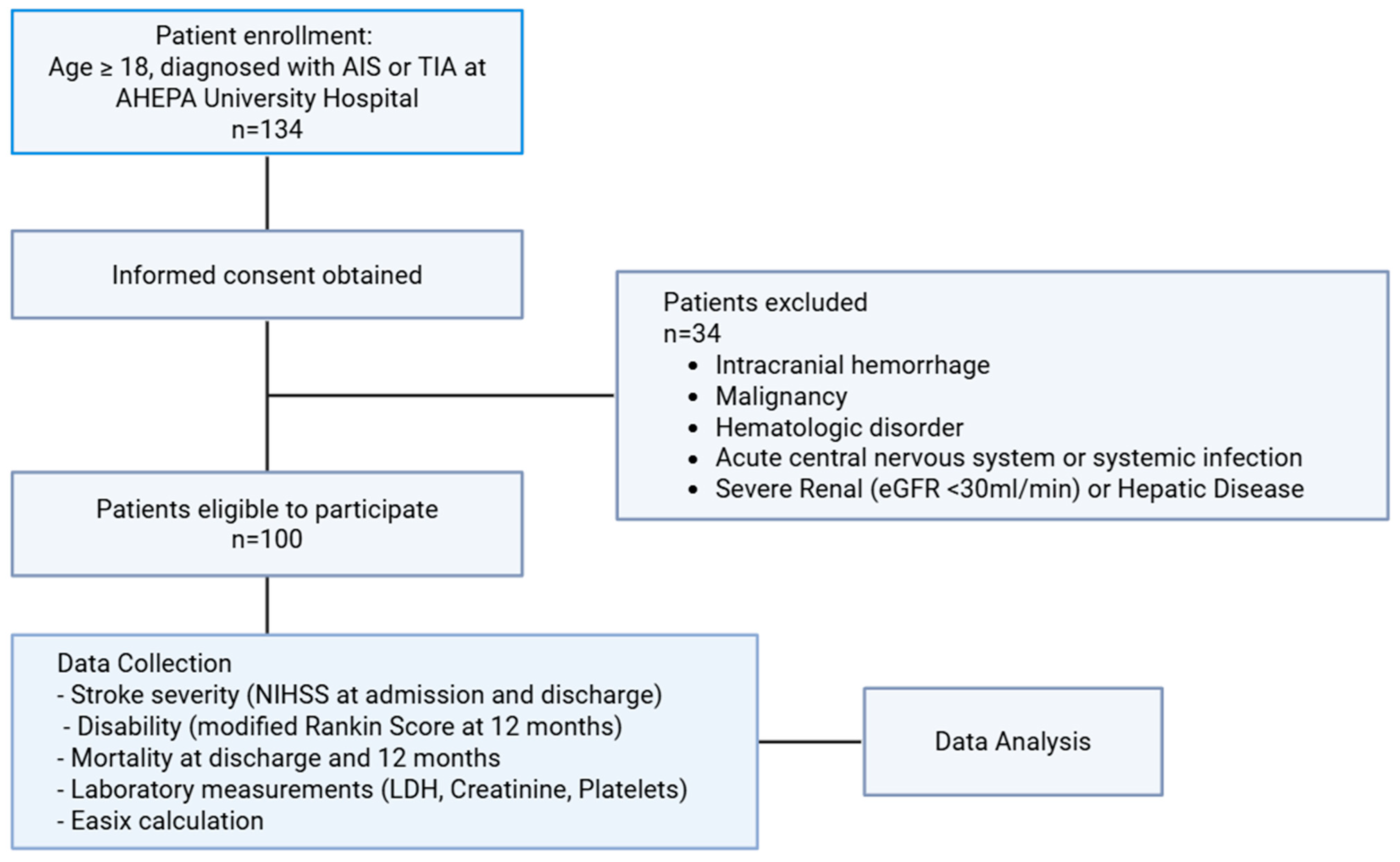
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Correlation of EASIX with Baseline Characteristics
3.3. Correlation of EASIX with Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.; Pandian, J.D.; Lindsay, P.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2024, 20, 132–144. [Google Scholar] [CrossRef]
- Krishnamurthi, R.V.; Barker-Collo, S.; Parag, V.; Parmar, P.; Witt, E.; Jones, A.; Mahon, S.; Anderson, C.S.; Barber, P.A.; Feigin, V.L. Stroke Incidence by Major Pathological Type and Ischemic Subtypes in the Auckland Regional Community Stroke Studies. Stroke 2018, 49, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Kleeberg, A.; Luft, T.; Golkowski, D.; Purrucker, J.C. Endothelial dysfunction in acute ischemic stroke: A review. J. Neurol. 2025, 272, 143. [Google Scholar] [CrossRef]
- Xu, S.-W.; Ilyas, I.; Weng, J.-P. Endothelial dysfunction in COVID-19: An overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol. Sin. 2022, 44, 695–709. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial Function in Cardiovascular Medicine: A Consensus Paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2020, 117, 29–42. [Google Scholar] [CrossRef]
- Yilmaz, G.; Granger, D.N. Leukocyte Recruitment and Ischemic Brain Injury. NeuroMol. Med. 2010, 12, 193–204. [Google Scholar] [CrossRef]
- Eidson, L.N.; Gao, Q.; Qu, H.; Kikuchi, D.S.; Campos, A.C.P.; Faidley, E.A.; Sun, Y.-Y.; Kuan, C.-Y.; Pagano, R.L.; Lassègue, B.; et al. Poldip2 controls leukocyte infiltration into the ischemic brain by regulating focal adhesion kinase-mediated VCAM-1 induction. Sci. Rep. 2021, 11, 5533. [Google Scholar] [CrossRef]
- Page, A.V.; Liles, W.C. Biomarkers of Endothelial Activation/Dysfunction in Infectious Diseases. Virulence 2013, 4, 507–516. [Google Scholar] [CrossRef]
- Pastorino, R.; Loreti, C.; Giovannini, S.; Ricciardi, W.; Padua, L.; Boccia, S. Challenges of Prevention for a Sustainable Personalized Medicine. J. Pers. Med. 2021, 11, 311. [Google Scholar] [CrossRef]
- Jin, H.; Bi, R.; Hu, J.; Xu, D.; Su, Y.; Huang, M.; Peng, Q.; Li, Z.; Chen, S.; Hu, B. Elevated Serum Lactate Dehydrogenase Predicts Unfavorable Outcomes After rt-PA Thrombolysis in Ischemic Stroke Patients. Front. Neurol. 2022, 13, 816216. [Google Scholar] [CrossRef]
- Zhong, Y.; Sun, H.; Jing, W.; Liao, L.; Huang, J.; Ma, J.; Chen, W. Association between serum creatinine and 30 days all-cause mortality in critically ill patients with non-traumatic subarachnoid hemorrhage: Analysis of the MIMIC-IV database. Front. Neurol. 2024, 15, 1359749. [Google Scholar] [CrossRef]
- Wang, L.; Liu, R.; Zhou, P.; Hu, H.; Deng, Z. Nonlinear relationship between platelet count and 30-day in-hospital mortality in intensive care unit stroke patients: A multicenter retrospective cohort study. Front. Neurol. 2024, 15, 1374159. [Google Scholar] [CrossRef]
- Luft, T.; Benner, A.; Jodele, S.; Dandoy, C.E.; Storb, R.; Gooley, T.; Sandmaier, B.M.; Becker, N.; Radujkovic, A.; Dreger, P.; et al. EASIX in patients with acute graft-versus-host disease: A retrospective cohort analysis. Lancet Haematol. 2017, 4, e414–e423. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Sakellari, I.; Chatzikonstantinou, T.; Mallouri, D.; Batsis, I.; Vardi, A.; Bousiou, Z.; Koravou, E.-E.; Masmanidou, M.; Touloumenidou, T.; et al. Endothelial and Complement Activation as Predictors of Survival in Adult Allogeneic Hematopoietic Cell Transplantation. HemaSphere 2020, 5, e487. [Google Scholar] [CrossRef]
- Park, S.; Go, S.I.; Lee, G.W. The Endothelial Activation and Stress Index (EASIX) score is an independent prognostic factor in patients with diffuse large B-cell lymphoma. BMC Cancer 2022, 22, 816. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Tzannou, I.; Batsis, I.; Tsonis, I.; Liga, M.; Gkirkas, K.; Ximeri, M.; Dolgyras, P.; Bampali, V.; Evangelidis, P.; et al. EASIX and m-EASIX predict severe cytokine release syndrome and overall survival after CAR T-cell therapy. Blood Vessels Thromb. Hemost. 2024, 1, 100025. [Google Scholar] [CrossRef]
- Finke, D.; Hund, H.; Frey, N.; Luft, T.; Lehmann, L.H. EASIX (endothelial activation and stress index) predicts mortality in patients with coronary artery disease. Clin. Res. Cardiol. 2024, 114, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Ma, X.; Zhu, F.; Zhu, C.; Ying, Z. Association between endothelial activation and stress index and 30-day mortality risk in acute myocardial infarction patients: A study based on the medical information mart for intensive care-IV database. BMC Cardiovasc. Disord. 2024, 24, 699. [Google Scholar] [CrossRef]
- Huang, Y.; Li, Z.; Wang, J.; Wang, D.; Yin, X. Endothelial activation and stress index is a reliable predictor for the prevalence and mortality outcomes of stroke. Sci. Rep. 2025, 15, 23285. [Google Scholar] [CrossRef]
- Brott, T.; Adams, H.P.; Olinger, C.P.; Marler, J.R.; Barsan, W.G.; Biller, J.; Spilker, J.; Holleran, R.; Eberle, R.; Hertzberg, V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke 1989, 20, 864–870. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Davis, P.H.; Leira, E.C.; Chang, K.-C.; Bendixen, B.H.; Clarke, W.R.; Woolson, R.F.; Hansen, M.S. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology 1999, 53, 126. [Google Scholar] [CrossRef]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Andrés, V.; Castiglioni, S.; Giudici, A.; Lau, E.S.; Nemcsik, J.; Seta, F.; Zaninotto, P.; Catalano, M.; Hamburg, N.M. Aging and Vascular Disease: A Multidisciplinary Overview. J. Clin. Med. 2023, 12, 5512. [Google Scholar] [CrossRef]
- Li, L.; Scott, C.A.; Rothwell, P.M. Association of Younger vs Older Ages with Changes in Incidence of Stroke and Other Vascular Events, 2002–2018. JAMA 2022, 328, 563–574. [Google Scholar] [CrossRef] [PubMed]
- The European Registers of Stroke (EROS) Investigators. Incidence of Stroke in Europe at the Beginning of the 21st Century. Stroke 2009, 40, 1557–1563. [CrossRef]
- Béjot, Y.; Duloquin, G.; Graber, M.; Garnier, L.; Mohr, S.; Giroud, M. Current Characteristics and Early Functional Outcome of Older Stroke Patients: A Population-Based Study (Dijon Stroke Registry). Age Ageing 2020, 50, 898–905. [Google Scholar] [CrossRef]
- Li, N.; Yin, J.; Zeng, Y.; Li, S.; Huang, C.; Chen, G.; Luo, H.; Jiang, Y. Association between endothelial activation and stress index and all-cause mortality risk in sepsis patients: A retrospective cohort analysis utilizing the MIMIC-IV database. BMC Infect. Dis. 2025, 25, 966. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Wang, K. Independent prognostic importance of endothelial activation and stress index (EASIX) in critically ill patients with heart failure: Modulating role of inflammation. Front. Med. 2025, 12, 1560947. [Google Scholar] [CrossRef]
- Ridker, P.M.; Cushman, M.; Stampfer, M.J.; Tracy, R.P. Inflammation, Aspirin, and the Risk of Cardiovascular Disease in Apparently Healthy Men. N. Engl. J. Med. 1997, 336, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Anrather, J.; Iadecola, C. Inflammation and Stroke: An Overview. Neurotherapeutics 2016, 13, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Lambertsen, K.L.; Finsen, B.; Clausen, B.H. Post-stroke inflammation—Target or tool for therapy? Acta Neuropathol. 2019, 137, 693–714. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wardlaw, J.M. Update on cerebral small vessel disease: A dynamic whole-brain disease. Stroke Vasc. Neurol. 2016, 1, 83–92. [Google Scholar] [CrossRef]
- Caplan, L.R. Lacunar Infarction and Small Vessel Disease: Pathology and Pathophysiology. J. Stroke 2015, 17, 2–6. [Google Scholar] [CrossRef]
- Makin, S.D.J.; Cook, F.A.B.; Dennis, M.S.; Wardlaw, J.M. Cerebral Small Vessel Disease and Renal Function: Systematic Review and Meta-Analysis. Cerebrovasc. Dis. 2014, 39, 39–52. [Google Scholar] [CrossRef]
- Knottnerus, I.L.H.; Ten Cate, H.; Lodder, J.; Kessels, F.; van Oostenbrugge, R.J. Endothelial Dysfunction in Lacunar Stroke: A Systematic Review. Cerebrovasc. Dis. 2009, 27, 519–526. [Google Scholar] [CrossRef]


| Variables | Acute Ischemic Stroke | Transient Ischemic Attack |
|---|---|---|
| n = 71 | n = 29 | |
| Age, years | 82 (77–88) | 82 (75–87) |
| Female sex | 43 (60.6) | 13 (44.8) |
| Arterial Hypertension | 64 (90.1) | 24 (82.8) |
| Diabetes Mellitus | 28 (39.4) | 7 (24.1) |
| Dyslipidemia | 50 (70.4) | 19 (65.5) |
| Atrial Fibrillation | 26 (36.6) | 8 (27.6) |
| Coronary Artery Disease | 19 (26.8) | 6 (20.7) |
| Peripheral artery disease | 17 (23.9) | 2 (6.9) |
| Previous Stroke | 22 (31.1) | 15 (51.7) |
| Current Smoking | 14 (19.7) | 6 (20.7) |
| LDH, U/L | 239 (195–294) | 233 (209–277) |
| Creatinine, mg/dL | 0.99 (0.83–1.27) | 0.89 (0.82–1.07) |
| Platelets, K/mL | 212 (175–278) | 203 (182–234) |
| EASIX | 1.06 (0.77–1.6) | 1.06 (0.93–1.39) |
| LN EASIX | 0.06 (−0.26–0.47) | 0.06 (−0.07–0.33) |
| WBC, K/mL | 8.51 (6.61–10.43) | 7.78 (6.69–9.42) |
| CRP, mg/dL | 0.58 (0.19–2.05) | 0.45 (0.11–1.82) |
| NIHSS at admission | 8 (3–15) | 2 (1–3) |
| mRS before admission | 2 (1–3) | 2 (1–3) |
| mRS at admission | 5 (4–5) | 3 (1–4) |
| Variables | B | p Value |
|---|---|---|
| Age | 0.001 | 0.76 |
| Sex (f) | −0.115 | 0.03 |
| Arterial Hypertension | 0.025 | 0.76 |
| Diabetes Mellitus | −0.042 | 0.46 |
| Dyslipidemia | 0.062 | 0.28 |
| Atrial Fibrillation | 0.001 | 0.99 |
| Peripheral Arterial Disease | −0.030 | 0.66 |
| Coronary Artery Disease | 0.095 | 0.12 |
| Stroke History | 0.046 | 0.41 |
| Current Smoking | 0.033 | 0.62 |
| CRP | 0.023 | 0.01 |
| WBC | 0.022 | <0.001 |
| LDL-cholesterol | 0.001 | 0.85 |
| Outcomes | AIS (n = 71) | TIA (n = 29) | ||
|---|---|---|---|---|
| B | p Value | B | p Value | |
| Days of Hospital Stay | 0.001 | 0.94 | 0.003 | 0.89 |
| NIHSS at Admission | −0.054 | 0.9 | −0.256 | 0.73 |
| NIHSS at Discharge | 0.594 | 0.7 | −0.661 | 0.32 |
| mRS at Admission | 0.001 | 0.99 | 0.314 | 0.48 |
| mRS at Discharge | −0.026 | 0.93 | 0.531 | 0.26 |
| In-Hospital Death | 0.08 | 0.35 | 0.054 | 0.48 |
| Death at 12 Months | 0.061 | 0.54 | 0.116 | 0.3 |
| Outcomes | Non-SVD (n = 61) | SVD (n = 10) | ||
|---|---|---|---|---|
| B | p Value | B | p Value | |
| Days of Hospital stay | 0.2 | 0.89 | 0.741 | 0.9 |
| NIHSS at Admission | −0.907 | 0.57 | 1.078 | 0.54 |
| NIHSS at Discharge | −0.309 | 0.85 | 2.287 | 0.3 |
| mRS at Admission | −0.066 | 0.75 | 0.175 | 0.78 |
| mRS at 12 Month | −0.287 | 0.433 | 2.383 | 0.02 |
| In-Hospital Death | 0.055 | 0.55 | 0.261 | 0.33 |
| Death at 12-month | −0.020 | 0.85 | 0.653 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Makris, M.; Ztriva, E.; Gavriilaki, E.; Patriarcheas, V.; Gougoula, V.; Giannakakis, M.; Tselepis, A.; Ntaios, G.; Savopoulos, C.; Kaiafa, G. Prognostic Role of the Endothelial Activation and Stress Index (EASIX) in Functional Outcomes and Mortality After Acute Ischemic Stroke: A Retrospective Pilot Cohort Study. J. Cardiovasc. Dev. Dis. 2026, 13, 66. https://doi.org/10.3390/jcdd13020066
Makris M, Ztriva E, Gavriilaki E, Patriarcheas V, Gougoula V, Giannakakis M, Tselepis A, Ntaios G, Savopoulos C, Kaiafa G. Prognostic Role of the Endothelial Activation and Stress Index (EASIX) in Functional Outcomes and Mortality After Acute Ischemic Stroke: A Retrospective Pilot Cohort Study. Journal of Cardiovascular Development and Disease. 2026; 13(2):66. https://doi.org/10.3390/jcdd13020066
Chicago/Turabian StyleMakris, Michail, Eleftheria Ztriva, Eleni Gavriilaki, Vasileios Patriarcheas, Vasiliki Gougoula, Michail Giannakakis, Alexandros Tselepis, Georgios Ntaios, Christos Savopoulos, and Georgia Kaiafa. 2026. "Prognostic Role of the Endothelial Activation and Stress Index (EASIX) in Functional Outcomes and Mortality After Acute Ischemic Stroke: A Retrospective Pilot Cohort Study" Journal of Cardiovascular Development and Disease 13, no. 2: 66. https://doi.org/10.3390/jcdd13020066
APA StyleMakris, M., Ztriva, E., Gavriilaki, E., Patriarcheas, V., Gougoula, V., Giannakakis, M., Tselepis, A., Ntaios, G., Savopoulos, C., & Kaiafa, G. (2026). Prognostic Role of the Endothelial Activation and Stress Index (EASIX) in Functional Outcomes and Mortality After Acute Ischemic Stroke: A Retrospective Pilot Cohort Study. Journal of Cardiovascular Development and Disease, 13(2), 66. https://doi.org/10.3390/jcdd13020066












