ADAMTS5 Orchestrates Cell Lineage Specific Patterning and Extracellular Matrix Organization During Semilunar Valve Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Gene-Targeted Mice
2.2. Histology, Immunohistochemistry and In Situ Hybridization
2.3. Three- Dimensional Reconstructions
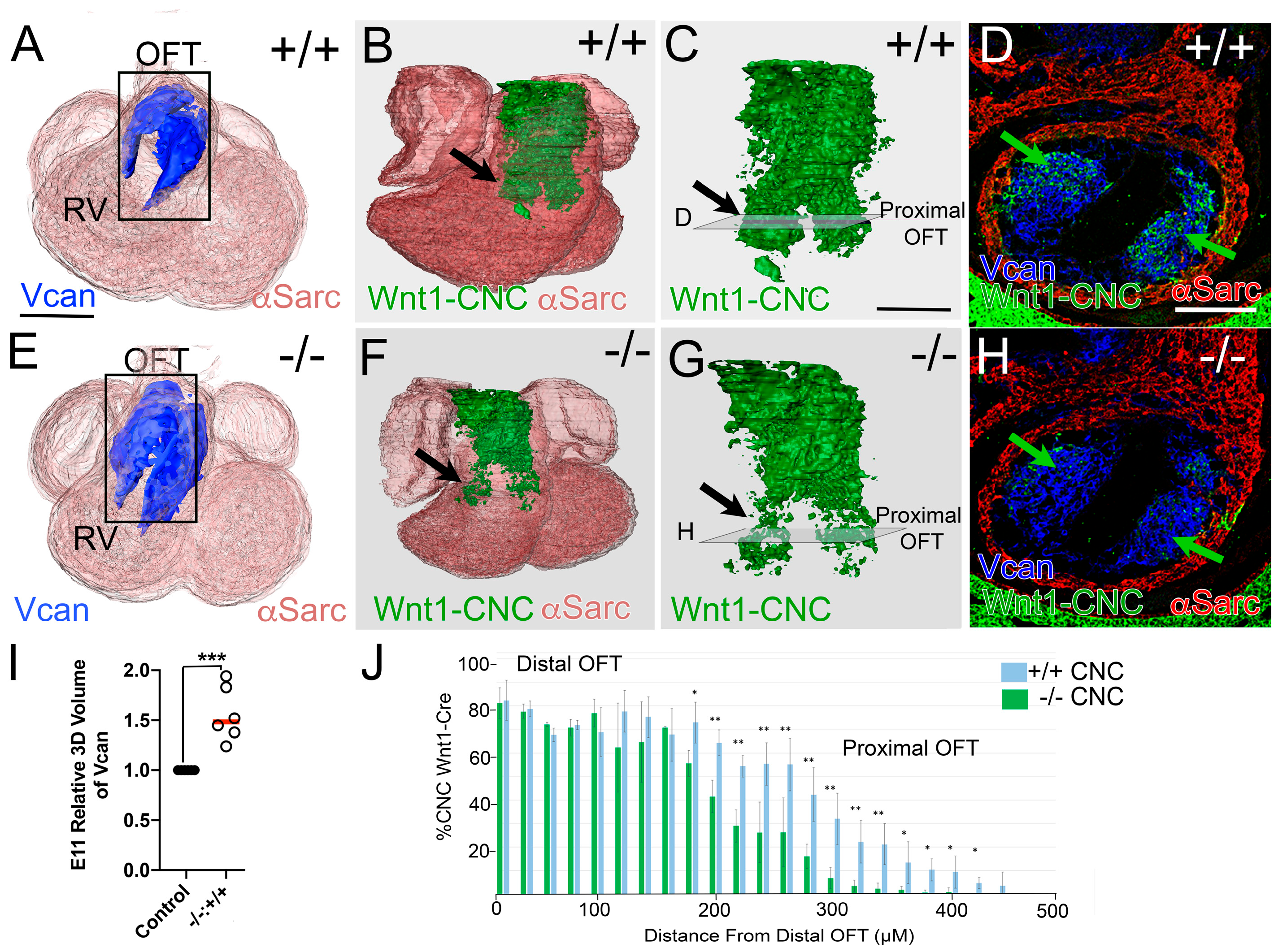
2.4. OFT CNC Lineage Contribution
2.5. Statistics
3. Results
3.1. CNC Were Reduced in the Proximal OFT of Adamts5−/− Hearts with Excess VCAN
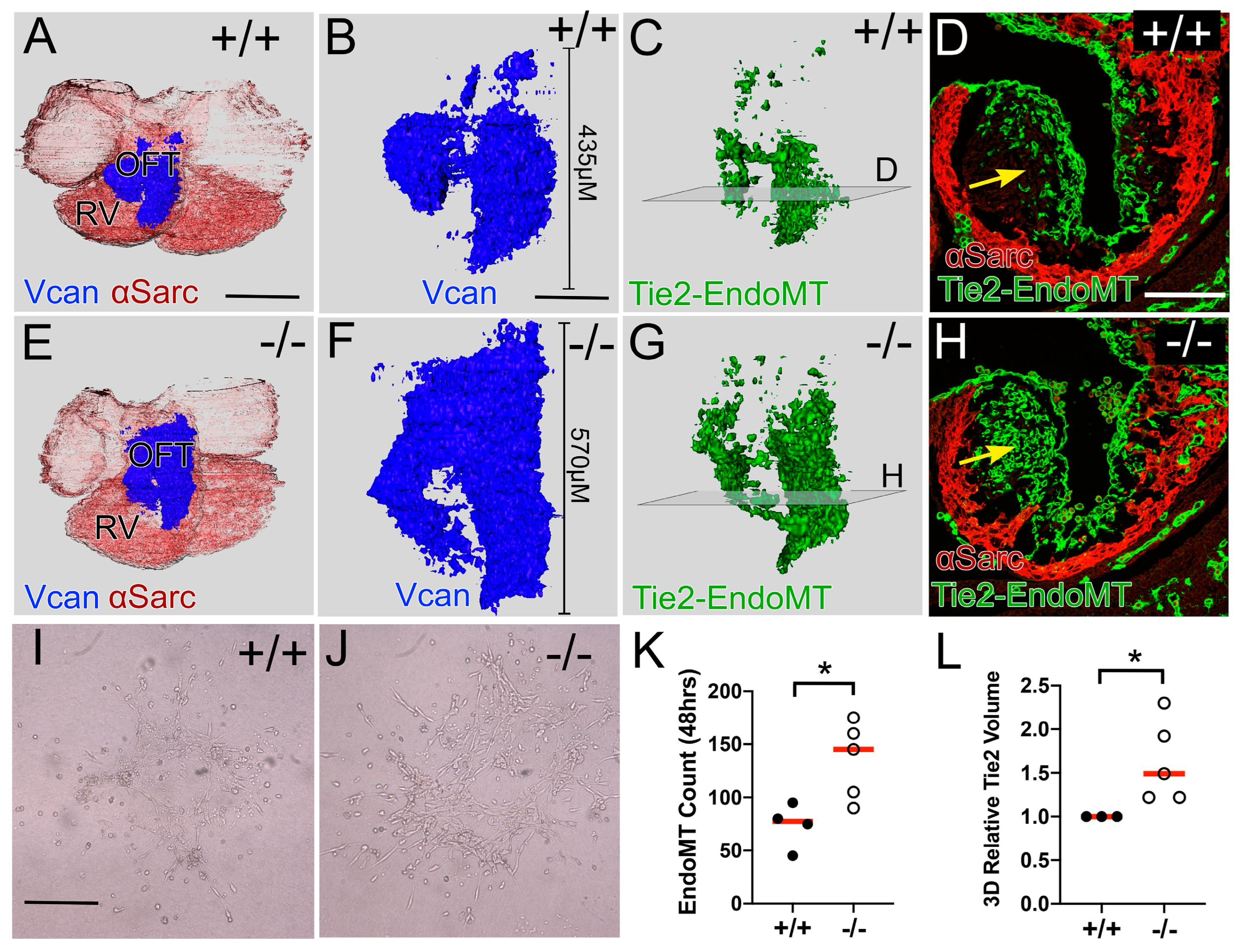
3.2. Reduction in CNC in the Adamts5−/− Proximal OFT Correlated with an Increase in the EndoMT Lineage
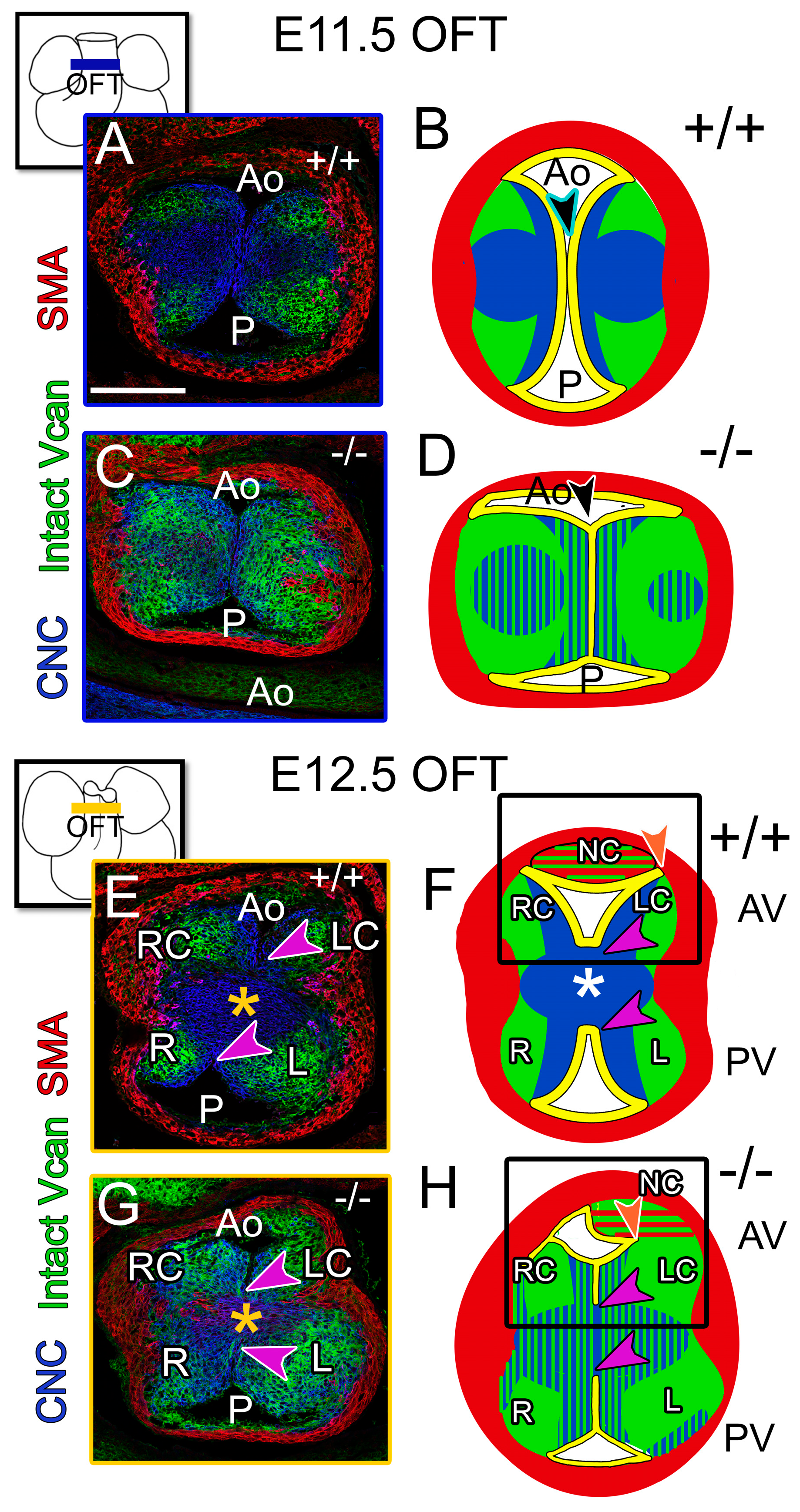
3.3. The Prevalvular Complex of the Developing OFT Was Altered by Loss of VCAN Cleavage by E11.5
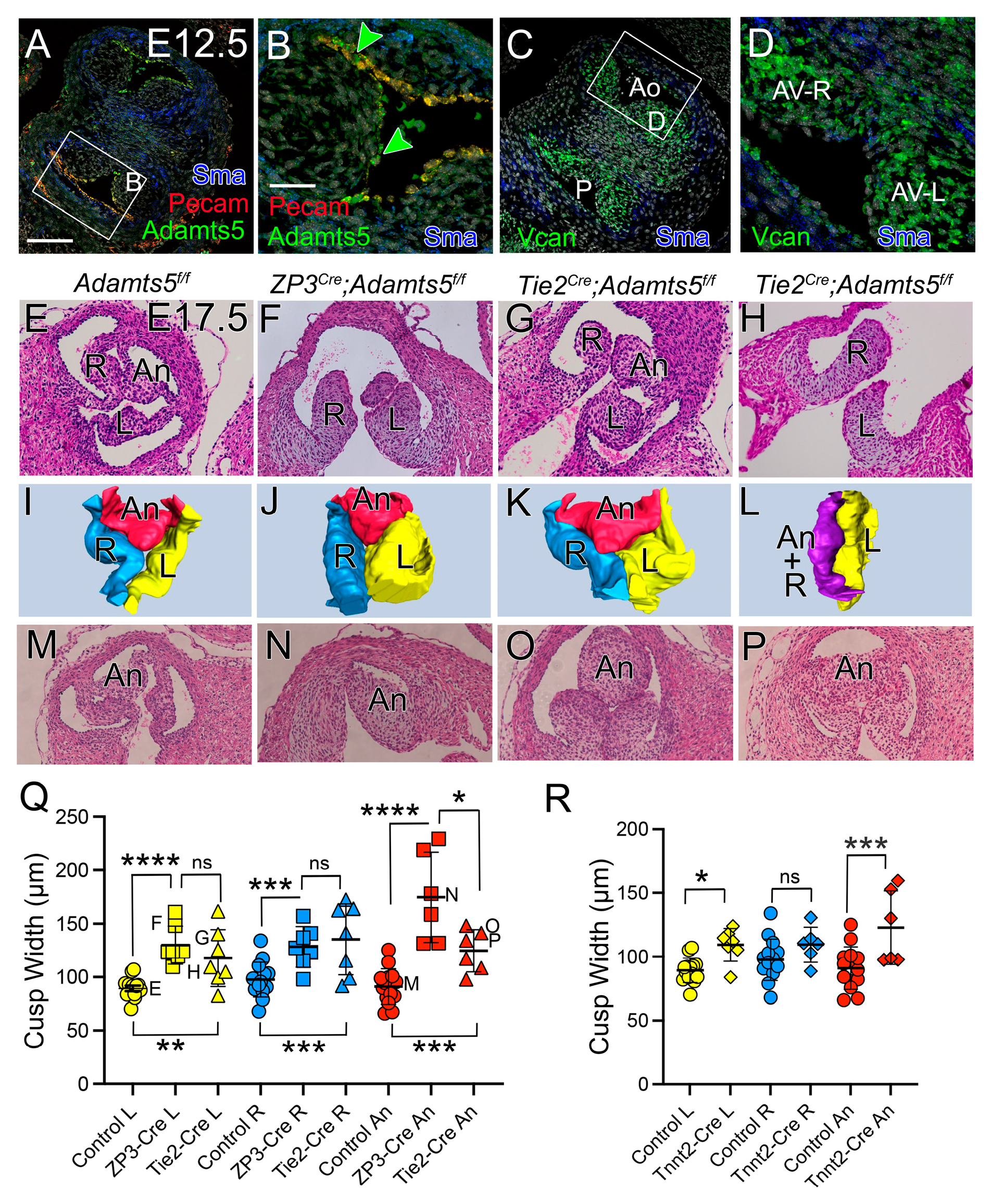
3.4. At E12.5, the CNC Patterning and Myocardial Lineage Contribution Were Disrupted in the Developing Valve Cusps in ADAMTS5-Deficient Distal OFTs
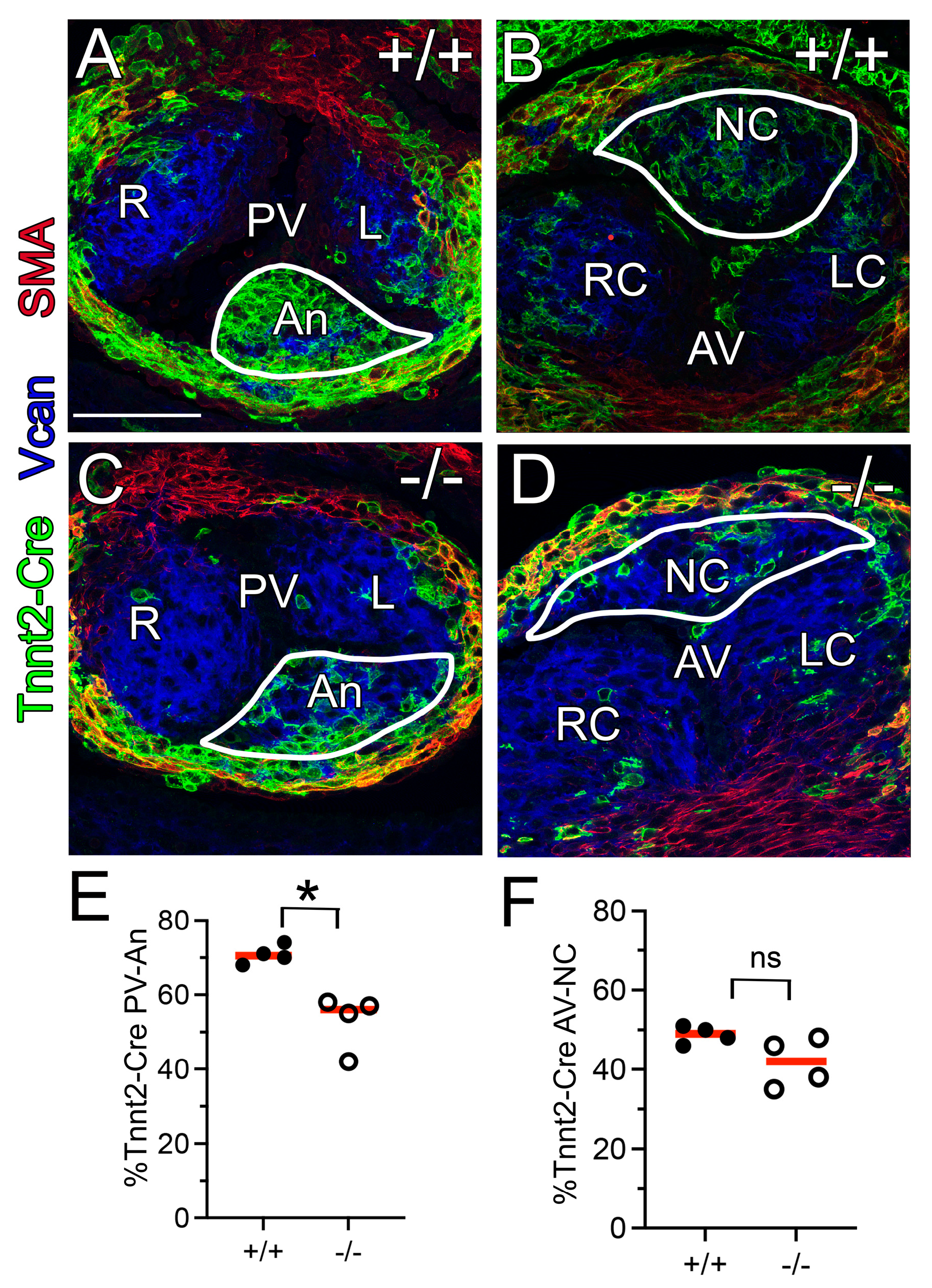
3.5. The Myocardial (Tnnt2-Cre) Population Was Reduced in the Adamts5−/− PV
3.6. Adamts5 mRNA, Expressed by the Endothelium and Myocardium, Were Required for Normal Semilunar Valve Formation
3.6.1. ADAMTS5 Was Required by the EndoMT Lineage for Normal SLV Development
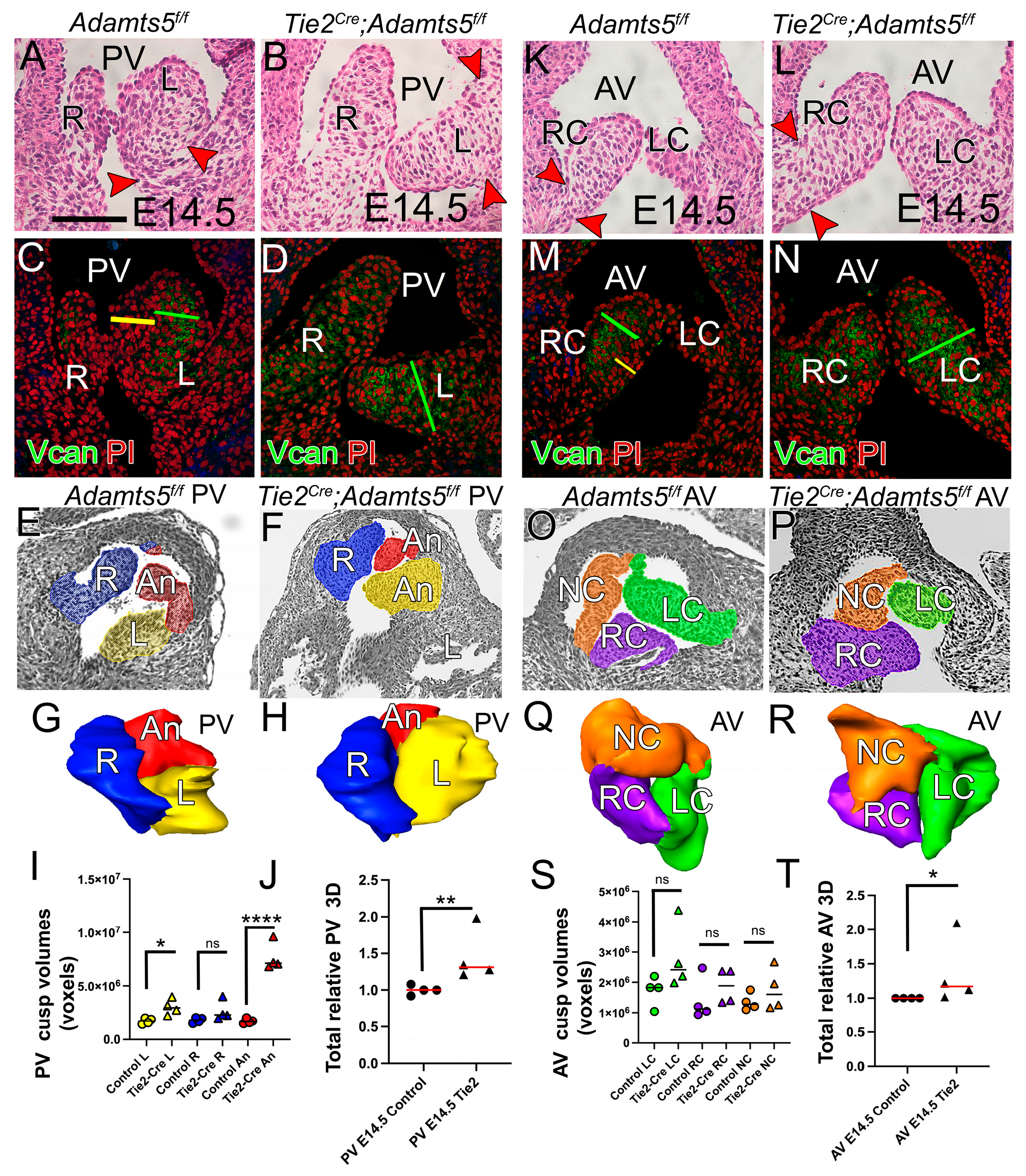
3.6.2. ADAMTS5 Was Required in the Myocardial Lineage for Normal SLV Formation
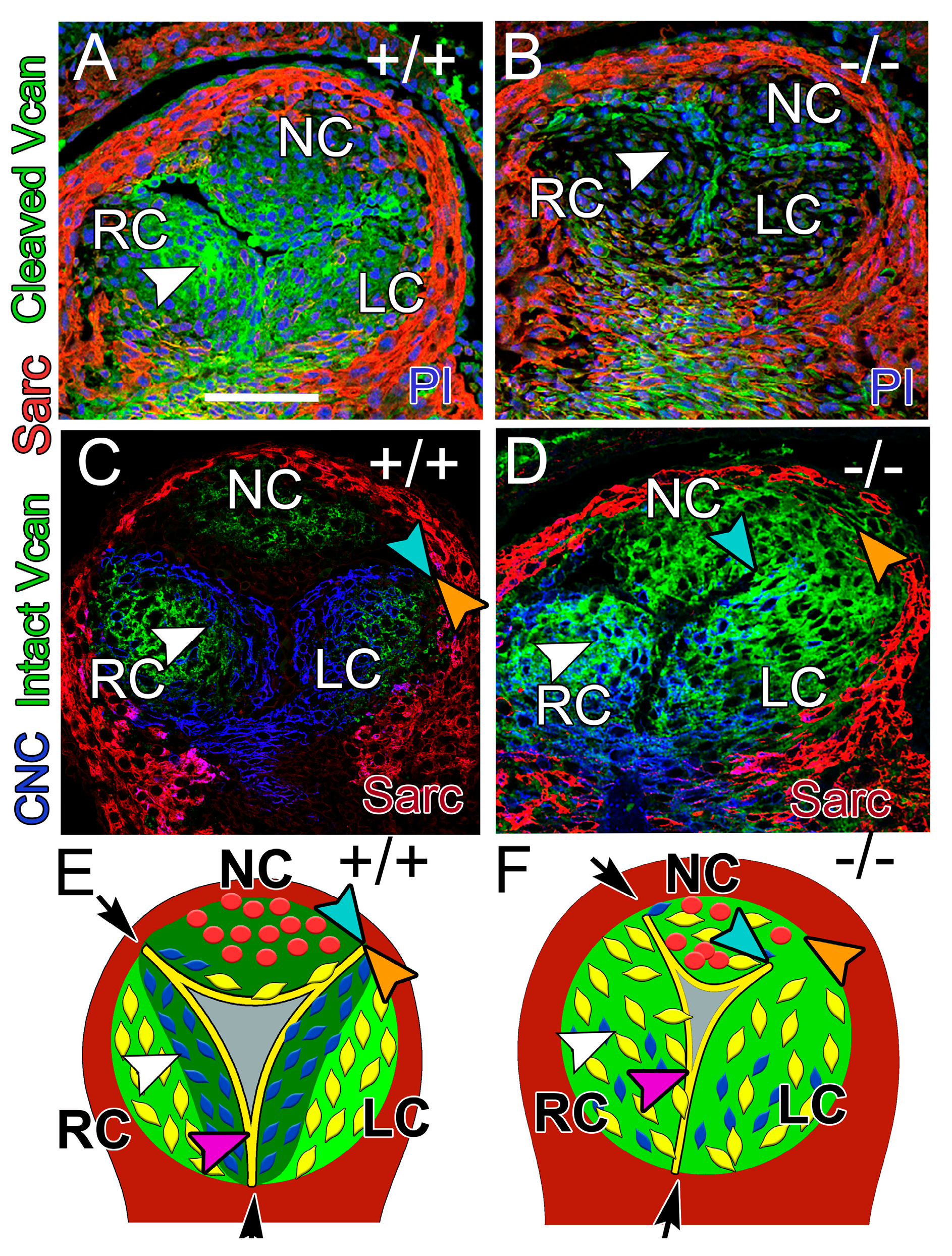
3.6.3. ADAMTS5 Expression in the EndoMT Lineage Was Required to Form the Narrow Hinge Regions of the SLV Cusps
4. Discussion
4.1. OFT Lineages Exhibit Changes in Patterning Due to Loss of ADAMTS5
4.2. Initial Stratification of Cardiac Valve ECM Involves CNC Patterning and Vcan Cleavage
4.3. Proteolytic Cleavage of Fibronectin May Contribute to the Remodeling ECM in the Cardiac OFT
4.4. Reciprocal Interactions of ECM Cleavage Events and Mechanical Force May Be Required for Early ECM Organization
4.5. Limitations of the Study
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAMTS5 | A disintegrin and metalloproteinase with thrombospondin motifs 5 |
| AV | aortic valve |
| ECM | extracellular matrix |
| VCAN | versican |
| SLV | semilunar valves (aortic and pulmonary valves) |
| CNC | cardiac neural crest |
| EndoMT | endothelial to mesenchymal transformation |
| BAV | bicuspid aortic valve |
| FN | fibronectin |
| OFT | outflow tract |
| VIC | valvular interstitial cells |
| SHF | secondary heart field |
| H&E | hematoxylin and Eosin |
| EGFP | enhanced green fluorescent protein |
References
- Vavilis, G.; Bäck, M.; Bárány, P.; Szummer, K. Epidemiology of Aortic Stenosis/Aortic Valve Replacement (from the Nationwide Swedish Renal Registry). Am. J. Cardiol. 2022, 163, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Cikach, F.S.; Koch, C.D.; Mead, T.J.; Galatioto, J.; Willard, B.B.; Emerton, K.B.; Eagleton, M.J.; Blackstone, E.H.; Ramirez, F.; Roselli, E.E.; et al. Massive aggrecan and versican accumulation in thoracic aortic aneurysm and dissection. J. Clin. Investig. 2018, 3. [Google Scholar] [CrossRef]
- Gupta, V.; Barzilla, J.E.; Mendez, J.S.; Stephens, E.H.; Lee, E.L.; Collard, C.D.; Laucirica, R.; Weigel, P.H.; Grande-Allen, K.J. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc. Pathol. 2009, 18, 191–197. [Google Scholar] [CrossRef]
- Rajamannan, N.M.; Evans, F.J.; Aikawa, E.; Grande-Allen, K.J.; Demer, L.L.; Heistad, D.D.; Simmons, C.A.; Masters, K.S.; Mathieu, P.; D O’Brien, K.; et al. Calcific aortic valve disease: Not simply a degenerative process A review and agenda for research from the National Heart and Lung and Blood Institute Aortic Stenosis Working Group. Circulation 2011, 124, 1783–1791. [Google Scholar] [CrossRef]
- Stephens, E.H.; Saltarrelli, J.G.; Baggett, L.S.; Nandi, I.; Kuo, J.J.; Davis, A.R.; Olmsted-Davis, E.A.; Reardon, M.J.; Morrisett, J.D.; Grande-Allen, K.J. Differential proteoglycan and hyaluronan distribution in calcified aortic valves. Cardiovasc. Pathol. 2011, 20, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Stephens, E.H.; Shangkuan, J.; Kuo, J.J.; Carroll, J.L.; Kearney, D.L.; Carberry, K.E.; Fraser, C.D.; Grande-Allen, K.J. Extracellular matrix remodeling and cell phenotypic changes in dysplastic and hemodynamically altered semilunar human cardiac valves. Cardiovasc. Pathol. 2011, 20, e157–e167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dupuis, L.E.; Osinska, H.; Weinstein, M.B.; Hinton, R.B.; Kern, C.B. Insufficient versican cleavage and Smad2 phosphorylation results in bicuspid aortic and pulmonary valves. J. Mol. Cell. Cardiol. 2013, 60, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.E.; Nelson, E.L.; Hozik, B.; Porto, S.C.; Rogers-DeCotes, A.; Fosang, A.; Kern, C.B. Adamts5−/− Mice Exhibit Altered Aggrecan Proteolytic Profiles That Correlate With Ascending Aortic Anomalies. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2067–2081. [Google Scholar] [CrossRef]
- Abu-Issa, R.; Smyth, G.; Smoak, I.; Yamamura, K.-I.; Meyers, E.N. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 2002, 129, 4613–4625. [Google Scholar] [CrossRef]
- Abu-Issa, R.; Waldo, K.; Kirby, M.L. Heart fields: One, two or more? Dev. Biol. 2004, 272, 281–285. [Google Scholar] [CrossRef]
- Evans, S.M.; Yelon, D.; Conlon, F.L.; Kirby, M.L. Myocardial Lineage Development. Circ. Res. 2010, 107, 1428–1444. [Google Scholar] [CrossRef]
- Okamoto, N.; Akimoto, N.; Hidaka, N.; Shoji, S.; Sumida, H. Formal genesis of the outflow tracts of the heart revisited: Previous works in the light of recent observations. Congenit. Anom. 2010, 50, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Mjaatvedt, C.; Nakaoka, T.; Moreno-Rodriguez, R.; Norris, R.; Kern, M.; Eisenberg, C.; Turner, D.; Markwald, R. The Outflow Tract of the Heart Is Recruited from a Novel Heart-Forming Field. Dev. Biol. 2001, 238, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Mjaatvedt, C.; Yamamura, H.; Capehart, A.; Turner, D.; Markwald, R. TheCspg2Gene, Disrupted in thehdfMutant, Is Required for Right Cardiac Chamber and Endocardial Cushion Formation. Dev. Biol. 1998, 202, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Kirby, M.L. Cellular and molecular contributions of the cardiac neural crest to cardiovascular development. Trends Cardiovasc. Med. 1993, 3, 18–23. [Google Scholar] [CrossRef]
- Jiang, X.; Rowitch, D.H.; Soriano, P.; McMahon, A.P.; Sucov, H.M. Fate of the mammalian cardiac neural crest. Development 2000, 127, 1607–1616. [Google Scholar] [CrossRef]
- Phillips, H.M.; Mahendran, P.; Singh, E.; Anderson, R.H.; Chaudhry, B.; Henderson, D.J. Neural crest cells are required for correct positioning of the developing outflow cushions and pattern the arterial valve leaflets. Cardiovasc. Res. 2013, 99, 452–460. [Google Scholar] [CrossRef]
- Restivo, A.; Piacentini, G.; Placidi, S.; Saffirio, C.; Marino, B. Cardiac outflow tract: A review of some embryogenetic aspects of the conotruncal region of the heart. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2006, 288A, 936–943. [Google Scholar] [CrossRef]
- Wu, B.; Wang, Y.; Lui, W.; Langworthy, M.; Tompkins, K.L.; Hatzopoulos, A.K.; Baldwin, H.S.; Zhou, B. Nfatc1 Coordinates Valve Endocardial Cell Lineage Development Required for Heart Valve Formation. Circ. Res. 2011, 109, 183–192. [Google Scholar] [CrossRef] [PubMed]
- de Lange, F.J.; Moorman, A.F.M.; Anderson, R.H.; Männer, J.; Soufan, A.T.; de Vries, C.d.G.; Schneider, M.D.; Webb, S.; van den Hoff, M.J.B.; Christoffels, V.M. Lineage and morphogenetic analysis of the cardiac valves. Circ. Res. 2004, 95, 645–654. [Google Scholar] [CrossRef]
- Mifflin, J.J.; Dupuis, L.E.; Alcala, N.E.; Russell, L.G.; Kern, C.B. Intercalated cushion cells within the cardiac outflow tract are derived from the myocardial troponin T type 2 (Tnnt2) Cre lineage. Dev. Dyn. 2018, 247, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Eley, L.; Alqahtani, A.M.; MacGrogan, D.; Richardson, R.V.; Murphy, L.; Salguero-Jimenez, A.; Pedro, M.S.R.S.; Tiurma, S.; McCutcheon, L.; Gilmore, A.; et al. A novel source of arterial valve cells linked to bicuspid aortic valve without raphe in mice. eLife 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Stanton, H.; Melrose, J.; Little, C.B.; Fosang, A.J. Proteoglycan degradation by the ADAMTS family of proteinases. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 1616–1629. [Google Scholar] [CrossRef]
- Malemud, C.J. Matrix metalloproteinases (MMPs) in health and disease: An overview. Front. Biosci. 2006, 11, 1696–1701. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, L.E.; McCulloch, D.R.; McGarity, J.D.; Bahan, A.; Wessels, A.; Weber, D.; Diminich, A.M.; Nelson, C.M.; Apte, S.S.; Kern, C.B. Altered versican cleavage in ADAMTS5 deficient mice; A novel etiology of myxomatous valve disease. Dev. Biol. 2011, 357, 152–164. [Google Scholar] [CrossRef]
- Stanton, H.; Rogerson, F.M.; East, C.J.; Golub, S.B.; Lawlor, K.E.; Meeker, C.T.; Little, C.B.; Last, K.; Farmer, P.J.; Campbell, I.K.; et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature 2005, 434, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Askew, R.; Sheppard, B.; Carito, B.; Blanchet, T.; Ma, H.-L.; Flannery, C.R.; Peluso, D.; Kanki, K.; Yang, Z.; et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 2005, 434, 644–648. [Google Scholar] [CrossRef]
- Kern, C.B.; Norris, R.A.; Thompson, R.P.; Argraves, W.S.; Fairey, S.E.; Reyes, L.; Hoffman, S.; Markwald, R.R.; Mjaatvedt, C.H. Versican proteolysis mediates myocardial regression during outflow tract development. Dev. Dyn. 2007, 236, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.B. Excess Provisional Extracellular Matrix: A Common Factor in Bicuspid Aortic Valve Formation. J. Cardiovasc. Dev. Dis. 2021, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Perris, R. The extracellular matrix in neural crest-cell migration. Trends Neurosci. 1997, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Melchionda, M.; Nastasi, G.; Woods, M.L.; Campo, S.; Perris, R.; Mayor, R. In vivo confinement promotes collective migration of neural crest cells. J. Cell Biol. 2016, 213, 543–555. [Google Scholar] [CrossRef]
- Patterson, R.A.; Cavanaugh, A.M.; Cantemir, V.; Brauer, P.R.; Reedy, M.V. MT2-MMP Expression During Early Avian Morphogenesis. Anat. Rec. 2012, 296, 64–70. [Google Scholar] [CrossRef]
- Waldo, K.L.; Kirby, M.L. Cardiac neural crest contribution to the pulmonary artery and sixth aortic arch artery complex in chick embryos aged 6 to 18 days. Anat. Rec. 1993, 237, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Akerberg, B.N.; Sarangam, M.L.; Stankunas, K. Endocardial Brg1 disruption illustrates the developmental origins of semilunar valve disease. Dev. Biol. 2015, 407, 158–172. [Google Scholar] [CrossRef]
- Combs, M.D.; Yutzey, K.E. Heart valve development: Regulatory networks in development and disease. Circ. Res. 2009, 105, 408–421. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Krantz, I.D.; Bason, L.; Piccoli, D.A.; Emerick, K.M.; Spinner, N.B.; Goldmuntz, E. Analysis of Cardiovascular Phenotype and Genotype-Phenotype Correlation in Individuals With a JAG1 Mutation and/or Alagille Syndrome. Circulation 2002, 106, 2567–2574. [Google Scholar] [CrossRef]
- High, F.; Epstein, J.A. Signalling pathways regulating cardiac neural crest migration and differentiation. Novartis Found. Symp. 2007, 283, 152–161. [Google Scholar] [CrossRef]
- High, F.A.; Zhang, M.; Proweller, A.; Tu, L.; Parmacek, M.S.; Pear, W.S.; Epstein, J.A. An essential role for Notch in neural crest during cardiovascular development and smooth muscle differentiation. J. Clin. Investig. 2007, 117, 353–363. [Google Scholar] [CrossRef]
- Varadkar, P.; Kraman, M.; Despres, D.; Ma, G.; Lozier, J.; McCright, B. Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle cells. Dev. Dyn. 2008, 237, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- MacGrogan, D.; D’aMato, G.; Travisano, S.; Martinez-Poveda, B.; Luxán, G.; del Monte-Nieto, G.; Papoutsi, T.; Sbroggio, M.; Bou, V.; Arco, P.G.-D.; et al. Sequential Ligand-Dependent Notch Signaling Activation Regulates Valve Primordium Formation and Morphogenesis. Circ. Res. 2016, 118, 1480–1497. [Google Scholar] [CrossRef] [PubMed]
- Kern, C.B.; Wessels, A.; McGarity, J.; Dixon, L.J.; Alston, E.; Argraves, W.S.; Geeting, D.; Nelson, C.M.; Menick, D.R.; Apte, S.S. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biol. 2010, 29, 304–316. [Google Scholar] [CrossRef]
- Wünnemann, F.; Ta-Shma, A.; Preuss, C.; Leclerc, S.; van Vliet, P.P.; Oneglia, A.; Thibeault, M.; Nordquist, E.; Lincoln, J.; Scharfenberg, F.; et al. Loss of ADAMTS19 causes progressive non-syndromic heart valve disease. Nat. Genet. 2019, 52, 40–47. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Q.; Lin, X.; Liu, X.; Qian, Y.; Xu, D.; Cao, N.; Han, X.; Zhu, Y.; Hu, W.; et al. Extracellular Matrix Disorganization Caused by ADAMTS16 Deficiency Leads to Bicuspid Aortic Valve With Raphe Formation. Circulation 2024, 149, 605–626. [Google Scholar] [CrossRef]
- McKeown-Longo, P.J.; Mosher, D.F. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J. Cell Biol. 1985, 100, 364–374. [Google Scholar] [CrossRef]
- Mosher, D.F.; McKeown-Longo, P.J. Assembly of fibronection-containing extracellular matrix: A glimpse of the machinery. Biopolymers 1985, 24, 199–210. [Google Scholar] [CrossRef]
- Schnellmann, R.; Sack, R.; Hess, D.; Annis, D.S.; Mosher, D.F.; Apte, S.S.; Chiquet-Ehrismann, R.A. Selective Extracellular Matrix Proteomics Approach Identifies Fibronectin Proteolysis by A Disintegrin-like and Metalloprotease Domain with Thrombospondin Type 1 Motifs (ADAMTS16) and Its Impact on Spheroid Morphogenesis. Mol. Cell. Proteom. 2018, 17, 1410–1425. [Google Scholar] [CrossRef]
- Mittal, A.; Pulina, M.; Hou, S.-Y.; Astrof, S. Fibronectin and integrin alpha 5 play essential roles in the development of the cardiac neural crest. Mech. Dev. 2010, 127, 472–484. [Google Scholar] [CrossRef]
- Mittal, A.; Pulina, M.; Hou, S.-Y.; Astrof, S. Fibronectin and integrin alpha 5 play requisite roles in cardiac morphogenesis. Dev. Biol. 2013, 381, 73–82. [Google Scholar] [CrossRef]
- Pham, D.H.; Dai, C.R.; Lin, B.Y.; Butcher, J.T. Local fluid shear stress operates a molecular switch to drive fetal semilunar valve extension. Dev. Dyn. 2021, 251, 481–497. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; Yu, Z.; Tan, C.; Pek, N.; O’dOnnell, A.; Wu, A.; Glass, I.; Winlaw, D.S.; Guo, M.; et al. APOE–NOTCH axis governs elastogenesis during human cardiac valve remodeling. Nat. Cardiovasc. Res. 2024, 3, 933–950. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Farrar, E.; Lui, W.; Lu, P.; Zhang, D.; Alfieri, C.M.; Mao, K.; Chu, M.; Yang, D.; et al. Notch-Tnf signalling is required for development and homeostasis of arterial valves. Eur. Hear. J. 2015, 38, 675–686. [Google Scholar] [CrossRef]
- Mead, T.J.; Yutzey, K.E. Notch pathway regulation of neural crest cell development in vivo. Dev. Dyn. 2011, 241, 376–389. [Google Scholar] [CrossRef]
- Dupuis, L.E.; Evins, S.E.; Abell, M.C.; Blakley, M.E.; Horkey, S.L.; Barth, J.L.; Kern, C.B. Increased Proteoglycanases in Pulmonary Valves after Birth Correlate with Extracellular Matrix Maturation and Valve Sculpting. J. Cardiovasc. Dev. Dis. 2023, 10, 27. [Google Scholar] [CrossRef]
- Goddard, L.M.; Duchemin, A.-L.; Ramalingan, H.; Wu, B.; Chen, M.; Bamezai, S.; Yang, J.; Li, L.; Morley, M.P.; Wang, T.; et al. Hemodynamic Forces Sculpt Developing Heart Valves through a KLF2-WNT9B Paracrine Signaling Axis. Dev. Cell 2017, 43, 274–289. [Google Scholar] [CrossRef]
- Li, Z.; Nardi, M.A.; Li, Y.-S.; Zhang, W.; Pan, R.; Dang, S.; Yee, H.; Quartermain, D.; Jonas, S.; Karpatkin, S. C-terminal ADAMTS-18 fragment induces oxidative platelet fragmentation, dissolves platelet aggregates, and protects against carotid artery occlusion and cerebral stroke. Blood 2009, 113, 6051–6060. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupuis, L.E.; Mifflin, J.J.; Marston, A.L.; Laxner, J.P.; Kern, C.B. ADAMTS5 Orchestrates Cell Lineage Specific Patterning and Extracellular Matrix Organization During Semilunar Valve Development. J. Cardiovasc. Dev. Dis. 2025, 12, 371. https://doi.org/10.3390/jcdd12090371
Dupuis LE, Mifflin JJ, Marston AL, Laxner JP, Kern CB. ADAMTS5 Orchestrates Cell Lineage Specific Patterning and Extracellular Matrix Organization During Semilunar Valve Development. Journal of Cardiovascular Development and Disease. 2025; 12(9):371. https://doi.org/10.3390/jcdd12090371
Chicago/Turabian StyleDupuis, Loren E., Joshua J. Mifflin, Amy L. Marston, Jeremy P. Laxner, and Christine B. Kern. 2025. "ADAMTS5 Orchestrates Cell Lineage Specific Patterning and Extracellular Matrix Organization During Semilunar Valve Development" Journal of Cardiovascular Development and Disease 12, no. 9: 371. https://doi.org/10.3390/jcdd12090371
APA StyleDupuis, L. E., Mifflin, J. J., Marston, A. L., Laxner, J. P., & Kern, C. B. (2025). ADAMTS5 Orchestrates Cell Lineage Specific Patterning and Extracellular Matrix Organization During Semilunar Valve Development. Journal of Cardiovascular Development and Disease, 12(9), 371. https://doi.org/10.3390/jcdd12090371




