1. Introduction
Visceral artery aneurysms (VAAs) and pseudoaneurysms (VAPAs) are rather uncommon however potentially life-threatening vascular pathologies, with a reported incidence rate per year ranging between 0.01% and 0.2% [
1]. VAAs are generally secondary to pre-existing arteriosclerotic damage of the vessel walls and mainly due to degeneration of the tunica media, more commonly seen in older patients. On the other hand, VAPAs are typically related to local inflammation (mainly pancreatitis), dissection or vascular trauma including iatrogenic causes [
2,
3,
4].
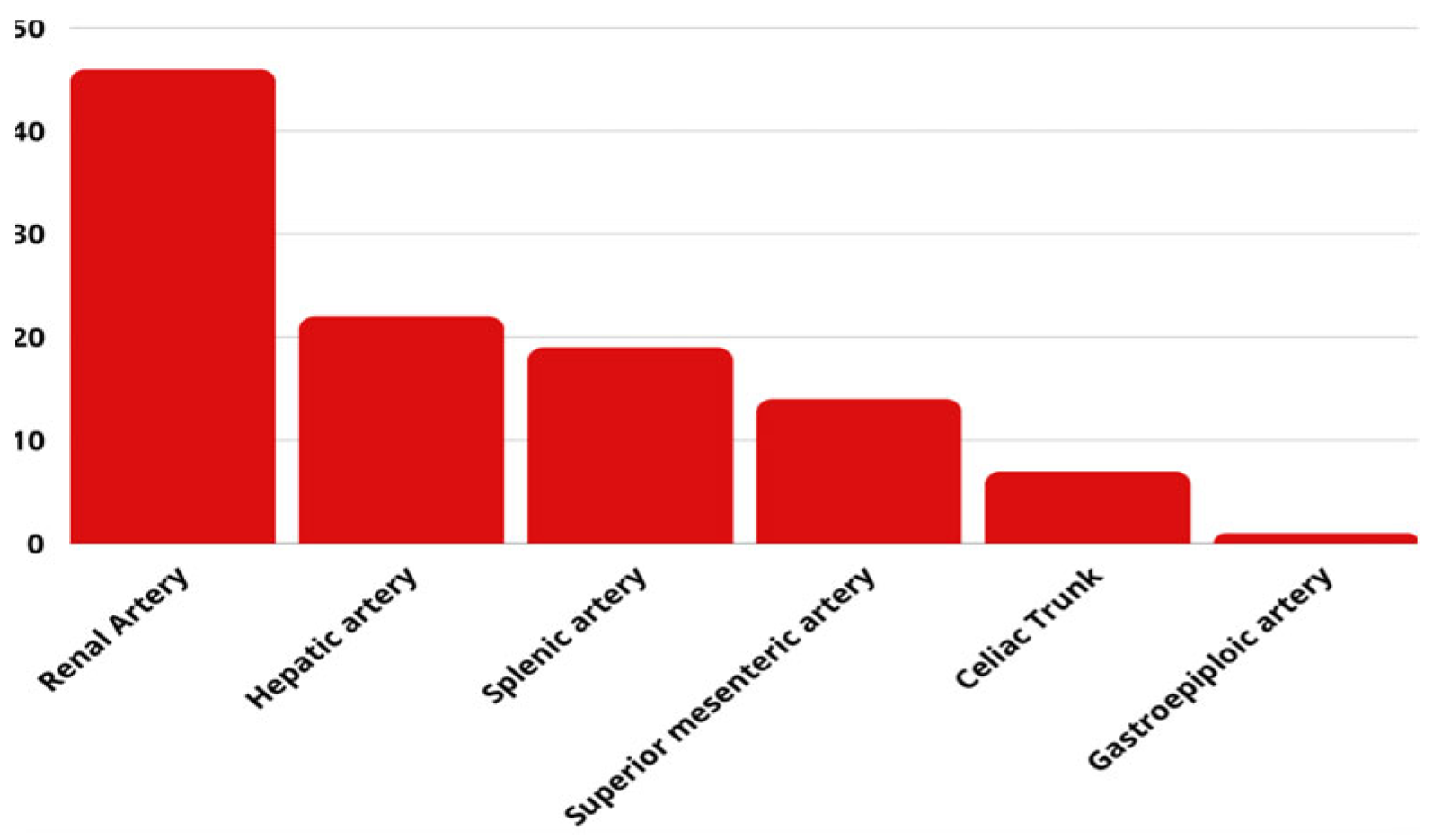
Splenic artery aneurysms have the highest prevalence among VAAs (60%), followed by hepatic (20–50%), superior mesenteric (6%) and celiac artery aneurysms (4%) [
5]. The majority of VAAs increase in size slowly over time, but in the presence of hyper-flow conditions (as pregnancy and infections) or hypertension, the risk of growth and subsequent rupture may increase significantly [
6]. The indications for treatment depend, mainly, on their location and size, with a threshold of 2 cm for most of the cases. Pseudoaneurysms, on the other hand, should be treated irrelevantly from their size, due to the higher risk of rupture [
7,
8,
9]. Due to the lower invasiveness and morbidity in comparison to traditional open surgery, the endovascular approach is the first line treatment for VAAs and VAPAs where the endovascular expertise is available [
10,
11,
12].
VAA can be classified into saccular, fusiform and complex. The most challenging scenario is to treat distal VAA with side-branches originating from the parent vessel and/or from the sac itself. In this case conventional treatment with coils or covered stents inevitably will cause the occlusion of one or more side-branches with consequent end-organ ischemia. This is fundamental in end-organ VAA such as kidney or mesenteric vessels where the collateral supply is insufficient or absent. Therefore, preserving both the feeding artery and the side branches is a desirable solution that was achieved with the flow-diversion techniques that were initially applied for the treatment of intracranial aneurysms and recently expanded to the peripheral circulation.
Flow-diverting stents (FDS) are structured with a greater metal surface area to redirect flow along the main vessel and minimize flow into the aneurysm, while maintaining enough flow through the porous stent to ensure branch vessels and perforators remain open [
13]. They facilitate aneurysm occlusion as following: firstly, by diminishing direct jet blood flow into the aneurysm and promoting organised flow along the stent, known as central diversion [
14]; secondly, by slowing the blood flow within the aneurysm, increasing stagnation, and favouring intra-aneurysmal thrombosis; and thirdly, by reducing wall stress and stagnant flow, leading to aneurysm remodeling and eventually promoting endothelialization across the aneurysm neck and arterial reconstruction [
15]. Dual antiplatelet therapy adherence is required to maintain stent patency, for a duration of 1 to 6 months, followed by life-long therapy with aspirin [
7]. Small differences have been observed in terms of the duration of dual antiplatelet therapy, with acetylsalicylic acid 100 mg/day and clopidogrel 75 mg/day.
Despite its appeal, the use of FDS is not widely adopted for the treatment of visceral aneurysms. One potential reason is the concern that was expressed with the first-generation devices is that the generated thrombosis may not sufficiently prevent aneurysm expansion [
16]. Therefore, the existing data are limited and mainly consist of case series and short reports. A meta-analysis with data from 10 cohort studies on 220 patients was published in 2020 reporting high rates of successful side branches patency, complete aneurysms thrombosis, and high primary stent patency rate [
17]. Nevertheless, the latter study includes evidence from the old generation of flow-diverting stents.
This comprehensive and systematic review aims to provide the most recent data on the efficacy and safety of FDS, and to compare the new generation of flow-diverting stents (NGFDS) with the old generation of flow-diverting stents (OGFDS) and with any other device or technique used for a flow diversion effect, i.e., the double layer nitinol stents or the Multi-Layered Bare Stent technique (MOUS) in the management of VAPAs and VAAs.
4. Discussion
Flow-diverting stents, originally developed for treating intracranial aneurysms, have been increasingly used in managing complex visceral arterial aneurysms with side-branches [
16,
30] with good results [
33], even though no consensus has been obtained internationally for their use, due to the lack of convincing evidence. No prospective randomized control data are available for analysis; however, a recent meta-analysis has reported high technical success rates, sac thrombosis, efficacy and safety [
17]. When branching vessels originate from a visceral aneurysm, covered stents may be unsuitable implying the coiling of the branches; in such cases, FDS can help preserve distal run-off and end-organ perfusion [
34]. This systematic review covers the most recent data on the use of FDS in peripheral VAA and VAPA and compares the efficacy and safety of the newest, oldest and multi-layered ones.
Flow-diverter technique arises from the device’s tight micromesh design to induce flow modulation rather than sealing it off, which depends on its porosity (the metal-free area relative to the total stent surface) and pore density (number of pores per unit area along the device-neck interface) [
33]. Both porosity and pore density influence the resistance to blood flow into the aneurysmal sac, and FDS promote slow sac thrombosis whilst preserving patency of collateral branches, crucial in territories with a high risk of distal ischaemia. Moreover, NFDS are more flexible and are easier to navigate than stent-grafts and could be a favourable choice when deploying in tortuous long vessels [
17,
35]. The primary advantage of FDS in preserving side-branches is particularly relevant when branches originating directly from the sac supply distal vital organs, where stent-grafts or coiling would not achieve the same conservative outcomes. Preserving the flow in visceral organ may be generally advised, when possible, to avoid distal ischemic sequels. This is particularly important in the renal or mesenteric arteries where the embolization of collaterals may lead to severe clinical complications.
Hemodynamic flow studies have shown that multiple overlapping uncovered stents manage to thrombose the sac whilst keeping the branch unobstructed [
36]. Zhang et al. discovered a “unique flow route” inside the thrombosed aneurysm sac that allows for branch runoff in these situations [
30]. Novel thin-film nitinol flow diverters, made from patterned sheets rather than braided wires, allows for a significantly higher pore density compared to traditional braided wire flow diverters and have shown promising results in preclinical tests [
37].
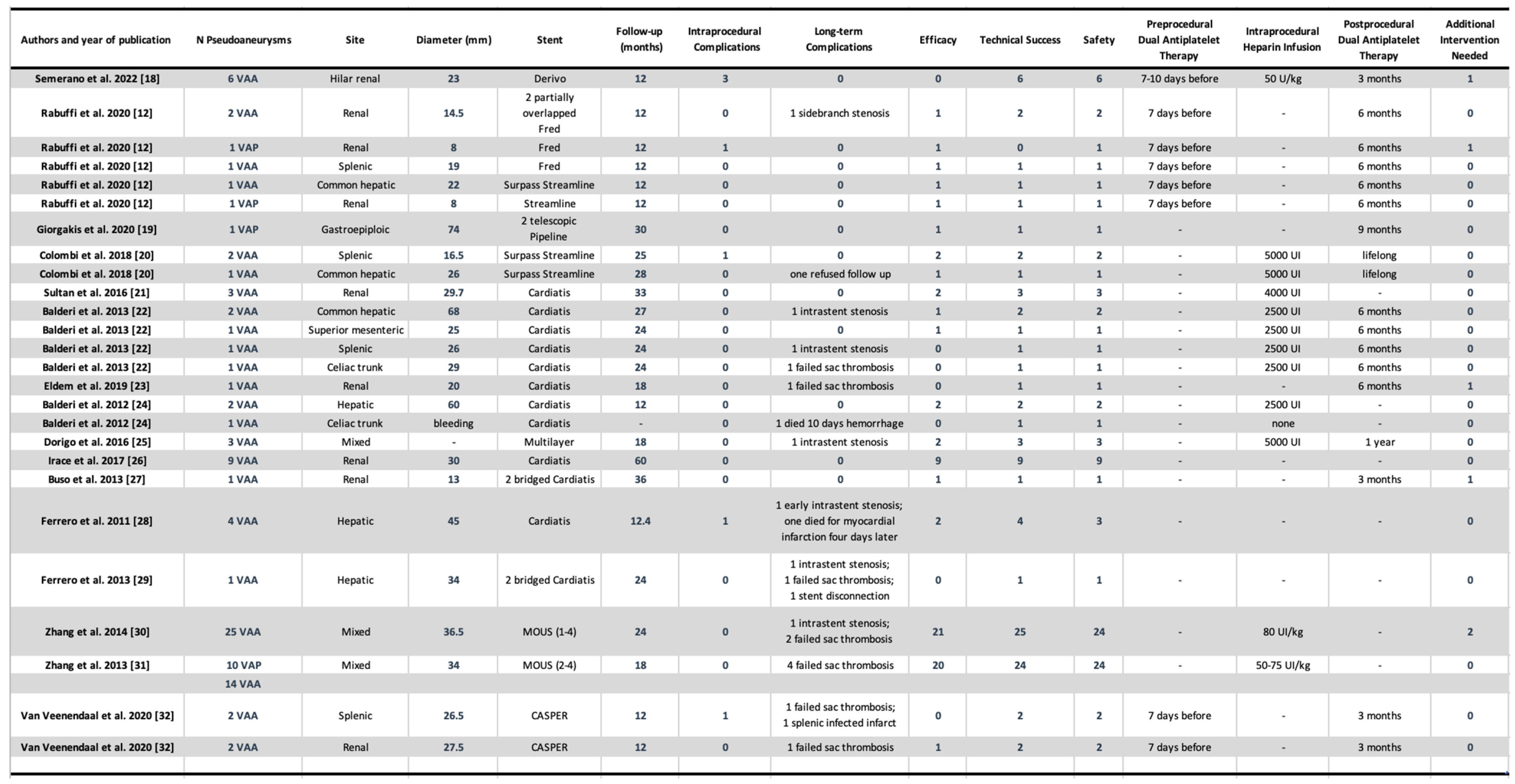
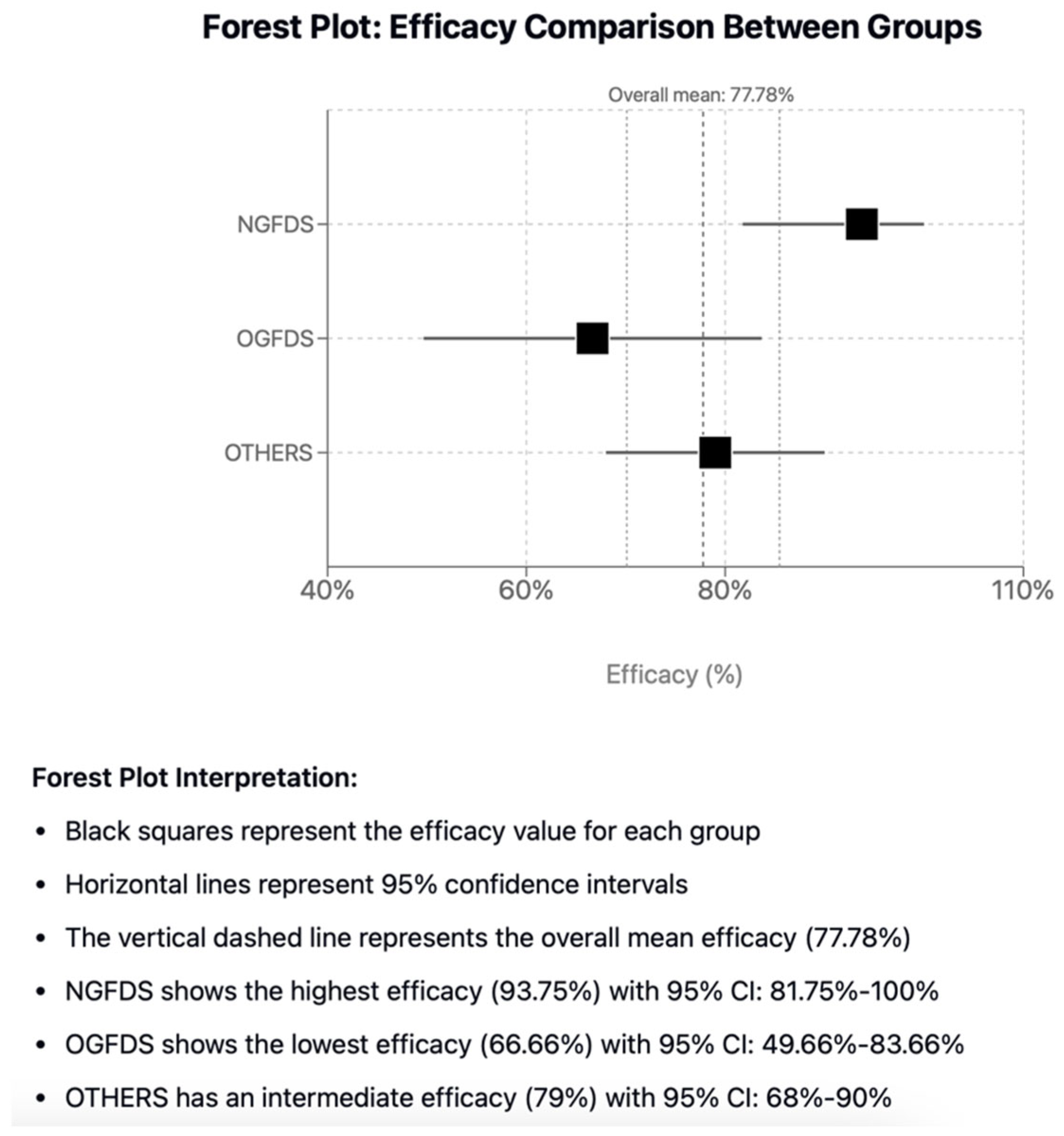
In this comprehensive and systematic review, the aim was to report the results of FDS in the management of VAA and VAPA and categorize enrolled studies into three groups for comparison: new generation FDS, old generation FDS, and other devices or techniques used to obtain a flow diversion effect. When considering the entire population, they were associated with significantly high, overall side-branch vessel patency (96.9%), treatment efficacy (78%) and safety (98%) and sac thrombosis (89%), which may be beneficial for sac stabilization and shrinkage [
17]. Stent induced thrombosis could be related with decreasing the wall’s tensile stress and eliminating local stress concentrations [
38]. Partial thrombosis has not been associated with reduced aneurysm shrinkage [
30,
38], although two case of partial thrombosis of the sac in the OGFDS were associated with increased size of the sac which eventually required re-intervention [
23,
29]. On the other hand, the 6 cases of partial thrombosis with MOUS technique did not show any increase in size nor cause of re-intervention compared to the complete thrombosis cases [
30,
31].
The new generation FDS have been associated with the highest rate of treatment efficacy (93.75%) compared to the other groups, however, despite the highest rate of intraprocedural complications (31.25%) and lowest technical success rates (75%). The cases reported having technical difficulties were due to prolapse of the stent in the aneurysm sac or to vessel dissection, 12% of which required re-intervention [
18,
23]. Intraprocedural issues were not seen in MOUS technique, only 1 case was observed in the use of double layer braided mesh stent which required reintervention with an additional stent positioning due to persistency of the aneurysm at 12 months CT angiogram [
32] and only 1 in OGFDS Cardiatis [
39], possibly due to the longer experience of doctors with these devices compared to the newer ones. The high rate of technical complications associated with the use of NGFDS can be partly explained by their predominant use in particularly complex cases, where they often represent the only available therapeutic option. This is especially true for tortuous and distal locations, where other techniques are not applicable. However, not all the articles analysed the exact location of the aneurysm, hence we could not clearly highlight this aspect, thus limiting our ability to directly correlate technical complications to the specific anatomical location of the treated aneurysms. Another issue regarding NGFDS, is the size available to date that limit their use in vessel with a dimeter > 7 mm.
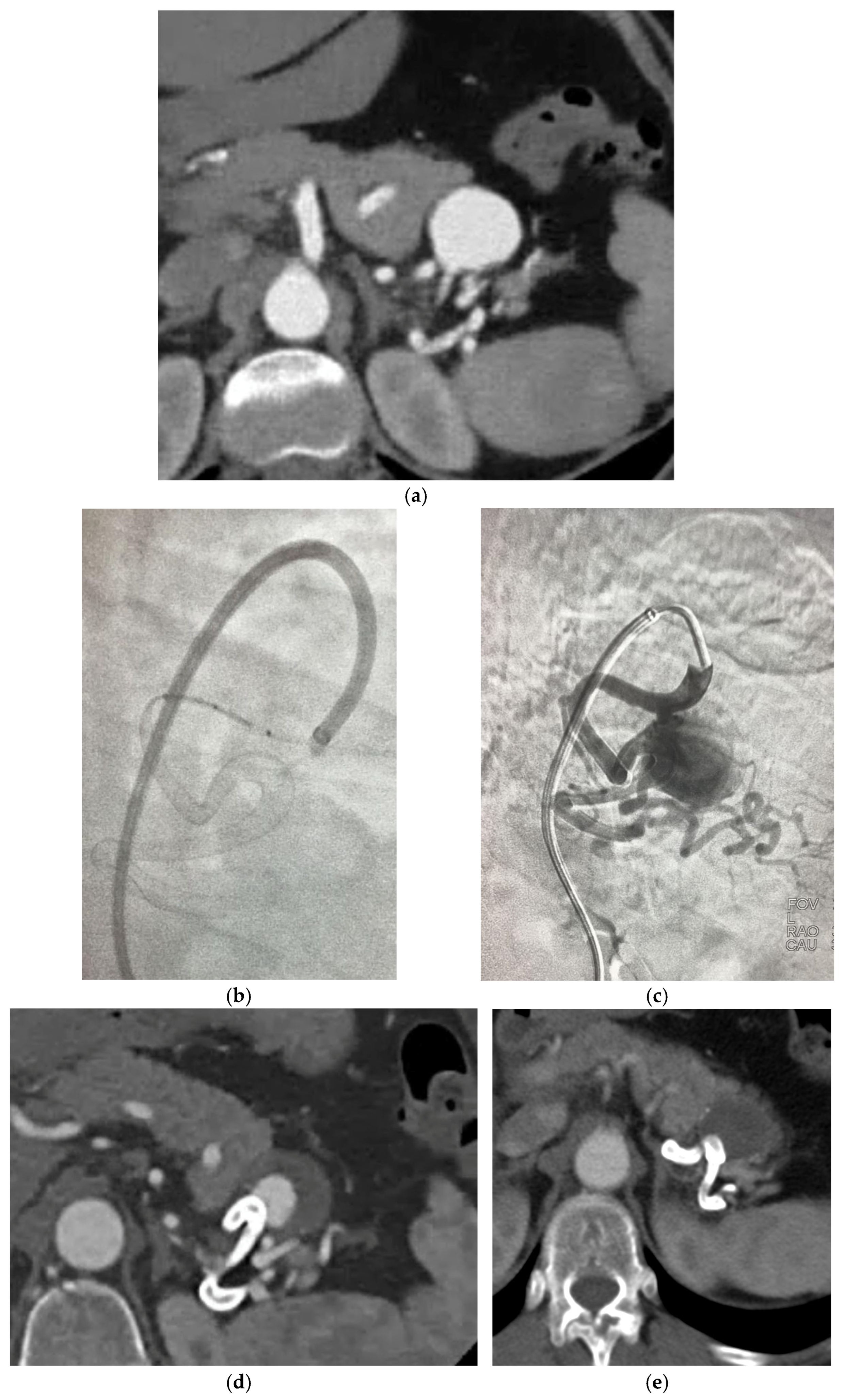
Figure 6a–e describe a case of NGFDS from our institution.
Stent occlusion is an issue observed in studies involving FDS [
39], partly associated to failure of patient’s compliance in undergoing correct anti-platelet therapy [
22,
25,
28,
30,
40]. In-stent thrombosis can occur anywhere from soon after placement to up to 24 months after the procedure. According to our data, the primary stent patency rate stands at 94.74% on average, and the only reported cases of stent thrombosis were associated with OGFDS, some of which required angioplasty [
23]. The stent thrombosis was not associated with sac perfusion.
Of importance, the only case of mortality associated to the procedure is reported by Balderi et al. in a case of a bleeding VAA of the celiac trunk as the FDS was inappropriately chosen as it did not manage to limit the haemorrhage [
24]. A similar case but with a successful outcome has been reported by Giorgakis et al. in the treatment of a sidewall bleeding VAPA of right Gastroepiploic artery with the use of two Pipeline stents embolization devices with a telescoping method in a patient after pancreatectomy, permitting to preserve the main arterial supply to the gastric conduit [
19].
The analysis of this systemic review data has limitations that must be accounted for. Firstly, the data retrieved were from small cohort, non-comparative, retrospective studies which lacked randomization, mainly due to the low prevalence of VAA\VAPAs. Furthermore, inconsistencies in the reporting standards of these studies made data retrieval challenging and imprecise for several parameters. Secondly, it was not possible to directly compare the different groups of stents based on the location of artery deployment, calibre, or underlying atheromatous disease. Moreover, many cases reported have not specified the patient’s adherence to anti-platelet therapy, which is very important in the management of these pathologies. For completeness of this systematic review we have included the analysis of FDS in VAPA’s, although we believe that these lesions have a high risk of bleeding and should therefore be treated with a fast and complete exclusion with front-and-backdoor coiling or with covered stenting. Lastly, older generation stents have been used for visceral aneurysms for over 10 years, whereas new generation stents have only been reported in cases from the past five years, creating a bias in comparing intraprocedural success and complications as doctors deploying older stents might have had more experience.
5. Conclusions
FDS represent a valid alternative for the management of complex peripheral VAAs and VAPAs, where other endovascular and surgical techniques are not feasible. Regarding the comparison between the flow diversion stent techniques described in the literature, it seems that the NGFDS are superior in terms of efficacy, despite less favourable data on their technical success and intra-procedural complications. Long-term complications were not present in new generation devices, promoting their role in avoiding intrastent thrombosis or incomplete sac exclusion, whilst preserving side-branches vessels.
To date, a major limitation in the use of flow diverters is their high cost. However, given the excellent results demonstrated by their application, increased use in the future may lead to cost reduction as happened with several devices that were transferred from neuro to peripheral intervention. Furthermore, dedicated large size devices are also now available overcoming a limitation of their past use. Further studies in this area may be required to shed light is this continuously developing field.