Abstract
Telomere dysfunction has emerged as a pivotal contributor to vascular senescence, a fundamental process in the pathogenesis of age-related cardiovascular diseases such as atherosclerosis. This connection underscores the therapeutic potential of targeting telomere biology to prevent or mitigate the progression of vascular aging. In this context, Astragalus membranaceus and its bioactive constituents, including astragaloside IV, cycloastragenol, and the commercial telomerase activator TA-65, demonstrate significant promise in attenuating vascular aging and atherosclerotic disease. These compounds exert a range of pleiotropic effects, including anti-inflammatory, antioxidant, endothelial-protective, and lipid-modulating actions, while also modulating telomerase activity and supporting telomere maintenance. This review provides an overview of the mechanistic basis underlying the anti-atherosclerotic effects of Astragalus-derived compounds and underscores critical key knowledge gaps. It also outlines future research directions necessary to validate their efficacy and therapeutic potential in the prevention and treatment of atherosclerosis and other age-related vascular disorders.
1. Introduction
Vascular senescence is now recognized as a key driver in the development and progression of atherosclerosis. Telomeres, the protective caps at the ends of chromosomes, play a crucial role in cellular aging and vascular health.
Indeed, progressive telomere attrition accelerates vascular senescence and atherosclerosis over time [1,2,3]. In endothelial and vascular smooth muscle cells, telomere shortening acts as a biological clock, triggering cells to enter replicative senescence or undergo apoptosis. Shortened telomeres initiate DNA damage responses, increase oxidative stress, and activate inflammatory signaling, fostering a pro-inflammatory secretory phenotype (SASP). This contributes to chronic low-grade inflammation within the vessel wall, exacerbating vascular dysfunction and plaque development [4,5].
Over the past few years, recent meta-analyses and Mendelian randomization studies across diverse ethnic populations have reported a significant association between shortened leukocyte telomeres and an increased risk of coronary atherosclerosis, myocardial infarction, and ischemic heart disease [6,7,8,9,10,11]. These findings provide strong evidence linking telomere length reduction to atherosclerosis progression. Since telomere shortening is closely linked to vascular aging, strategies to preserve telomere length or counteract telomere-associated dysfunction may hold significant therapeutic potential [12,13]. These approaches offer promising avenues for combating the atherosclerotic disease and promoting healthy vascular aging. Various interventions aimed at enhancing telomere length, through genetic manipulation or pharmacological means, have already demonstrated potential in delaying cellular and tissue aging [12,13].
Astragalus, a plant widely used in traditional Chinese medicine, has attracted considerable attention for its ability to activate telomerase and extend telomere length, making it a promising natural supplement for promoting healthy aging [14]. Recently, research on the therapeutic effects and mechanisms of Astragalus membranaceus (AM) and its active compounds has also gained significant interest in the cardiovascular field [15,16,17,18].
The cardioprotective effects of Astragalus are mediated through multiple pharmacological actions, including anti-inflammatory, antifibrotic, antioxidative, antidiabetic, immunoregulatory, and cardioprotective mechanisms, acting via numerous signaling pathways [15,16,17,18]. These properties highlight its potential as a complementary strategy for cardiovascular disease prevention and treatment.
However, its comprehensive effects on vascular aging and atherosclerosis and plausible mechanisms remain unclear.
To shed light on the mechanisms of action and promote future research, this paper reviews and discusses current evidence on the anti-atherosclerotic effects of AM and its active compounds, with a particular focus on their impact on telomere biology and vascular aging.
2. Overview of Telomere Biology
Telomeres are nucleoprotein structures located at the ends of chromosomes. They are essential for protecting chromosomes from degradation, fusion, and inappropriate recombination. They consist of a variable number of double-stranded telomeric repeats, 5′-(TTAAGGG)n-3′, together with a terminal region containing a single-stranded G-rich 3′ overhang. This overhang folds into higher-order structures to enhance chromosome end protection [19,20].
Telomere repeats are associated with the Shelterin multi-protein complex. This complex includes factors that bind specifically to different telomeric regions; the telomere protection 1 (Pot1)-TTP1 heterodimer binds directly to the G-strand overhang, while the double-stranded telomeric region is recognized by the telomere repeat binding factors 1 (TRF1) and the telomere repeat binding factors 2 (TRF2). In addition, these factors interact with proteins such as repressor activator protein 1 (RAP1) and TRF1-interacting nuclear protein 2 (TIN2) [20].
During DNA replication, telomeres gradually shorten in most somatic cells due to the end-replication problem. Because of this progressive shortening, telomeres function as a biological clock, with their length correlating closely with cellular aging. When telomeres become critically short, the cell activates a DNA damage response, which can lead to cellular senescence (permanent cell cycle arrest) or apoptosis (programed cell death). However, certain cells, such as stem cells, germ cells, and many cancer cells, express telomerase, a reverse transcriptase enzyme that adds telomeric repeats to chromosome ends, thereby counteracting telomere shortening and enabling continued cell division [19,20].
This enzyme comprises a catalytic subunit, telomerase reverse transcriptase (TERT), which utilizes the telomerase RNA component (TERC). TERC contains a sequence complementary to the telomeric repeats, enabling the synthesis of new telomeric DNA at the overhang [19,20].
Improving our understanding of telomere length and maintenance mechanisms, including the controlled and targeted reactivation of telomerase, offers a promising strategy for extending healthy lifespan and treating degenerative diseases by counteracting telomere-driven cellular aging (Figure 1).
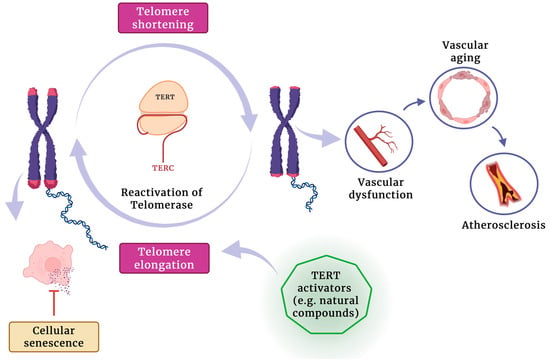
Figure 1.
The potential role of telomerase activators in promoting telomere elongation, reducing cellular senescence, and mitigating vascular aging and atherosclerosis development.
3. Astragalus membranaceus and Its Active Compounds
Astragalus radix, the dried root of Astragalus membranaceus, has been used for centuries as an herbal remedy to counteract oxidative stress, inflammation, and aging [21]. The pharmacological effects of Astragalus radix result from a multitude of its chemical constituents. Over 200 compounds have been identified in Astragalus-based herbal formulations [22], the most notable being saponins, polysaccharides, and flavonoids; but it also includes components such as anthraquinones, alkaloids, amino acids, β-sitosterol, and metallic elements [21]. The main active constituents in Astragalus supplements are the triterpenoid saponins, mainly astragalosides. Among these, astragaloside IV is the primary active component of the medicinal Astragalus plant. Astragaloside IV (AS IV) is a lanolin alcohol-derived tetracyclic triterpene saponin that possesses a range of pharmaceutical properties, including anti-inflammatory, anti-insulin resistance, antitumor, and neuroprotective effects [23]. Its molecular formula is C4H6O14, and its structure is similar to that of steroidal drugs, with very low solubility.
Cycloastragenol (CAG) is another bioactive molecule derived from various species of the Astragalus genus [24]. It is an aglycone derivative of AS IV and a triterpenoid saponin compound formed through the hydrolysis of AS IV [24].
TA-65 is a patented, natural, encapsulated form of cycloastragenol that has gained attention as a safe and effective dietary supplement for promoting healthy aging, with no reported toxicity [25]. Another active ingredient of Astragalus radix is astragalus polysaccharide (APS), a heteropolysaccharide with a complex chemical structure and water solubility [17]. It contains heteropolysaccharides, dextran, and fractions of both neutral and acidic polysaccharides, which have demonstrated significant immunomodulatory effects and anticancer activity [26].
The structure and the detailed physicochemical properties of the main Astragalus-derived compounds are summarized in Figure 2.
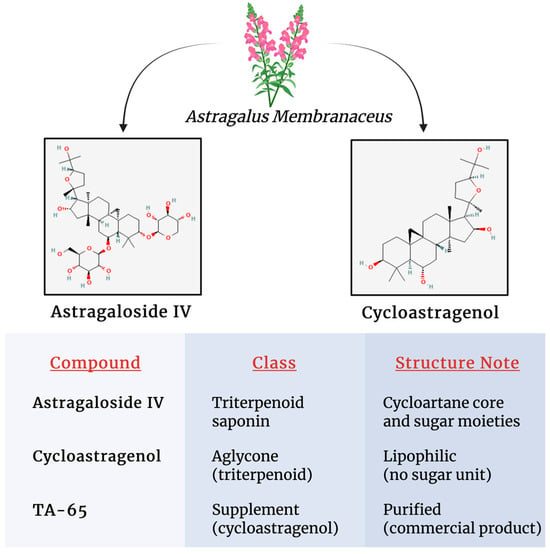
Figure 2.
Structural characteristics and detailed physicochemical properties of major Astragalus constituents.
4. Anti-Senescence Mechanisms and Anti-Aging-Related Effects of Astragalus and Its Chemical Constituents
4.1. Preclinical and Animal Evidence
Several studies have demonstrated that CAG and/or AS IV can delay or mitigate cellular senescence and apoptosis under various experimental conditions [27,28]. Both compounds have been identified as telomerase-stimulating agents [27,28]. Telomerase activity is crucial for maintaining the proliferative capacity and longevity of somatic cells [29], but it declines significantly in adult organisms compared to neonates [29].
The CAG has been shown to enhance telomerase activity in neuronal cells via activation of the cyclic adenosine monophosphate response element-binding protein (CREB), a key transcription factor required for upregulating TERT expression and downstream telomerase function [28]. Similarly, AS IV has been reported to increase telomerase expression by modulating several intracellular pathways, including the Mitogen-Activated Protein Kinase (MAPK), Janus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT), and CREB signaling cascades [24]. In addition, AS IV is capable of activating telomerase in a range of cell types, particularly in mouse embryonic fibroblasts (MEFs, G3 Terc+/−) and hematopoietic progenitor cells. Systemic supplementation of AS IV has been shown to boost TERT activation in various tissues, including the brain, liver, heart, lungs, and bone marrow, thereby alleviating telomere attrition in aged mice [30,31].
Furthermore, co-administration of AS IV and CAG has been shown to enhance telomerase activity via activation of the proto-oncogene tyrosine-protein kinase Src/Mitogen-Activated Protein Kinase Kinase/Extracellular Signal-Regulated Kinase (Src/MEK/ERK) signaling cascade [27]. A study demonstrated that both CAG and AS IV play a significant role in preventing high glucose (HG)-induced intervertebral disc degeneration by attenuating cellular senescence, both replicative and stress-induced premature senescence. These compounds may also inhibit apoptosis in nucleus pulposus cells by upregulating telomerase activity and promoting telomere elongation [23]. The activation mediated by CAG is primarily associated with the MAPK and Protein Kinase B pathways (Akt). Specifically, Akt enhances telomerase enzymatic activity by promoting post-translational phosphorylation of the TERT subunit [32].
The CAG has been found to induce regulated protein kinase (ERK) phosphorylation in Human Embryonic Kidney 293 cells (HEK293) and neonatal keratinocytes by engaging the Src/MEK/ERK pathway [27].
Like CAG and AS-IV, TA-65 is a small-molecule telomerase activator, commercially available on the market since 2008. Since its introduction, it has been investigated in preclinical and early clinical work for its potential role in promoting telomere maintenance and cellular health [33]. For instance, it has been shown to increase average telomere length and reduce both the frequency of critically short telomeres and DNA damage in haplo-insufficient mouse embryonic fibroblasts (MEFs, G3 Terc+/−). Indeed, these cells exhibit telomere shortening due to their possession of a single copy of the telomerase RNA component gene [30].
In addition, the dietary supplementation of 25 mg/kg per day of TA-65 is capable of inducing an improvement of certain health-span indicators, including glucose tolerance, osteoporosis and skin fitness, without significantly increasing global cancer incidence [30].
Moreover, TA-65 showed protective effects in mouse models of chronic obstructive pulmonary disease (COPD) and cigarette-smoke damage. This protective effect was associated with a decreased expression of the pro-fibrotic cytokine transforming growth factor beta 1 (TGF-β1) in the small airway walls of CS-exposed mice, together with a protection against fibroblast-to-myofibroblast differentiation in response to TGF-β1 in lung primary fibroblast in vitro [34].
4.2. Human Investigation Studies
Human studies have also supported these in vitro findings, indicating that TA-65 can positively influence telomere length in healthy individuals [33].
A study of 117 relatively healthy individuals aged 53–87 years and positive for cytomegalovirus found that a low dose of TA-65 (250 U) significantly increased telomere length over 12 months, whereas participants in the placebo group experienced significant telomere shortening [35].
A placebo-controlled study recruiting a large number of healthy adults (500) showed that oral intake of TA-65 across all doses (100 U, 250 U, and 500 U) for nine months significantly decreased CD8+CD28− T cells, suggesting a potential benefit by decreasing senescent T cells [36].
Similarly, in a randomized, double-blind, placebo-controlled six-month trial involving 40 healthy volunteers, participants taking an Astragalus-based supplement exhibited significantly increased median and short telomere lengths, while no change was observed in the placebo group [37].
Although these data suggest that telomerase therapies and pharmacological interventions can counteract the effects of telomere attrition, their role in vascular biology and atherosclerosis is still largely unknown.
5. Astragalus in Vascular Cells and Atherosclerosis
5.1. Effects on HUVEC
Endothelial cells, which are essential elements of the arterial intima, play a key role in regulating vascular function and preserving internal homeostasis.
Endothelial dysfunction, characterized by reduced nitric oxide production, oxidative stress, and proinflammatory signaling, drives atherosclerosis by promoting plaque formation, immune cell recruitment, and lipid accumulation in the arterial wall [38].
As previously mentioned, telomere shortening is strongly associated with vascular dysfunction by promoting cellular senescence, which impairs vascular repair, elevates oxidative stress, and contributes to the development of atherosclerosis [1,2,3]
Numerous studies have demonstrated the protective effects of AS IV against vascular endothelial dysfunction [39], although evidence regarding its influence on telomere biology in endothelial cells remains lacking. In vitro studies have shown that AS IV at 80 μmol/L promotes proliferation and angiogenic activity in human umbilical vein endothelial cells (HUVECs), primarily through the suppression of phosphatase and tensin homolog (PTEN) expression and activation of the Phosphoinositide 3-Kinase (PI3K)/Akt signaling pathway [40]. In addition, Zhang et al. showed that a low concentration of AS IV (0.25 μM) facilitates capillary-like tube formation in HUVECs through PI3K/Akt pathway activation and upregulation of hypoxia-inducible factor 1-alpha (HIF-1α), suggesting a role in neovascularization [41].
In agreement with these data, Wang et al. demonstrated that AS IV (10, 40, and 120 μM) significantly promotes proliferation, migration, and tube formation in HUVECs via ERK1/2 phosphorylation and JAK2/STAT3 pathway activation [42].
AS IV also exhibits antioxidant properties in oxidative injury models. Xu et al. reported that AS IV (20–100 μmol/L) increased nitric oxide (NO) bioavailability in H2O2-stimulated HUVECs by inhibiting reactive oxygen species (ROS)/Nuclear Factor kappa-light-chain-enhancer of activated B (NF-κB) signaling and reducing endothelial nitric oxide synthase (eNOS) uncoupling [43]. Zhu et al. further reported that AS IV at concentrations of 10, 20, and 50 μM significantly enhances oxidized Low Density Lipoprotein (LDL)-induced HUVECs migration and motility while suppressing the generation of ROS and NADPH oxidase (NOX). These effects are mediated by activation of the nuclear factor erythroid 2-related factor 2 (NRF2)/heme oxygenase-1 (HO-1) signaling axis, a key regulator of oxidative stress responses [44].
Qiu et al. observed that AS IV at doses of 50 and 100 mg/mL mitigated Homocysteine (HCY)-induced endothelial dysfunction, a known risk factor for atherosclerosis, by reducing ROS accumulation and enhancing superoxide dismutase (SOD) activity, thereby restoring cellular homeostasis [45]. Similar observations were reported by Shao et al. They investigated the role of AS IV in a circular RNA-mediated regulatory mechanism using an in vitro atherosclerosis model in which HUVECs were exposed to ox-LDL. AS IV (100 μM) reduced apoptosis, oxidative stress, and inflammation, while enhancing cell viability and migration. These effects were mediated through modulation of the circ_0000231/miR-135a-5p/Chloride Intracellular Channel 4 (CLIC4) axis [46].
Complementary to these findings, Chen et al. explored the molecular basis of AS-IV’s protective effects against ox-LDL-induced endothelial injury. AS IV treatment inactivated the NF-κB pathway by regulating the histone deacetylase 9 (HDAC9), leading to reduced apoptosis, oxidative stress, and inflammatory signaling [47].
Together, these findings highlight AS IV as a multifunctional compound with significant potential in the prevention and treatment of endothelial dysfunction and atherosclerosis through different molecular pathways.
5.2. Effects on VSMCs
Like endothelial cells, vascular smooth muscle cells (VSMCs) play a key role in the development and progression of atherosclerosis by contributing to plaque formation, vascular wall remodeling, and the regulation of inflammation and calcification [48].
However, studies investigating the protective effects of Astragalus membranaceus in this context remain limited. Zhang et al. reported that AS IV at a concentration of 10 μM inhibited Angiotensin II-induced proliferation of A10 cells (a rat VSMC line) by reducing the cyclin-dependent kinase 2 (CDK2) activity [49]. CDK2 is essential for the transition from the G1 to the S phase of the cell cycle, as well as for regulating the G2 phase, thereby promoting cell proliferation [50].
Further supporting evidence comes from Lu et al., who found that AS IV (50 μg/mL) restored Adenosine Triphosphate (ATP) production in Angiotensin II-induced VSMCs. Additionally, AS IV reversed mitochondrial dysfunction by enhancing oxygen consumption rates, increasing mitochondrial membrane potential, and boosting mitochondrial DNA content. These mitochondrial improvements were accompanied by a reduction in ROS production, increased SOD activity, and stimulation of mitochondrial biogenesis and mitophagy [51].
Consistent with these findings, Li et al. reported that AS IV attenuated senescence in bleomycin-induced VSMCs by restoring mitochondrial membrane potential, improving mitochondrial integrity, and promoting mitophagy. This anti-senescent effect was mediated via Parkin upregulation, highlighting the relevance of AS-IV-induced mitophagy in preserving VSMCs homeostasis [52].
However, in another study, Song et al. demonstrated that AS IV (50 μg/mL) can inhibit the autophagy and mineralization of VSMCs by increasing the expression of long non-coding RNA H19 (lncRNA H19) and decreasing the expression of dual-specificity phosphatase 5 (DUSP5) [53].
Table 1 presents a summary of studies investigating the beneficial effects of astragaloside IV, whereas Figure 3 illustrates a schematic overview of the effects of Astragalus-derived compounds on vascular cells.

Table 1.
Studies investigating the beneficial effects of astragaloside IV on vascular cells.
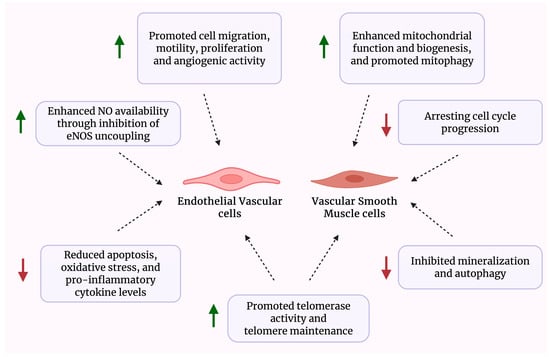
Figure 3.
Schematic overview of the biological effects of Astragalus-derived compounds on vascular cells.
5.3. In Vivo Effects on Atherosclerosis and Plaque
Several in vivo studies have investigated the anti-atherosclerotic effects of astragaloside IV, particularly in murine models of atherosclerosis (Table 2). These studies have provided valuable insights into the compound’s pharmacological mechanisms and its potential for modulating plaque development and vascular inflammation. In an in vivo model of atherosclerosis, ApoE−/− mice were fed a high-fat diet (HFD) ad libitum and treated daily with AS IV (25 mg/kg) for eight weeks. This model exhibited increased autophagy and mineralization in VSMCs of the thoracic aorta. AS IV administration significantly attenuated these effects, suggesting a protective role against VSMCs dysfunction [53].

Table 2.
Studies investigating the beneficial effects of astragaloside IV in animal models of atherosclerosis.
Similarly, Wang et al. reported that AS IV treatment at 40 mg/kg reduced lipid-rich areas in atherosclerotic plaques of ApoE−/− mice. This was accompanied by increased collagen content and fibrous cap thickness, effects linked to regulation of the PI3K/Akt and Toll-Like Receptor 4 (TLR4)/NF-κB pathways, inhibition of Matrix Metalloproteinase-9 (MMP-9) expression, and anti-inflammatory activity [54]. In LDLR−/− mice, Zhang et al. demonstrated that AS IV mitigated atherosclerosis by targeting the MAPK/NF-κB signaling pathway. This intervention reduced NF-κB p65 expression in the aortic root and decreased inflammatory cytokine levels in serum, aortic, and liver tissues. The anti-inflammatory effects involved inhibition of MAPK components (JNK, ERK1/2, and p38), suppression of NF-κB signaling, and reduced phosphorylation of inflammatory proteins, including inducible Nitric Oxide Synthase (iNOS), Vascular Cell Adhesion Molecule-1 (VCAM-1), and Interleukin-6 (IL-6) [55].
Moreover, Sun et al. found that daily AS IV administration at 20 mg/kg in rats upregulated Peroxisome Proliferator-Activated Receptor gamma (PPAR-γ) through NF-κB inhibition, resulting in decreased serum concentrations of ox-LDL, Tumor Necrosis Factor alpha (TNF-α), IL-6, and IL-18, thereby suppressing atherosclerosis progression [56]. Similarly, Qin et al. showed that AS IV at 40 mg/kg/day reduced atherosclerotic severity in ApoE−/− mice via modulation of the stromal-cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) pathway [57]. Interestingly, a meta-analysis of 22 animal I/R model studies showed consistent reduction in infarct size, affirming strong reproducibility across models [58].
Beyond direct vascular and anti-inflammatory effects, modulation of lipid metabolism represents another key mechanism by which Astragalus compounds may protect against atherosclerosis. Hypercholesterolemia is a major driver of atherosclerosis. In experimental models of diet-induced hypercholesterolemia, APS significantly lowered plasma cholesterol, triglycerides, and LDL-cholesterol, while also reducing hepatic lipid accumulation, enhancing fecal bile acid and sterol excretion, inhibiting intestinal cholesterol absorption, and upregulating hepatic cholesterol-7α-hydroxylase and LDL receptor expression [59]. In parallel, inflammatory pathways contribute to lipid dysregulation; TNF-α impairs reverse cholesterol transport by downregulating ATP-binding cassette transporter A1 (ABCA1), promoting foam cell formation [60]. APS counteracts this process by restoring ABCA1 expression, promoting cholesterol efflux, and suppressing NF-κB activation, thereby limiting inflammation-driven lipid accumulation [61]. These findings suggest that, Astragalus-derived compounds such as AS IV and APS may exert complementary anti-atherosclerotic actions through lipid-lowering and cholesterol-transport modulation.
5.4. Human Clinical Studies
Limited but promising clinical data have also demonstrated improvements in cardiac function and oxidative stress markers in acute myocardial infraction and ischemic heart disease.
Indeed, some preliminary Chinese observational studies have reported positive effects of Astragalus on symptoms such as dyspnea, chest pain, and angina, alongside improvements in electrocardiographic parameters and cardiac output in patients with ischemic heart disease [62,63].
A double-blind, randomized, placebo-controlled trial in which patients with metabolic syndrome were allocated to consume either 16 mg daily of a TA-65 supplement or a placebo for 12 weeks showed that there was an improvement in risk factors for cardiovascular disease, with reduced inflammatory levels (low TNF-α levels) and a parallel reduction in body mass index, waist circumference, and atherosclerotic ratio LDL-C/High-Density Lipoprotein (HDL) [64].
More recently, the Telomerase ACTivator to reverse Immunosenescence in Acute Coronary Syndrome (TACTIC) study, a double-blinded, randomized controlled trial, evaluated for the first time whether TA-65 can reduce immune cell aging in patients following myocardial infarction. The study demonstrated that one year of treatment with TA-65 significantly enhanced telomerase activity in immune cells, resulting in a marked reduction in systemic inflammatory markers such as IL-6 and TNF-α. Additionally, it improved lymphocyte proliferation and decreased signs of immune senescence. These findings highlight the promising therapeutic role for TA-65 in modulating post-infarction inflammation and immune function [65].
6. Conclusions and Perspectives
Telomere dysfunction has emerged as a critical contributor to vascular senescence, a key process in the development of age-related cardiovascular diseases such as atherosclerosis. This strong association highlights the potential of therapeutic strategies that enhance telomere biology to prevent or slow the progression of atherosclerosis.
Within this context, Astragalus membranaceus and its bioactive compounds, including astragaloside IV, cycloastragenol, and the commercially available telomerase activator TA-65 demonstrate considerable promise in reducing vascular aging and atherosclerosis. Further supporting their relevance in cardiovascular disease, astragaloside IV and related compounds have also demonstrated protective roles in a range of cardiac pathologies, such as heart failure, hypertrophy, fibrosis, and ischemia–reperfusion injury.
As discussed above, these agents exert pleiotropic effects on vascular biology, including anti-inflammatory, antioxidant, endothelial-protective, and lipid-regulating actions. Mechanistically, they influence key signaling pathways such as PI3K/Akt and ROS/NF-κB, while also modulating telomerase activity and telomere maintenance, both increasingly recognized as central regulators of vascular senescence and plaque instability.
Despite these encouraging findings, the precise molecular targets of Astragalus membranaceus-derived compounds in vascular cells remain only partially understood. The reactivation of endogenous telomerase activity may represent a fundamental mechanism underlying their atheroprotective effects. However, the direct involvement of telomere–telomerase regulation in mediating these benefits remains speculative.
A promising avenue for future research is to determine whether shelterin-related complexes act as intermediaries or modulators of the effects of Astragalus membranaceus and its derivatives on telomerase function. The shelterin complex, a group of six telomere-binding proteins, TRF1, TRF2, POT1, TIN2, telomere protection protein 1 (TPP1) and RAP1, is essential for telomere protection and serves as a key negative regulator of telomerase access to chromosome ends [66]. A deeper understanding of the specific roles and regulatory mechanisms of each shelterin component could reveal how Astragalus compounds interact with telomere biology and identify novel molecular targets for the controlled activation of telomerase in vascular cells.
In addition, telomeric repeat-containing RNA (TERRA), a long non-coding RNA transcribed from telomeric regions, has emerged as a significant regulator of telomere integrity and telomerase activity [67]. Future studies should investigate whether Astragalus membranaceus influences TERRA expression or its interaction with telomerase, and how this may contribute to telomere homeostasis and vascular cell aging.
Moreover, it is becoming increasingly evident that telomerase exerts multiple non-canonical functions beyond telomere length maintenance, including roles in DNA damage response, transcriptional regulation, and mitochondrial function.
Notably, recent studies suggest that astragaloside IV also modulates mitochondrial biogenesis and mitophagy, particularly in VSMCs exposed to senescence-inducing stimuli such as angiotensin II. AS IV promotes Parkin-mediated mitophagy and supports mitochondrial homeostasis, factors now recognized as critical in the regulation of vascular aging [52]. Given the well-established bidirectional crosstalk between telomere dysfunction and mitochondrial decline, exploring how Astragalus-derived compounds affect the telomere–mitochondrion axis may provide novel mechanistic insights into their vasculoprotective properties.
Consequently, future studies should elucidate the impact of Astragalus membranaceus on telomerase-associated pathways to better understand its full therapeutic potential in the context of vascular aging.
To elucidate these relationships, future in vitro studies should investigate the effects of astragaloside IV, cycloastragenol, and TA-65 on telomerase activity, telomere length maintenance, mitochondrial dynamics, and senescence markers in both HUVECs and VSMCs, particularly under conditions of oxidative or inflammatory stress.
Although preliminary preclinical and clinical data are encouraging, comprehensive randomized controlled trials and long-term safety assessments are still required.
Future research should also aim to define dose–response relationships, explore potential synergistic effects with standard therapies, and validate robust biomarkers of vascular rejuvenation.
Additionally, given that these compounds act as telomerase activators and that telomerase reactivation is implicated in more than 80% of human tumors [68], the potential carcinogenic risk cannot be ruled out and should be rigorously assessed before considering clinical applications.
These efforts will be essential for establishing therapeutic efficacy, safety, and clinical utility of Astragalus-derived compounds in the prevention and treatment of atherosclerosis and other age-related vascular disorders.
Author Contributions
Conceptualization, M.G.A.; methodology, P.C.; resources, M.G.A.; writing—original draft preparation, P.C.; writing—review and editing, P.C. and M.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge co-funding from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8-Project Age-It: “Ageing Well in an Ageing Society”. The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
All the figures of this manuscript were created with biorender.com.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABCA1 | ATP-Binding Cassette Transporter A1 |
| Akt | Protein Kinase B pathway |
| AM | Astragalus membranaceus |
| APS | Astragalus Polysaccharide |
| AS IV | Astragaloside IV |
| ATP | Adenosine Triphosphate |
| CAG | Cycloastragenol |
| CDK2 | Cyclin-Dependent Kinase 2 |
| CLIC4 | Chloride Intracellular Channel 4 |
| COPD | Chronic Obstructive Pulmonary Disease |
| CREB | cAMP Response Element-Binding Protein |
| CXCR4 | CXC Chemokine Receptor 4 |
| DUSP5 | Dual Specificity Phosphatase 5 |
| eNOS | Endothelial Nitric Oxide Synthase |
| ERK | Regulated Protein Kinase |
| HCY | Homocysteine |
| HDAC9 | Histone Deacetylase 9 |
| HDL | High-Density Lipoprotein |
| HEK293 | Human Embryonic Kidney 293 Cells |
| HFD | High-Fat Diet |
| HIF-1α | Hypoxia-Inducible Factor 1-alpha |
| HO-1 | Heme Oxygenase-1 |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| IL-6 /IL-18/IL-1β | Interleukin-6/-18/-1 beta |
| iNOS | inducible Nitric Oxide Synthase |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| LDL | Low Density Lipoprotein |
| lncRNA H19 | Long non-coding RNA H19 |
| MAPK | Mitogen-Activated Protein Kinase |
| MEFs | Mouse Embryonic Fibroblasts |
| MEK | Mitogen-Activated Protein Kinase Kinase |
| MMP-9 | Matrix Metalloproteinase-9 |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NO | Nitric Oxide |
| NOX | NADPH Oxidase |
| NRF2 | Nuclear factor erythroid 2-Related Factor 2 |
| PI3K | Phosphoinositide 3-Kinase |
| POT1 | Telomere Protection 1 |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor gamma |
| PTEN | Phosphatase and Tensin Homolog |
| RAP1 | Repressor Activator Protein 1 |
| ROS | Reactive Oxygen Species |
| SASP | Senescence-Associated Secretory Phenotype |
| SDF-1 | Stromal cell-Derived Factor-1 |
| SOD | Superoxide Dismutase |
| Src | Proto-oncogene tyrosine-protein kinase Src |
| TA-65 | Telomerase Activator-65 |
| TERC | Telomerase RNA Component |
| TERRA | Telomeric Repeat-Containing RNA |
| TERT | Telomerase Reverse Transcriptase |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TIN2 | TRF1-Interacting Nuclear Protein 2 |
| TLR4 | Toll-Like Receptor 4 |
| TNF-α | Tumor Necrosis Factor-alpha |
| TPP1 | Telomere Protection Protein 1 |
| TRF1 | Telomere Repeat Binding Factors 1 |
| TRF2 | Telomere Repeat Binding Factors 1 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| VSMCs | Vascular Smooth Muscle Cells |
References
- Andreassi, M.G. DNA damage, vascular senescence and atherosclerosis. J. Mol. Med. 2008, 86, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Minamino, T.; Komuro, I. Role of telomeres in vascular senescence. Front. Biosci. 2008, 13, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 3, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, W.; Herrmann, M. The Importance of Telomere Shortening for Atherosclerosis and Mortality. J. Cardiovasc. Dev. Dis. 2020, 7, 29. [Google Scholar] [CrossRef]
- Yin, H.; Pickering, J.G. Telomere Length: Implications for Atherogenesis. Curr. Atheroscler. Rep. 2023, 25, 95–103. [Google Scholar] [CrossRef]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef]
- D’Mello, M.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between shortened leukocyte telomere length and cardiometabolic outcomes: Systematic review and meta-analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef]
- Emami, M.; Agbaedeng, T.A.; Thomas, G.; Middeldorp, M.E.; Thiyagarajah, A.; Wong, C.X.; Elliott, A.D.; Gallagher, C.; Hendriks, J.M.L.; Lau, D.H.; et al. Accelerated Biological Aging Secondary to Cardiometabolic Risk Factors Is a Predictor of Cardiovascular Mortality: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2022, 38, 365–375. [Google Scholar] [CrossRef]
- Li, C.; Stoma, S.; Lotta, L.A.; Warner, S.; Albrecht, E.; Allione, A.; Arp, P.P.; Broer, L.; Buxton, J.L.; Alves, A.D.S.C.; et al. Genome-wide Association Analysis in Humans Links Nucleotide Metabolism to Leukocyte Telomere Length. Am. J. Hum. Genet. 2020, 106, 389–404. [Google Scholar] [CrossRef]
- Deng, Y.; Li, Q.; Zhou, F.; Li, G.; Liu, J.; Lv, J.; Li, L.; Chang, D. Telomere length and the risk of cardiovascular diseases: A Mendelian randomization study. Front. Cardiovasc. Med. 2022, 9, 1012615. [Google Scholar] [CrossRef]
- Qin, S.; Sheng, Z.; Chen, C.; Cao, Y. Genetic relationship between ageing and coronary heart disease: A Mendelian randomization study. Eur. Geriatr. Med. 2024, 15, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.K.; Lin, M.H.; Wang, C.Y. Telomeres as Therapeutic Targets in Heart Disease. JACC Basic. Transl. Sci. 2019, 4, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Sagris, M.; Theofilis, P.; Antonopoulos, A.S.; Tsioufis, K.; Tousoulis, D. Telomere Length: A Cardiovascular Biomarker and a Novel Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 16010. [Google Scholar] [CrossRef]
- Liu, Y.X.; Song, X.; Dan, L.W.; Tang, J.M.; Jiang, Y.; Deng, C.; Zhang, D.D.; Li, Y.Z.; Wang, W. Astragali Radix: Comprehensive review of its botany, phytochemistry, pharmacology and clinical application. Arch. Pharm. Res. 2024, 47, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.Q.; Chen, H.W.; Li, J. Astragaloside IV: An Effective Drug for the Treatment of Cardiovascular Diseases. Drug. Des. Devel. Ther. 2020, 14, 3731–3746. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, H.; Zhang, Y.; Cheng, Z.; Wan, M.; Qin, W.; Li, P.; Feng, J.; Shao, S.; Xue, W.; et al. Recent pharmacological advances in the treatment of cardiovascular events with Astragaloside IV. Biomed. Pharmacother. 2023, 168, 115752. [Google Scholar] [CrossRef]
- Li, M.; Han, B.; Zhao, H.; Xu, C.; Xu, D.; Sieniawska, E.; Lin, X.; Kai, G. Biological active ingredients of Astragali Radix and its mechanisms in treating cardiovascular and cerebrovascular diseases. Phytomedicine 2022, 98, 153918. [Google Scholar] [CrossRef]
- Yang, C.; Pan, Q.; Ji, K.; Tian, Z.; Zhou, H.; Li, S.; Luo, C.; Li, J. Review on the protective mechanism of astragaloside IV against cardiovascular diseases. Front. Pharmacol. 2023, 14, 1187910. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Blasco, M.A. Telomere length, stem cells and aging. Nat. Chem. Biol. 2007, 3, 640–649. [Google Scholar] [CrossRef]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A review of recent research progress on the astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Li, C.; Lu, L.; Zhang, Q.; Zhu, R.; Wang, W. A Review of Chinese Herbal Medicine for the Treatment of Chronic Heart Failure. Curr. Pharm. Des. 2017, 23, 5115–5124. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Xiao, J.; Guo, Q.; Du, J.; Jiang, Z.; Lu, S.; Zhang, H.; Zhang, X.; Wang, X. Cycloastragenol and Astragaloside IV activate telomerase and protect nucleus pulposus cells against high glucose-induced senescence and apoptosis. Exp. Ther. Med. 2021, 22, 1326. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhou, L.; Yang, Y.; Liu, Y. Cycloastragenol: An exciting novel candidate for age-associated diseases. Exp. Ther. Med. 2018, 16, 2175–2182. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Y.; Wang, G.; Gu, W.; Zhao, S.; Hu, X.; Liu, W.; Cai, Y.; Ma, Z.; Gautam, R.K.; et al. Research progress of small-molecule drugs in targeting telomerase in human cancer and aging. Chem. Biol. Interact. 2023, 382, 110631. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Chen, Y.; Liang, C.L.; Liu, H.; Qiu, F.; Dai, Z. Antitumor effects of immunity-enhancing traditional Chinese medicine. Biomed. Pharmacother. 2020, 121, 109570. [Google Scholar] [CrossRef]
- Yung, L.Y.; Lam, W.S.; Ho, M.K.; Hu, Y.; Ip, F.C.; Pang, H.; Chin, A.C.; Harley, C.B.; Ip, N.Y.; Wong, Y.H. Astragaloside IV and cycloastragenol stimulate the phosphorylation of extracellular signal-regulated protein kinase in multiple cell types. Planta Med. 2012, 78, 115–121. [Google Scholar] [CrossRef]
- Ip, F.C.; Ng, Y.P.; An, H.J.; Dai, Y.; Pang, H.H.; Hu, Y.Q.; Chin, A.C.; Harley, C.B.; Wong, Y.H.; Ip, N.Y. Cycloastragenol is a potent telomerase activator in neuronal cells: Implications for depression management. Neurosignals 2014, 22, 52–63. [Google Scholar] [CrossRef]
- Hemann, M.T.; Strong, M.A.; Hao, L.Y.; Greider, C.W. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell 2001, 107, 67–77. [Google Scholar] [CrossRef]
- Bernardes de Jesus, B.; Schneeberger, K.; Vera, E.; Tejera, A.; Harley, C.B.; Blasco, M.A. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell 2011, 10, 604–621. [Google Scholar] [CrossRef]
- Le Saux, C.J.; Davy, P.; Brampton, C.; Ahuja, S.S.; Fauce, S.; Shivshankar, P.; Nguyen, H.; Ramaseshan, M.; Tressler, R.; Pirot, Z.; et al. A novel telomerase activator suppresses lung damage in a murine model of idiopathic pulmonary fibrosis. PLoS ONE 2013, 8, 58423. [Google Scholar] [CrossRef]
- Breitschopf, A.M.; Zeiher, S.; Dimmeler, S. Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett. 2001, 493, 21–25. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, H.; Luo, Y. Anti-aging implications of Astragalus membranaceus (Huangqi): A well-known Chinese tonic. Aging Dis. 2017, 8, 868. [Google Scholar] [CrossRef] [PubMed]
- Tiendrébéogo, A.J.F.; Soumagne, T.; Pellegrin, F.; Dagouassat, M.; Tran Van Nhieu, J.; Caramelle, P.; Paul, E.N.; Even, B.; Zysman, M.; Julé, Y.; et al. The telomerase activator TA-65 protects from cigarette smoke-induced small airway remodeling in mice through extra-telomeric effects. Sci. Rep. 2023, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.; Singaravelu, G.; Harley, C.B.; Flom, P.; Suram, A.; Raffaele, J.M. A Natural Product Telomerase Activator Lengthens Telomeres in Humans: A Randomized, Double Blind, and Placebo Controlled Study. Rejuvenation Res. 2016, 19, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, G.; Harley, C.B.; Raffaele, J.M.; Sudhakaran, P.; Suram, A. Double-blind, placebo-controlled, randomized clinical trial demonstrates telomerase activator TA-65 decreases immunosenescent CD8+ CD28− T cells in humans. OBM Geriatr. 2021, 5, 1–26. [Google Scholar] [CrossRef]
- De Jaeger, C.; Kruiskamp, S.; Voronska, E.; Lamberti, C.; Baramki, H.; Beaudeux, J.L.; Cherin, P. A Natural Astragalus-Based Nutritional Supplement Lengthens Telomeres in a Middle-Aged Population: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2024, 16, 2963. [Google Scholar] [CrossRef]
- Davignon, J.; Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation 2004, 109, III-27–III-32. [Google Scholar] [CrossRef]
- Leng, B.; Tang, F.; Lu, M.; Zhang, Z.; Wang, H.; Zhang, Y. Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Life Sci. 2018, 209, 111–121. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, X.; Feng, Q.; Chen, J.; Shen, L.; Yu, P.; Yang, L.; Chen, D.; Zhang, H.; Sun, W.; et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. 2019, 227, 82–93. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Lu, L.; Zhao, X.; Gao, X.; Wang, Y. Astragaloside IV stimulates angiogenesis and increases hypoxia-inducible factor-1α accumulation via phosphatidylinositol 3-kinase/Akt pathway. J. Pharmacol. Exp. Ther. 2011, 338, 485–491. [Google Scholar] [CrossRef]
- Wang, S.G.; Xu, Y.; Chen, J.D.; Yang, C.H. Astragaloside IV stimulates angiogenesis and increases nitric oxide accumulation via JAK2/STAT3 and ERK1/2 pathway. Molecules 2013, 18, 12809–12819. [Google Scholar] [CrossRef]
- Xu, C.; Tang, F.; Lu, M.; Yang, J.; Han, R.; Mei, M.; Hu, J.; Wang, H. Pretreatment with Astragaloside IV protects human umbilical vein endothelial cells from hydrogen peroxide induced oxidative stress and cell dysfunction via inhibiting eNOS uncoupling and NADPH oxidase–ROS–NF-κB pathway. Can. J. Physiol. Pharmacol. 2016, 94, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Li, J.; Zhang, X. Astragaloside IV protects against oxidized low- density lipoprotein (ox-LDL)-Induced endothelial cell injury by reducing oxidative stress and inflammation. Med. Sci. Monit. 2019, 25, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.H.; Xie, X.J.; Zhang, B.Q. Astragaloside IV improves homocysteine-induced acute phase endothelial dysfunction via antioxidation. Biol. Pharm. Bull. 2010, 33, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Liu, Z.; Liu, S.; Lin, N.; Deng, Y. Astragaloside IV alleviates atherosclerosis through targeting circ_0000231/miR-135a-5p/CLIC4 axis in AS cell model in vitro. Mol. Cell. Biochem. 2021, 476, 1783–1795. [Google Scholar] [CrossRef]
- Chen, D.; Du, Y.; Ye, S.; Yu, J. Astragaloside IV protects against oxidized low-density lipoprotein-induced injury in human umbilical vein endothelial cells via the histone deacetylase 9 (HDAC9)/NF-κB axis. Environ. Toxicol. 2023, 38, 534–544. [Google Scholar] [CrossRef]
- Doran, A.C.; Meller, N.; McNamara, C.A. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 812–819. [Google Scholar] [CrossRef]
- Zhang, Y.; Rong, H.; Zhang, F.X.; Wu, K.; Mu, L.; Meng, J.; Xiao, B.; Zamponi, G.W.; Shi, Y. A Membrane Potential- and Calpain-Dependent Reversal of Caspase-1 Inhibition Regulates Canonical NLRP3 Inflammasome. Cell Rep. 2018, 24, 2356–2369.e5. [Google Scholar] [CrossRef]
- De Boer, L.; Oakes, V.; Beamish, H.; Giles, N.; Stevens, F.; Somodevilla-Torres, M.; Desouza, C.; Gabrielli, B. Cyclin A/cdk2 coordinates centrosomal and nuclear mitotic events. Oncogene 2008, 27, 4261–4268. [Google Scholar] [CrossRef]
- Lu, Y.; Li, S.; Wu, H.; Bian, Z.; Xu, J.; Gu, C.; Chen, X.; Yang, D. Beneficial effects of astragaloside IV against angiotensin II-induced mitochondrial dysfunction in rat vascular smooth muscle cells. Int. J. Mol. Med. 2015, 36, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, J.; Zhang, Y.; Hong, L.; He, Z.; Zeng, Z.; Zhang, L. Astragaloside IV alleviates senescence of vascular smooth muscle cells through activating Parkin-mediated mitophagy. Hum. Cell 2022, 35, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wei, D.; Chen, Y.; Chen, L.; Bian, Y.; Shen, Y.; Chen, J.; Pan, Y. Association of astragaloside IV-inhibited autophagy and mineralization in vascular smooth muscle cells with lncRNA H19 and DUSP5-mediated ERK signaling. Toxicol. Appl. Pharmacol. 2019, 364, 45–54. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, X.; Ma, Z.; Niu, J.; Ma, S.; Wenjie, W.; Chen, J. Combination of tanshinone IIA and astragaloside IV attenuate atherosclerotic plaque vulnerability in ApoE−/− mice by activating PI3K/AKT signaling and suppressing TRL4/NF-κB signaling. Biomed. Pharmacother. 2020, 123, 109729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Du, M.; Wang, J.; Liu, P. Astragaloside IV Relieves Atherosclerosis and Hepatic Steatosis viaMAPK/NF-κB Signaling Pathway in LDLR-/- Mice. Front Pharmacol. 2022, 13, 828161. [Google Scholar] [CrossRef]
- Sun, B.; Rui, R.; Pan, H.; Zhang, L.; Wang, X. Effect of combined use of astragaloside IV (asIV) and atorvastatin (AV) on expression of PPAR-γ and inflammation-associated cytokines in atherosclerosis rats. Med. Sci. Monit. 2018, 24, 6229–6236. [Google Scholar] [CrossRef]
- Qin, H.; Liu, P.; Lin, S. Effects of astragaloside IV on the SDF-1/CXCR4 expression in atherosclerosis of ApoE−/− mice induced by hyperlipaemia. Evid. Based Complement. Altern. Med. 2015, 2015, 385154. [Google Scholar]
- Zheng, Q.; Zhu, J.Z.; Bao, X.Y.; Zhu, P.C.; Tong, Q.; Huang, Y.Y.; Zhang, Q.H.; Zhang, K.J.; Zheng, G.Q.; Wang, Y. A Preclinical Systematic Review and Meta-Analysis of Astragaloside IV for Myocardial Ischemia/Reperfusion Injury. Front Physiol. 2018, 9, 795. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, K.; Wu, S.; Liu, L.; Qiang, C.; Lin, X.; Liu, B. Astragalus polysaccharides lowers plasma cholesterol through mechanisms distinct from statins. PLoS ONE 2011, 6, e27437. [Google Scholar] [CrossRef]
- Mei, C.L.; Chen, Z.J.; Liao, Y.H.; Wang, Y.F.; Peng, H.Y.; Chen, Y. Interleukin-10 inhibits the down-regulation of ATP binding cassette transporter A1 by tumour necrosis factor-alpha in THP-1 macrophage-derived foam cells. Cell Biol. Int. 2007, 31, 1456–1461. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.F.; Yang, X.F.; Wang, Z.H.; Lian, Y.T.; Yang, Y.; Li, X.W.; Gao, X.; Chen, J.; Shu, Y.W.; et al. Specific Kv1.3 blockade modulates key cholesterol-metabolism-associated molecules in human macrophages exposed to ox-LDL. J. Lipid Res. 2013, 54, 34–43. [Google Scholar] [CrossRef]
- Li, S.Q.; Yuan, R.X.; Gao, H. Clinical observation on the treatment of ischemic heart disease with Astragalus membranaceus. Chin. J. Integr. Med. 1995, 15, 77–80. [Google Scholar]
- Lei, Z.Y.; Qin, H.; Liao, J.Z. Action of Astragalus membranaceus on left ventricular function of angina pectoris. Chin. J. Integr. Med. 1994, 14, 199–202. [Google Scholar]
- Fernandez, M.L.; Thomas, M.S.; Lemos, B.S.; DiMarco, D.M.; Missimer, A.; Melough, M.; Chun, O.K.; Murillo, A.G.; Alyousef, H.M.; Medina-Vera, I. TA-65, A Telomerase Activator improves Cardiovascular Markers in Patients with Metabolic Syndrome. Curr. Pharm. Des. 2018, 24, 1905–1911. [Google Scholar] [CrossRef] [PubMed]
- Bawamia, B.; Spray, L.; Wangsaputra, V.K.; Bennaceur, K.; Vahabi, S.; Stellos, K.; Kharatikoopaei, E.; Ogundimu, E.; Gale, C.P.; Keavney, B.; et al. Activation of telomerase by TA-65 enhances immunity and reduces inflammation post myocardial infarction. GeroScience 2023, 45, 2689–2705. [Google Scholar] [CrossRef]
- Mir, S.M.; Samavarchi Tehrani, S.; Goodarzi, G.; Jamalpoor, Z.; Asadi, J.; Khelghati, N.; Qujeq, D.; Maniati, M. Shelterin Complex at Telomeres: Implications in Ageing. Clin. Interv. Aging 2020, 15, 827–839. [Google Scholar] [CrossRef]
- Canale, P.; Campolo, J.; Borghini, A.; Andreassi, M.G. Long Telomeric Repeat-Containing RNA (TERRA): Biological Functions and Challenges in Vascular Aging and Disease. Biomedicines 2023, 11, 3211. [Google Scholar] [CrossRef]
- Aschacher, T.; Wolf, B.; Enzmann, F.; Kienzl, P.; Messner, B.; Sampl, S.; Svoboda, M.; Mechtcheriakova, D.; Holzmann, K.; Bergmann, M. LINE-1 induces hTERT and ensures telomere maintenance in tumour cell lines. Oncogene 2016, 35, 94–104. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).