Comparison of Immunomodulatory Therapies for Cardiovascular Clinical and Inflammatory Markers Outcomes in Mild to Moderately Ill Hospitalized Multisystem Inflammatory Syndrome in Children Patients
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CDC | Centers for Disease Control and Prevention |
| CRP | C Reactive Protein |
| CTSE | Council of State and Territorial Epidemiologists |
| GC | Glucocorticoids |
| IVIG | Intravenous immunoglobulins |
| LVEF | Left Ventricular Ejection Fraction |
| MIS-C | Multisystem inflammatory syndrome in children |
| NT-proBNP | N-Terminal pro Brain Natriuretic Peptide |
| PICU | Pediatric intensive care unit |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| VIS | Vasoactive-Inotrope Score |
References
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Wessels, P.A.; Bingler, M.A. A comparison of Kawasaki Disease and multisystem inflammatory syndrome in children. Prog. Pediatr. Cardiol. 2022, 65, 101516. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Porritt, R.A.; Cheng, M.H.; Bahar, I.; Arditi, M. COVID-19-associated multisystem inflamma-tory syndrome in children (MIS-C): A novel disease that mimics toxic shock syndrome-the superantigen hy-pothesis. J. Allergy Clin. Immunol. 2021, 147, 57–59. [Google Scholar] [CrossRef]
- Kumar, D.; Rostad, C.A.; Jaggi, P.; Nunez, D.S.V.; Prince, C.; Lu, A.; Hussaini, L.; Nguyen, T.H.; Malik, S.; Ponder, L.A.; et al. Distinguishing immune activation and inflammatory signatures of multisystem inflammatory syndrome in children (MIS-C) versus hemophagocytic lymphohistiocytosis (HLH). J. Allergy Clin. Immunol. 2022, 149, 1592–1606.e16. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Paediatrics and Child Health. Guidance: Paediatric Multisystem Inflammatory Syndrome Temporally Associated with COVID-19. Available online: https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19 (accessed on 15 April 2025).
- for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19 Summary and Recommendations 2020. Available online: https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 15 April 2025).
- World Health Organization. Multisystem Inflammatory Syndrome in Children and Adolescents with COVID-19. Available online: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 (accessed on 15 April 2025).
- Centers for Disease Control and Prevention. National Notifiable Diseases Surveillance System. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with SARS-CoV-2 Infection 2023 Case Definition. Available online: https://ndc.services.cdc.gov/case-definitions/multisystem-inflammatory-syndrome-in-children-mis-c-2023 (accessed on 15 April 2025).
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS–CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 1. Arthritis Rheumatol. 2020, 72, 1791–1805. [Google Scholar] [CrossRef]
- Harwood, R.; Allin, B.E.; Jones, C.; Whittaker, E.; Ramnarayan, P.; Ramanan, A.V.; Kaleem, M.; Tulloh, R.; Peters, M.J.; Almond, S.; et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): Results of a national Delphi process. Lancet Child Adolesc. Health 2021, 5, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hennon, T.R.; Penque, M.D.; Abdul-Aziz, R.; Alibrahim, O.S.; McGreevy, M.B.; Prout, A.J.; Schaefer, B.A.; Ambrusko, S.J.; Pastore, J.V.; Turkovich, S.J.; et al. COVID-19 associated Multisystem Inflammatory Syndrome in Children (MIS-C) guidelines; a Western New York approach. Prog. Pediatr. Cardiol. 2020, 57, 101232. [Google Scholar] [CrossRef] [PubMed]
- Ouldali, N.; Toubiana, J.; Antona, D.; Javouhey, E.; Madhi, F.; Lorrot, M.; Leger, P.-L.; Galeotti, C.; Claude, C.; Wiedemann, A.; et al. Association of intravenous im-munoglobulins plus methylprednisolone vs. immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 2021, 325, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Son, M.B.F.; Murray, N.; Friedman, K.; Young, C.C.; Newhams, M.M.; Feldstein, L.R.; Loftis, L.L.; Tarquinio, K.M.; Singh, A.R.; Heidemann, S.M.; et al. Multisystem inflamma-tory syndrome in children: Initial therapy and outcomes. N. Engl. J. Med. 2021, 385, 23–34. [Google Scholar] [CrossRef]
- Belhadjer, Z.; Auriau, J.; Méot, M.; Oualha, M.; Renolleau, S.; Houyel, L.; Bonnet, D. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation 2020, 142, 2282–2284. [Google Scholar] [CrossRef]
- McArdle, A.J.; Vito, O.; Patel, H.; Seaby, E.G.; Shah, P.; Wilson, C.; Broderick, C.; Nijman, R.; Tremoulet, A.H.; Munblit, D.; et al. Treatment of multisystem inflammatory syndrome in children. New Engl. J. Med. 2021, 385, 11–22. [Google Scholar] [CrossRef]
- Villacis-Nunez, D.S.; Jones, K.; Jabbar, A.; Fan, F.; Moore, W.; Peter, A.S.; Henderson, M.; Xiang, Y.; Kelleman, M.S.; Sherry, W.; et al. Short-term outcomes of corticosteroid monotherapy in multisystem inflammatory syn-drome in children. JAMA Pediatr. 2022, 176, 576–584. [Google Scholar] [CrossRef]
- Channon-Wells, S.; Vito, O.; McArdle, A.J.; Seaby, E.G.; Patel, H.; Shah, P.; Pazukhina, E.; Wilson, C.; Broderick, C.; D’SOuza, G.; et al. Immunoglobulin, glucocorticoid, or combination therapy for multisystem inflammatory syndrome in children: A propensity-weighted cohort study. Lancet Rheumatol. 2023, 5, e184–e199. [Google Scholar] [CrossRef]
- Ouldali, N.; Son, M.B.F.; McArdle, A.J.; Vito, O.; Vaugon, E.; Belot, A.; Leblanc, C.; Murray, N.L.; Patel, M.M.; Levin, M.; et al. Immunomodulatory therapy for MIS-C. Pediatrics 2023, 152, e2022061173. [Google Scholar] [CrossRef] [PubMed]
- Welzel, T.; Atkinson, A.; Schöbi, N.; Andre, M.C.; Bailey, D.G.N.; Blanchard-Rohner, G.; Buettcher, M.; Grazioli, S.; Koehler, H.; Perez, M.; et al. Methylprednisolone versus intravenous immunoglobulins in children with pae-diatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS): An open-label, multicentre, randomised trial. Lancet Child. Adolesc. Health. 2023, 7, 238–248. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group. Immunomodulatory therapy in children with paediatric inflammatory mul-tisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS, MIS-C; RECOVERY): A randomised, controlled, open-label, platform trial. Lancet Child Adolesc. Health 2024, 8, 190–200. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Kernan, K.F.; Schulert, G.S.; Seo, P.; et al. American College of Rheumatology clinical guidance for multisystem inflam-matory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: Version 3. Arthritis Rheumatol. 2022, 74, 1–20. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Multisystem Inflammatory Syndrome in Children (MIS-C) Interim Guidance. Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/clinical-guidance/multisystem-inflammatory-syndrome-in-children-mis-c-interim-guidance (accessed on 15 April 2025).
- World Health Organization. Living Guidance for Clinical Management of COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 15 April 2025).
- Whittaker, E.; Bamford, A.; Kenny, J.; Kaforou, M.; Jones, C.E.; Shah, P.; Ramnarayan, P.; Fraisse, A.; Miller, O.; Davies, P.; et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020, 324, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Jonat, B.; Gorelik, M.; Boneparth, A.; Geneslaw, A.S.; Zachariah, P.; Shah, A.; Broglie, L.; Duran, J.; Morel, K.D.; Zorrilla, M.; et al. Multisystem inflammatory syndrome in children associated with coronavirus disease 2019 in a Children’s Hospital in New York City: Patient characteristics and an institutional protocol for evaluation, management, and follow-up. Pediatr. Crit. Care Med. 2020, 22, e178–e191. [Google Scholar] [CrossRef]
- Vukomanovic, V.; Krasic, S.; Prijic, S.; Ninic, S.; Popovic, S.; Petrovic, G.; Ristic, S.; Simic, R.; Cerovic, I.; Nesic, D. Recent Experience: Corticosteroids as a First-line Therapy in Children With Multisystem Inflammatory Syndrome and COVID-19-related Myocardial Damage. Pediatr. Infect. Dis. J. 2021, 40, e390–e394. [Google Scholar] [CrossRef]
- Sütçü, M.; Kara, E.M.; Yıldız, F.; Gül, D.; Yıldız, R.; Yılmaz, D.; Atik, F.; Özkaya, O. MIS-C Treatment: Is gluco-corticoid monotherapy enough for mild cases? Am. J. Emerg. Med. 2024, 83, 95–100. [Google Scholar] [CrossRef]
- Kaushik, S.; Aydin, S.I.; Derespina, K.R.; Bansal, P.B.; Kowalsky, S.; Trachtman, R.; Gillen, J.K.; Perez, M.M.; Soshnick, S.H.; Conway, E.E.; et al. Multisystem Inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection (MIS-C): A multi-institutional study from New York City. J. Pediatr. 2020, 224, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Levy, N.; Koppel, J.H.; Kaplan, O.; Yechiam, H.; Shahar-Nissan, K.; Cohen, N.K.; Shavit, I. Severity and inci-dence of Multisystem Inflammatory Syndrome in children during 3 SARS-CoV-2 pandemic waves in Israel. JAMA 2022, 327, 2452–2454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Almeida, F.J.; Baillie, J.K.; Bowen, A.C.; Britton, P.N.; Brizuela, M.E.; Buonsenso, D.; Burgner, D.; Chew, K.Y.; Chokephaibulkit, K.; et al. International pediatric COVID-19 severity over the course of the pandemic. JAMA Pediatr. 2023, 177, 1073–1084. [Google Scholar] [CrossRef]
- Radia, T.; Williams, N.; Agrawal, P.; Harman, K.; Weale, J.; Cook, J.; Gupta, A. Multi-system inflammatory syndrome in children & adolescents (MIS-C): A systematic review of clinical features and presentation. Paediatr. Respir. Rev. 2021, 38, 51–57. [Google Scholar]
- Jonat, B.; Geneslaw, A.S.; Capone, C.A.; Shah, S.; Bartucca, L.; Sewell, T.B.; Acker, K.P.; Mitchell, E.; Cheung, E.W. Early treatment of multisystem inflammatory syndrome in children. Pediatrics 2023, 152. [Google Scholar] [CrossRef] [PubMed]
- Tonge, J.J.; Stevens, O.; Dawson, J.; Hawley, D.; Kerrison, C.; Krone, N.; Maltby, S.L.; McMahon, A.-M.; Shackley, F.; Talekar, R.; et al. Assessing the response of biomarkers to anti-inflammatory medications in PIMS-TS by longitudinal multilevel modeling: Real-world data from a UK Tertiary Center. Pediatr. Allergy, Immunol. Pulmonol. 2023, 36, 94–103. [Google Scholar] [CrossRef]
- McIntosh, A.M.; Tong, S.; Deakyne, S.J.; Davidson, J.A.; Scott, H.F. Validation of the vasoactive-inotropic score in pediatric sepsis*. Pediatr. Crit. Care Med. 2017, 18, 750–757. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Tagarro, A.; Domínguez-Rodríguez, S.; Mesa, J.M.; Epalza, C.; Grasa, C.; Iglesias-Bouzas, M.I.; Fernán-dez-Cooke, E.; Calvo, C.; Villaverde, S.; Torres-Fernández, D.; et al. Treatments for multi-system inflammatory syndrome in children - discharge, fever, and second-line therapies. Eur. J. Pediatr. 2023, 182, 461–466. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Lopez, L.; Colan, S.D.; Stylianou, M.; Granger, D.; Trachtenberg, F.; Frommelt, P.; Pearson, G.; Camarda, J.; Cnota, J.; Cohen, M.; et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: The Pediatric Heart Network Normal Echocardiogram Database. Circ. Cardiovasc. Imaging 2017, 10, e006979. [Google Scholar] [CrossRef] [PubMed]
- Caorsi, R.; Consolaro, A.; Speziani, C.; Sozeri, B.; Ulu, K.; Faugier-Fuentes, E.; Menchaca-Aguayo, H.; Ozen, S.; Sener, S.; Akhter Rahman, S.; et al. The HyperPed-COVID international registry: Impact of age of onset, disease presentation and geographical distri-bution on the final outcome of MIS-C. J. Autoimmun. 2024, 147, 103265. [Google Scholar] [CrossRef] [PubMed]
- Lampidi, S.; Maritsi, D.; Charakida, M.; Eleftheriou, I.; Farmaki, E.; Spyridis, N.; Charisi, K.; Vantsi, P.; Filippatos, F.; Skourti, K.; et al. Multisystem inflammatory syndrome in children (MIS-C): A nationwide collaborative study in the Greek population. Eur. J. Pediatr. 2024, 183, 1693–1702. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Lee, E.H.; Miller, A.D.; Lim, S.; Brown, C.M.; Yousaf, A.R.; Zambrano, L.D.; Belay, E.D.; God-fred-Cato, S.; Abrams, J.Y.; et al. Council of State and Territorial Epidemiologists/CDC Surveillance Case Defi-nition for Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection—United States. MMWR Recomm. Rep. 2022, 71, 1–14. [Google Scholar] [CrossRef]
- Ramcharan, T.; Nolan, O.; Lai, C.Y.; Prabhu, N.; Krishnamurthy, R.; Richter, A.G.; Jyothish, D.; Kanthimathi-nathan, H.K.; Welch, S.B.; Hackett, S.; et al. Paediatric inflammatory multisystem syndrome: Temporally associated with SARS-CoV-2 (PIMS-TS): Cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr. Cardiol. 2020, 41, 1391–1401. [Google Scholar] [CrossRef]
- Abbas, Q.; Ali, H.; Amjad, F.; Hussain, M.Z.H.; Rahman, A.R.; Khan, M.H.A.; Padhani, Z.; Abbas, F.; Imam, D.; Alikhan, Z.; et al. Clinical presentation, diagnosis and management of multisystem inflammatory syndrome in children (MIS-C): A systematic review. BMJ Paediatr. Open 2024, 8, e002344. [Google Scholar] [CrossRef]
- Tong, T.; Jin, Y.-H.; Wang, M.; Gong, F.-Q. Treatment of multisystem inflammatory syndrome in children. World J. Pediatr. 2024, 20, 325–339. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef]
- Kavurt, A.V.; Bağrul, D.; Gül, A.E.K.; Özdemiroğlu, N.; Ece, I.; Çetin, I.I.; Özcan, S.; Uyar, E.; Emeksiz, S.; Çelikel, E.; et al. Echocardiographic findings and correlation with laboratory values in multisystem inflammatory syndrome in children (MIS-C) Associated with COVID-19. Pediatr. Cardiol. 2021, 43, 413–425. [Google Scholar] [CrossRef]
- Butters, C.; Benede, N.; Moyo-Gwete, T.; Richardson, S.I.; Rohlwink, U.; Shey, M.; Ayres, F.; Manamela, N.P.; Makhado, Z.; Balla, S.R.; et al. Comparing the immune abnormalities in MIS-C to healthy children and those with inflammatory disease reveals distinct inflammatory cytokine production and a monofunctional T cell response. Clin. Immunol. 2023, 259, 109877. [Google Scholar] [CrossRef]
- Rajamanickam, A.; Kumar, N.P.; Venkataraman, A.; Varadarjan, P.; Selladurai, E.; Sankaralingam, T.; Thiruvengadam, K.; Selvam, R.; Thimmaiah, A.; Natarajan, S.; et al. Sex-specific differences in systemic immune responses in MIS-C children. Sci. Rep. 2024, 14, 1720. [Google Scholar] [CrossRef]
- Schmitz, A.; Wood, K.E.; Badheka, A.; Burghardt, E.; Wendt, L.; Sharathkumar, A.; Koestner, B. NT-proBNP lev-els following IVIG treatment of multisystem inflammatory syndrome in children. Hosp. Pediatr. 2022, 12, e261–e265. [Google Scholar] [CrossRef]
- Visa-Reñé, N.; Rubio-Páez, A.; Mitjans-Rubies, N.; Paredes-Carmona, F. Comparison of plasma inflammatory biomarkers between MIS-C and potentially serious infections in pediatric patients. Reumatol. Clin. (Engl. Ed.) 2024, 20, 84–91. [Google Scholar] [CrossRef]
- Harthan, A.A.; Nadiger, M.; McGarvey, J.S.; Hanson, K.; Gharpure, V.P.; Bjornstad, E.C.; Chiotos, K.; Miller, A.S.; Reikoff, R.A.; Gajic, O.; et al. Early combination therapy with im-munoglobulin and steroids is associated with shorter ICU length of stay in Multisystem Inflammatory Syn-drome in Children (MIS-C) associated with COVID-19: A retrospective cohort analysis from 28 U.S. Hospitals. Pharmacotherapy 2022, 42, 529–539. [Google Scholar] [CrossRef]
- Cole, L.D.; Osborne, C.M.; Silveira, L.J.; Rao, S.; Lockwood, J.M.; Kunkel, M.J.; MacBrayne, C.E.; Heizer, H.R.; Anderson, M.S.; Jone, P.N.; et al. IVIG compared with IVIG plus infliximab in Multisystem Inflammatory Syn-drome in Children. Pediatrics. 2021, 148, e2021052702. [Google Scholar] [CrossRef]
- Stasiak, A.; Perdas, E.; Smolewska, E. Risk factors of a severe course of pediatric multi-system inflammatory syndrome temporally associated with COVID-19. Eur. J. Pediatr. 2022, 181, 3733–3738. [Google Scholar] [CrossRef]
- Stasiak, A.; Kędziora, P.; Kierzkowska, B.; Niewiadomska-Jarosik, K.; Perdas, E.; Smolewska, E. Changes in the cardiovascular system in children with pediatric multisystem inflammatory syndrome temporally associated with COVID-19—A single center experience. Int. J. Cardiol. 2022, 361, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh Esslami, G.; Mamishi, S.; Pourakbari, B.; Mahmoudi, S. Systematic review and meta-analysis on the serological, immunological, and cardiac parameters of the multisystem inflammatory syndrome (MIS-C) associated with SARS-CoV-2 infection. J. Med. Virol. 2023, 95, e28927. [Google Scholar] [CrossRef] [PubMed]
- Pandit, M.; Frishman, W.H. Multisystem inflammatory syndrome in children during the COVID-19 pandemic: A review of clinical manifestations, Cardiac Complications and Medical Management. Cardiol. Rev. 2024. [Google Scholar] [CrossRef] [PubMed]
- Shioji, N.; Sumie, M.; Englesakis, M.; Gilfoyle, E.; Maynes, J.T.; Aoyama, K. Multisystem inflammatory syndrome in children: An Umbrella review. J. Anesthesia 2024, 38, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yin, L.; Patel, J.; Tang, L.; Huang, Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J. Med Virol. 2021, 93, 4358–4369. [Google Scholar] [CrossRef]
- Sugunan, S.; Bindusha, S.; Geetha, S.; Niyas, H.R.; Kumar, A.S. Clinical profile and short-term outcome of chil-dren with SARS-CoV-2 related Multisystem Inflammatory Syndrome (MIS-C) treated with pulse methylpred-nisolone. Indian Pediatr. 2021, 58, 718–722. [Google Scholar] [CrossRef]
- Gruber, C.N.; Patel, R.S.; Trachtman, R.; Lepow, L.; Amanat, F.; Krammer, F.; Wilson, K.M.; Onel, K.; Geanon, D.; Tuballes, K.; et al. Mapping Systemic Inflammation and Antibody Responses in Multisystem Inflammatory Syndrome in Children (MIS-C). Cell 2020, 183, 982–995.e14. [Google Scholar] [CrossRef]
- Moshage, H.J.A.; Janssen, J.; Franssen, J.H.; Hafkenscheid, J.C.; Yap, S.H. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J. Clin. Investig. 1987, 79, 1635–1641. [Google Scholar] [CrossRef]
- Pathak, A.; Agrawal, A. Evolution of C-Reactive Protein. Front. Immunol. 2019, 10, 943. [Google Scholar] [CrossRef]
- Kerr, R.; Stirling, D.; Ludlam, C.A. Interleukin-6 and hemostasis. Br. J. Haematol. 2001, 115, 3–12. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Pilania, R.K.; Karkhele, R.; Kumar, T.S.; Danda, D.; Singh, S. Severe COVID-19, multisystem in-flammatory syndrome in children, and Kawasaki disease: Immunological mechanisms, clinical manifestations and management. Rheumatol. Int. 2021, 41, 19–32. [Google Scholar] [CrossRef]
- Oragui, C.C. Cardiovascular manifestations of multisystem Inflammatory Syndrome in Children (MIS-C) associated with COVID-19. Cureus 2023, 15, e41950. [Google Scholar] [CrossRef] [PubMed]
- Davies, P.; Evans, C.; Kanthimathinathan, H.K.; Lillie, J.; Brierley, J.; Waters, G.; Johnson, M.; Griffiths, B.; du Pré, P.; Mohammad, Z.; et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: A multicentre observational study. Lancet Child Adolesc. Health 2020, 4, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Bichali, S.; Ouldali, N.; Godart, F.; Maboudou, P.; Houeijeh, A.; Leteurtre, S. NT-proBNP course during MIS-C post-COVID-19: An observational study. Eur. J. Pediatr. 2024, 183, 1667–1674. [Google Scholar] [CrossRef]
- Koteda, Y.; Suda, K.; Kishimoto, S.; Ito, S.; Kudo, Y.; Nishino, H.; Ishii, H.; Iemura, M.; Matuishi, T. Impact of intravenous immunoglobulin infusion on longitudinal left ventricular performance in patients with acute Kawasaki disease of usual course. J. Cardiol. 2009, 54, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Raynor, A.; Vallée, C.; Belkarfa, A.-L.; Lunte, K.; Laney, M.; Belhadjer, Z.; Vicca, S.; Boutten, A.; Bonnet, D.; Nivet-Antoine, V. Multisystem inflammatory syndrome in children: Inputs of BNP, NT-proBNP and Galectin-3. Clin. Chim. Acta 2022, 529, 109–113. [Google Scholar] [CrossRef]
- Beaver, M.; Jepson, B.; Binka, E.; Truong, D.; Crandall, H.; McFarland, C.; Williams, R.; Ou, Z.; Treemarcki, E.; Jensen, D.; et al. Baseline echocardiography and laboratory findings in MIS-C and associations with clinical illness severity. Pediatr. Cardiol. 2024, 45, 560–569. [Google Scholar] [CrossRef]
- Schlapbach, L.J.; Andre, M.C.; Grazioli, S.; Schöbi, N.; Ritz, N.; Aebi, C.; Agyeman, P.; Albisetti, M.; Bailey, D.G.N.; Berger, C.; et al. Best practice recommendations for the diagnosis and management of children with Pediatric Inflammatory Multisystem Syndrome temporally associated with SARS-CoV-2 (PIMS-TS.; Multisystem Inflammatory Syn-drome in Children, MIS-C) in Switzerland. Front. Pediatr. 2021, 9, 667507. [Google Scholar] [CrossRef]
- Anderson, E.M.; Diorio, C.; Goodwin, E.C.; McNerney, K.O.; Weirick, M.E.; Gouma, S.; Bolton, M.J.; Arevalo, C.P.; Chase, J.; Hicks, P.; et al. Severe acute respiratory syndrome-Coronavirus-2 (SARS-CoV-2) antibody re-sponses in children With Multisystem Inflammatory Syndrome in Children (MIS-C) and mild and severe coro-navirus disease 2019 (COVID-19). J. Pediatric Infect. Dis. Soc. 2021, 10, 669–673. [Google Scholar] [CrossRef]
- Esteve-Sole, A.; Anton, J.; Pino-Ramirez, R.M.; Sanchez-Manubens, J.; Fumadó, V.; Fortuny, C.; Rios-Barnes, M.; Sanchez-De-Toledo, J.; Girona-Alarcón, M.; Mosquera, J.M.; et al. Similarities and differences between the immunopathogenesis of COVID-19–related pediatric multisystem inflammatory syndrome and Kawasaki disease. J. Clin. Investig. 2021, 131, e144554. [Google Scholar] [CrossRef]
- Vella, L.A.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Diorio, C.; Kuri-Cervantes, L.; Alanio, C.; Pampena, M.B.; Wu, J.E.; Chen, Z.; et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared with adult and pediatric COVID-19. Sci. Immunol. 2021, 6, eabf7570. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Fish, M.; Jennings, A.; Doores, K.J.; Wellman, P.; Seow, J.; Acors, S.; Graham, C.; Timms, E.; Kenny, J.; et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat. Med. 2020, 26, 1701–1707. [Google Scholar] [CrossRef]
- Consiglio, C.R.; Cotugno, N.; Sardh, F.; Pou, C.; Amodio, D.; Rodriguez, L.; Tan, Z.; Zicari, S.; Ruggiero, A.; Pascucci, G.R.; et al. The Immunology of Multisystem Inflammatory Syndrome in Children with COVID-19. Cell. 2020, 183, 968–981.e97. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.Y.; Day-Lewis, M.; Henderson, L.A.; Friedman, K.G.; Lo, J.; Roberts, J.E.; Lo, M.S.; Platt, C.D.; Chou, J.; Hoyt, K.J.; et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflamma-tory syndrome in children. J. Clin. Invest. 2020, 130, 5942–5950. [Google Scholar] [CrossRef]
- Benvenuto, S.; Avcin, T.; Taddio, A. Multisystem inflammatory syndrome in children: A review. Acta Paediatr. 2024, 113, 2011–2023. [Google Scholar] [CrossRef]
- Sinha, A.; Bagga, A. Pulse steroid therapy. Indian J. Pediatr. 2008, 75, 1057–1066. [Google Scholar] [CrossRef]
- Stahn, C.; Buttgereit, F. Genomic and nongenomic effects of glucocorticoids. Nat. Clin. Pr. Rheumatol. 2008, 4, 525–533. [Google Scholar] [CrossRef]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Yao, X.; Lin, Z.; Tao, Y.; Xu, J.; Xu, X.; Fang, Z.; Geng, Z.; Fu, S.; Wang, W.; et al. Similarities and differences between MIS-C and KD: A systematic review and meta-analysis. Pediatr. Rheumatol. 2022, 20, 112. [Google Scholar] [CrossRef]
- Ferrara, G.; Petrillo, M.G.; Giani, T.; Marrani, E.; Filippeschi, C.; Oranges, T.; Simonini, G.; Cimaz, R. Clinical Use and Molecular Action of Corticosteroids in the Pediatric Age. Int. J. Mol. Sci. 2019, 20, 444. [Google Scholar] [CrossRef] [PubMed]
- Kazatchkine, M.D.; Kaveri, S.V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N. Engl. J. Med. 2001, 345, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Ballow, M. The IgG molecule as a biological immune response modifier: Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J. Allergy Clin. Immunol. 2011, 127, 315–323. [Google Scholar] [CrossRef]
- Norris, P.A.; Kaur, G.; Lazarus, A.H. New insights into IVIg mechanisms and alternatives in autoimmune and inflammatory diseases. Curr. Opin. Hematol. 2020, 27, 392–398. [Google Scholar] [CrossRef]
- Dove, M.L.; Jaggi, P.; Kelleman, M.; Abuali, M.; Ang, J.Y.; Ballan, W.; Basu, S.K.; Campbell, M.J.; Chikkabyrappa, S.M.; Choueiter, N.F.; et al. Multisystem Inflammatory Syndrome in children: Survey of protocols for early hospital evaluation and management. J. Pediatr. 2021, 229, 33–40. [Google Scholar] [CrossRef]
- Moraleda, C.; Serna-Pascual, M.; Soriano-Arandes, A.; Simó, S.; Epalza, C.; Santos, M.; Grasa, C.; Rodríguez, M.; Soto, B.; Gallego, N.; et al. Multi-inflammatory Syndrome in children related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Spain. Clin. Infect. Dis. 2020, 72, e397–e401. [Google Scholar] [CrossRef]
- Diaz, F.B.; Yagnam, F.; Karsies, T.J.; Vásquez-Hoyos, P.; Jaramillo-Bustamante, J.-C.; Gonzalez-Dambrauskas, S.; Drago, M.; Cruces, P. Comparison of Interleukin-6 plasma concentration in Multisystem Inflammatory Syndrome in children associated With SARS-CoV-2 and Pediatric Sepsis. Front. Pediatr. 2021, 9, 756083. [Google Scholar] [CrossRef]
- Kumar, N.P.; Venkataraman, A.; Nancy, A.; Selvaraj, N.; Moideen, K.; Ahamed, S.F.; Renji, R.M.; Sasidaran, K.; Kumar, S.; Periyakuppan, M.; et al. Immune profiles in Multisystem Inflammatory Syndrome in Children with cardiovascular abnormalities. Viruses 2023, 15, 2162. [Google Scholar] [CrossRef]
- Bartha-Tatár, A.; Sinkovits, G.; Schnur, J.; Maráczi, V.; Dávid, M.; Zsigmond, B.; Rimanóczy, É.; Szalay, B.; Biró, E.; Bekő, G.; et al. Prognostic Value of baseline serum pro-inflammatory cytokines in severe Multisystem Inflammatory Syndrome in children. J. Clin. Med. 2024, 13, 7177. [Google Scholar] [CrossRef]
- Day-Lewis, M.; Berbert, L.; Baker, A.; Dionne, A.; Newburger, J.W.; Son, M.B.F. Updated case definition of MIS-C and implications for clinical care. Pediatrics 2024, 153, e2023063259. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Friedman, K.G.; Gorelik, M.; Lapidus, S.K.; Bassiri, H.; Behrens, E.M.; Ferris, A.; Kernan, K.F.; Schulert, G.S.; et al. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in children associated with SARS–CoV-2 and hyperinflammation in pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021, 73, e13–e29. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020, 395, 1607–1608. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriou, I.; Maritsi, D.; Lampidi, S.; Charisi, K.; Vantsi, P.; Skourti, K.; Filippatos, F.; Amplianitis, I.; Dimou, D.; Papadopoulou-Legbelou, K.; et al. Decreasing incidence of the Multisystem Inflammatory Syndrome in children over 3 pandemic waves. Pediatr. Infect. Dis. J. 2022, 42, 122–124. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All Patients (n = 39) | IVIG Treatment (n = 17) | IVIG and Corticosteroid Treatment (n = 22) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 7.6 ± 4.6 | 7.9 ± 4.6 | 7.3 ± 4.7 | 0.70 * |
| Weight (kilograms) | 30 [18, 52] | 32 [17, 52] | 29 [21, 46] | 0.98 † |
| Gender: Male | 25 (64) | 12 (71) | 13 (59) | 0.46 ‡ |
| Race and ethnicity | 0.84 § | |||
| Asian | 3 (7.7) | 1 (5.9) | 2 (9.1) | |
| Black | 13 (33) | 7 (41) | 6 (27) | |
| Hispanic or Latino | 4 (10) | 2 (12) | 2 (9.1) | |
| White | 19 (49) | 7 (41) | 12 (55) | |
| Presence of comorbidities | 0.61 § | |||
| Obesity | 15 (38) | 7 (41) | 8 (36) | 0.76 ‡ |
| Chronic medical illness a | 13 (33) | 4 (24) | 9 (41) | 0.25 ‡ |
| Presence of SARS-CoV-2 infection | 17 (100) | 22 (100) | ||
| Positive SARS-CoV-2 NAAT or antigen | 4 (10) | 1 (6) | 3 (17) | 0.79 § |
| Positive SARS-CoV-2 antibody | 10 (26) | 3 (18) | 7 (32) | 0.52 § |
| Exposure to suspected or confirmed COVID-19 case | 18 (46) | 13 (76) | 15 (68) | 0.83 § |
| Organ system involvement per 2020 CDC MIS-C definition [6] | ||||
| Cardiac | 32 (82) | 11 (65) | 21 (95) | 0.04 § |
| Hematologic | 27 (69) | 11 (65) | 16 (73) | 0.59 § |
| Gastrointestinal | 37 (95) | 17 (100) | 20 (91) | 0.59 § |
| Mucocutaneous | 30 (77) | 12 (71) | 18 (82) | 0.46 § |
| Neurologic | 14 (36) | 5 (29) | 9 (41) | 0.46 § |
| Musculoskeletal | 8 (21) | 3 (18) | 5 (23) | 0.99 § |
| Renal | 1 (2.6) | 0 (0) | 1 (4.5) | 0.99 § |
| Respiratory | 9 (23) | 1 (5.9) | 8 (36) | 0.052 § |
| Number of organ systems involved | 4 [3, 5] | 4 [3, 4] | 4 [4, 5] | 0.025 † |
| Multisystem involvement per 2023 CSTE/CDC MIS-C surveillance definition [8] | ||||
| Cardiac | 21 (54) | 7 (41) | 14 (64) | 0.28 § |
| Hematologic | 34 (87) | 15/17 (88) | 19/22 (90) | 0.99 § |
| Gastrointestinal | 37 (95) | 17 (100) | 20 (91) | 0.59 § |
| Mucocutaneous | 30 (78) | 12 (71) | 18 (82) | 0.46 § |
| Number of organ systems involved | 3 [2, 4] | 3 [2, 4] | 3 [2, 4] | 0.28 § |
| Shock | 10 (26) | 2 (12) | 8 (36) | 0.16 ‡ |
| Severity of illness | 0.23 ‡ | |||
| Mild | 21 (54) | 11 (65) | 10 (45) | |
| Moderate | 18 (46) | 6 (35) | 12 (55) |
| Characteristic | All Patients (n = 39) | IVIG Treatment (n = 17) | IVIG and Corticosteroid Treatment (n = 22) | p Value |
|---|---|---|---|---|
| Clinical features | ||||
| Duration of illness (days) | 5.0 [4.0, 6.0] | 6.0 [5.0, 6.0] | 5.0 [4.0, 5.0] | 0.17 † |
| Duration of fever (days) | 5.0 [4.0, 6.0] | 5.0 [4.0, 6.0] | 5.0 [4.0, 5.0] | 0.59 † |
| Clinical symptoms | ||||
| Presence of fever | 39 (100) | 17 (100) | 22 (100) | |
| Clinical signs | ||||
| Heart rate (rate/min) | 129 [103, 139] | 116 [101, 139] | 137 [115, 137] | 0.15 † |
| Tachycardic patients | 38 (97) | 16 (94) | 22 (100) | 0.44 § |
| Mean arterial blood pressure (mmHg) | 65 [54, 72] | 67 [57, 72] | 61 [54, 73] | 0.81 † |
| Hypotension | 6 (15) | 2 (12) | 4 (18) | 0.91 ‡ |
| Shock | 10 (26) | 2 (12) | 8 (36) | 0.16 ‡ |
| Clinical symptoms and findings per 2023 CSTE/CDC MIS-C surveillance definition [8] | ||||
| Cardiac | 21 (54) | 7 (41) | 14 (64) | 0.28 § |
| LVEF < 55% in patients | 15 (38) | 5 (29) | 10 (45) | 0.30 § |
| Coronary artery dilatation | 3 (8) | 1 (6.7) | 2 (9.1) | 0.99 § |
| Elevated troponin levels | 5 (13) | 1 (6.7) | 4 (18) | 0.43 § |
| Gastrointestinal | 37 (95) | 17 (100) | 20 (91) | 0.59 § |
| Abdominal pain | 33 (85) | 14 (82) | 19 (86) | 0.92 § |
| Vomiting | 34 (87) | 16 (94) | 18 (81) | 0.51 § |
| Diarrhea | 22 (56) | 7 (41) | 15 (68) | 0.17 § |
| Hematological | 34 (87) | 15/17 (88) | 19/22 (90) | 0.99 § |
| Absolute Lymphocyte count < 1000/mcL | 34 (87) | 15/17 (88) | 19/22 (90) | 0.99 § |
| Platelet count < 150,000/mcL | 7 (18) | 2/17 (12) | 5/22 (23) | 0.38 § |
| Mucocutaneous | 30 (77) | 12 (71) | 18 (82) | 0.46 § |
| Skin rash | 22 (56) | 10 (59) | 12 (57) | 0.92 § |
| Inflammation of oral mucosa | 18 (46) | 8 (47) | 10 (45) | 0.82 § |
| Conjunctivitis | 18 (46) | 9 (53) | 9 (41) | 0.45 § |
| Edema/peeling of peripheral extremities | 13 (33) | 4 (24) | 9 (41) | 0.42 § |
| Characteristic | All Patients (n = 39) | IVIG Treatment (n = 17) | IVIG and Corticosteroid Treatment (n = 22) | p Value |
|---|---|---|---|---|
| Inflammatory markers | ||||
| Number of inflammatory markers tested | 8 [7, 9] | 8 [7.7, 8] | 8 [7, 9] | 0.98 † |
| Number of positive inflammatory markers | 6 [5, 8] | 5 [4, 7.5] | 7 [5, 8] | 0.23 † |
| Albumin level ≤ 3 g/dL †† | 24/39 (61) | 11/17 (65) | 13/22 (59) | 0.72 ‡ |
| Albumin level (g/dL) | 2.8 [2.3, 3.2] | 2.8 [2.65, 3.1] | 2.8 [2.2, 3.2] | 0.58 † |
| C-reactive protein levels ≥ 3/dL †† | 39/39 (100) | 17/17 (100) | 22/22 (100) | |
| C-reactive protein levels (mg/dL) | 13.5 [10.2, 19.7] | 13.5 [8.05, 11.1] | 14.2 [10.6, 21.5] | 0.39 † |
| D-dimer levels > 3000 ng/mL †† | 25/39 (64) | 8/17 (47) | 17/22 (77) | 0.051 ‡ |
| D-dimer levels (ng/mL) | 4110 [2050, 7390] | 2700 [2050, 5270] | 4505 [3570, 7390] | 0.32 † |
| Ferritin levels > 500 ng/mL †† | 22/38 (58) | 8/17 (47) | 14/21 (67) | 0.22 ‡ |
| Ferritin levels (ng/mL) | 569 [318, 1018] | 494 [228, 663] | 576 [433, 1050] | 0.11 † |
| Fibrinogen levels > 400 mg/dL †† | 26/30 (87) | 11/13 (85) | 15/17 (88) | 0.99 § |
| Fibrinogen levels (mg/dL) | 552 [450, 638] | 577 [459, 744] | 486 [430, 609] | 0.17 * |
| Interleukin-6 levels > 7 pg/mL †† | 23/23 (100) | 11/11 (100) | 12/12 (100) | |
| Interleukin-6 levels (pg/mL) | 173 [42, 411] | 57 [31, 318] | 196 [139, 675] | 0.074 † |
| Lymphocyte count < 1 × 109/L †† | 34/39 (87) | 15/17 (88) | 19/22 (90) | 0.99 § |
| Lymphocyte count (×109/L) | 0.84 [0.63, 1.06] | 0.89 [0.68, 1.06] | 0.74 [0.60, 0.96] | 0.36 † |
| Neutrophil count > 7.7 × 109/L †† | 28/39 (72) | 10/17 (59) | 18/22 (82) | 0.16 § |
| Neutrophil count (×109/L) | 10.16 [7.32, 14.29] | 9.54 [4.4, 11.29] | 10.63 [9.21, 15.48] | 0.046 † |
| NT-proBNP level > 400 pg/mL | 34/38 (89) | 12/16 (75) | 22/22 (100) | 0.024 § |
| NT-proBNP levels (pg/mL) | 2102 [760, 5762] | 792 [437, 6132] | 2986 [1084, 5741] | 0.047 † |
| Echocardiographic results | ||||
| LVEF < 55% in patients | 15 (38) | 5 (29) | 10 (45) | 0.30 ‡ |
| LVEF on admission | 57 [49, 64] | 63 [49, 66] | 58.5 [49, 61] | 0.13 † |
| Coronary artery aneurysm | 3 (7.6) | 1 (6) | 2 (9) | 0.81 |
| Pericarditis/pericardial effusion | 19 (49) | 7 (41) | 12 (54) | 0.40 |
| Radiographic results | ||||
| Pleural effusion | 10 (26) | 2 (12) | 8 (36) | 0.16 |
| Characteristic | IVIG Treatment (n = 17) | IVIG and Corticosteroid Treatment (n = 22) | p Value |
|---|---|---|---|
| General features | |||
| Duration of symptoms before initiation of therapy (days) | 6 [4.5, 6.5] | 5 [4, 5] | 0.64 * |
| Time lag between admission and start of therapy (days) | 1.0 [0, 1.0] | 0.50 [0, 2.0] | 0.88 † |
| Supportive Care | |||
| Fluid resuscitation | 4 (24) | 12 (55) | 0.051 |
| Fluid resuscitation before therapy (mL/kg) | 0 [0, 10] | 10 [0, 23] | 0.064 † |
| Fluid resuscitation after starting therapy (mL/kg) | 0 [0, 0] | 0 [0, 0] | 0.99 † |
| Vasoactive medications | 1 (6) | 6 (27) | 0.19 § |
| Nasal cannula oxygen | 1 (5.9) | 8 (36) | 0.052 § |
| Non-invasive ventilation | 1 (5.9) | 2 (9.1) | 0.99 § |
| Medications | |||
| IVIG | |||
| IVIG dose (gms/kg) | 2.0 [2.0, 2.0] | 2.0 [2.0, 2.0] | 0.99 † |
| IVIG duration (days) | 1.0 [1.0, 1.0] | 1.0 [1.0, 1.0] | 0.88 † |
| Corticosteroids | |||
| Steroid dose | 0.49 § | ||
| Low dose (2 mg/kg) | 6 (86) | 15 (68) | |
| High dose (10 mg/kg) | 1 (14) | 7 (32) | |
| Steroid dose distribution | 0.99 § | ||
| Single | 2 (28) | 7 (32) | |
| Divided | 5 (62) | 15 (68) | |
| Immunomodulation | |||
| IL-1 inhibitor therapy | 2 (18) | 5 (24) | 0.99 § |
| IL-1 inhibitor dose (mg/kg/day) | 2.3 ± 0.35 | 2.9 ± 1.1 | 0.29 * |
| IL-1 inhibitor therapy duration (days) | 4.5 ± 0.71 | 6.2 ± 2.8 | 0.26 * |
| Additional therapy (steroids and immunomodulators) | 9 (53) | 5 (24) | 0.051 § |
| Additional steroids after 24 h | 7 (41) | 0 (0) | 0.001 § |
| Other medications | |||
| Antiplatelet therapy | 14 (82) | 20 (91) | 0.64 § |
| Anticoagulation therapy | 5 (29) | 18 (82) | <0.001 ‡ |
| Characteristic | IVIG Treatment (n = 17) | IVIG and Corticosteroid Treatment (n = 22) | 95% CI [Lower Limit, Upper Limit] | p Value |
|---|---|---|---|---|
| Cardiovascular features | ||||
| Delta ↓ in HR (rate/min) | ||||
| Day 0 to day 1 | 11 [3, 19] | 32 [27, 41] | 17.8 [9.74, 25.8] | <0.001 * |
| Day 0 to day 3 | 25 [6.5, 41.5] | 44.5 [31, 61] | 21.3 [6.6, 36.1] | 0.009 * |
| Day 0 to day 5 | 37 [28, 55] | 64 [45, 75] | 22.3 [8.7, 35.9] | 0.032 * |
| Delta ↑ in mean BP (mmHg) | ||||
| Day 0 to day 1 | 5 [1, 7] | 8.5 [6, 13] | 5.63 [1.61, 9.64] | 0.007 * |
| Day 0 to day 3 | 7 [3, 16.5] | 12 [5, 19] | 3.42 [0.8, 10.67] | 0.049 * |
| Day 0 to day 5 | 9.5 [2.5, 19] | 17 [12, 22] | 3.71 [−2.5, 9.92] | 0.13 * |
| General features | ||||
| Fever resolution (days) | 2.0 [0, 3.0] | 0 [0, 0] | 1.48 [0.79, 2.16] | <0.001 * |
| Delta ↓ in temperature (°C) | ||||
| Day 0 to day 1 | 0.9 [0.25, 1.3] | 2.3 [1.5, 2.9] | 1.45 [0.94, 1.95] | <0.001 * |
| Day 0 to day 3 | 1.9 [1.3, 2.5] | 2.6 [2, 3.1] | 0.74 [0.21, 1.26] | 0.005 |
| Clinical outcomes: | ||||
| PICU admission following initiation of therapy | 5 (29) | 5 (22) | 0.55 † | |
| PICU length of stay (days) following initiation of therapy | 2 [0.5, 7] | 4 [2.5, 5.5] | 0.01 [−3.21, 3.23] | 0.74 * |
| Hospital length of stay (days) | 6 [4, 7] | 7.5 [5, 11] | 1.66 [−0.44, 3.76] | 0.079 * |
| Characteristic | IVIG Treatment (n = 17) | IVIG and Corticosteroid Treatment (n = 22) | 95% CI [Lower Limit, Upper Limit] | p Value |
|---|---|---|---|---|
| Inflammatory markers | ||||
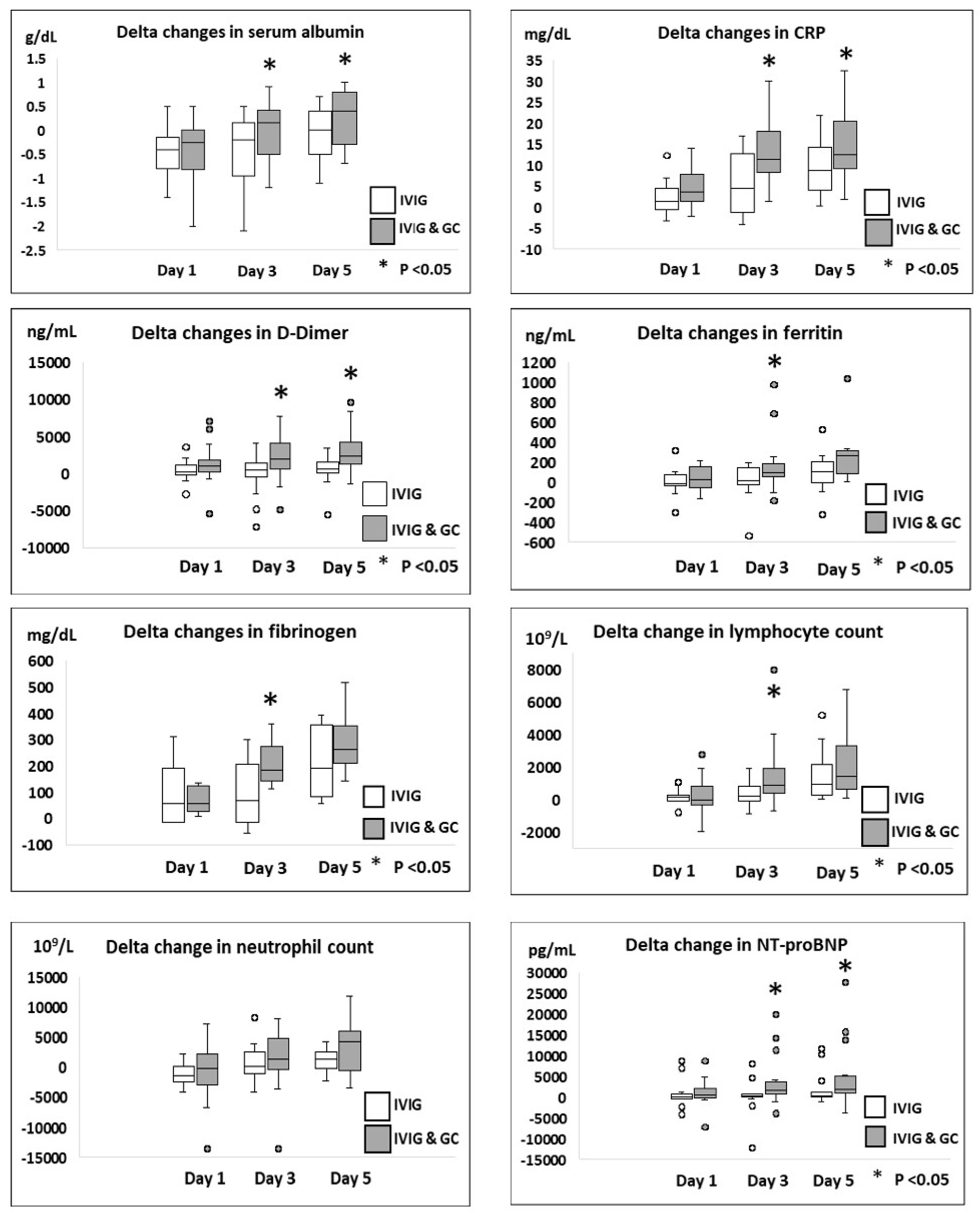
| Delta ↑ in albumin levels | ||||
| Day 0 to day 1 | −0.4 [−0.85, −0.1] | −0.25 [−0.8, 0] | 0.01 [−0.34, 0.36] | 0.80 * |
| Day 0 to day 3 | −0.2 [−0.9, 0] | 0.15 [−0.5, 0.4] | 0.43 [0.2, 0.84] | 0.035 * |
| Day 0 to day 5 | 0 [−0.5, 0.25] | 0.4 [−0.3, 0.75] | 0.37 [0.1, 0.70] | 0.037 * |
| Delta ↓ in CRP levels | ||||
| Day 0 to day 1 | 1.9 [−0.45, 4.4] | 3.6 [1.4, 6.8] | 2.32 [−0.35, 4.99] | 0.14 * |
| Day 0 to day 3 | 4.6 [−0.75, 13] | 10 [8.2, 18] | 7.56 [3.0, 12.11] | 0.019 * |
| Day 0 to day 5 | 5.1 [2.9, 14] | 13 [9.8, 21] | 4.6 [0.2, 9.4] | 0.03 * |
| Delta ↓ in D Dimer levels | ||||
| Day 0 to day 1 | 30 [−220, 1060] | 1010 [270, 1710] | 985 [−374.1, 2344.6] | 0.053 * |
| Day 0 to day 3 | 395 [−355, 1345] | 1840 [655, 3990] | 2344 [488.7, 4200.2] | 0.015 * |
| Day 0 to day 5 | 630 [−30, 2080] | 2900 [1270, 4160] | 2486 [854.6, 4117.3] | 0.048 * |
| Delta ↓ in ferritin levels | ||||
| Day 0 to day 1 | −13 [−56, 60] | 31 [−16, 210] | 416 [−186, 1019.9] | 0.16 * |
| Day 0 to day 3 | 12 [−33, 162] | 137 [54, 257] | 1448 [−609.4, 3505.5] | 0.049 * |
| Day 0 to day 5 | 128 [1.5, 255] | 296 [93, 330] | 2350 [−284.1, 4948.1] | 0.35 * |
| Delta ↓ in fibrinogen levels | ||||
| Day 0 to day 1 | 32 [−16, 154] | 55 [32, 120] | 13.4 [−40.1, 16.8] | 0.47 * |
| Day 0 to day 3 | 66 [−16, 206.5] | 184 [141, 271] | 110 [44.4, 176] | 0.027 * |
| Day 0 to day 5 | 291 [190, 293] | 284 [223, 320] | 72.3 [−3.4, 147.9] | 0.52 * |
| Delta ↑ in lymphocyte count | ||||
| Day 0 to day 1 | 110 [−130, 280] | −60 [−340, 680] | 101.9 [−466.5, 670.3] | 0.83 * |
| Day 0 to day 3 | 515 [−45, 1095] | 1050 [390, 1890] | 1006 [63.5, 1948] | 0.048 * |
| Day 0 to day 5 | 1150 [400, 3740] | 1915 [605, 3420] | 715.9 [−428.6, 1860] | 0.65 * |
| Delta ↓ in neutrophil count | ||||
| Day 0 to day 1 | 1700 [−230, 2460] | 125 [−2180, 2600] | 531 [−1819, 2882] | 0.52 * |
| Day 0 to day 3 | −40 [−2475, 2430] | −1375 [−4660, 160] | 939 [−1717, 3596] | 0.23 * |
| Day 0 to day 5 | −940 [−6340, 2225] | −3295 [−5450, 1220] | 787 [−2657, 4231] | 0.61 * |
| Delta ↓ in NT - proBNP levels | ||||
| Day 0 to day 1 | −23 [−391, 182] | 597 [−84, 1842] | 58.6 [−2104.7, 2222] | 0.20 * |
| Day 0 to day 3 | 411 [103, 719] | 1660 [916, 3640] | 2901 [−349.3, 6153] | 0.020 * |
| Day 0 to day 5 | 697 [66, 1193] | 1999 [1032, 5285] | 3113 [−851.4 7078] | 0.048 * |
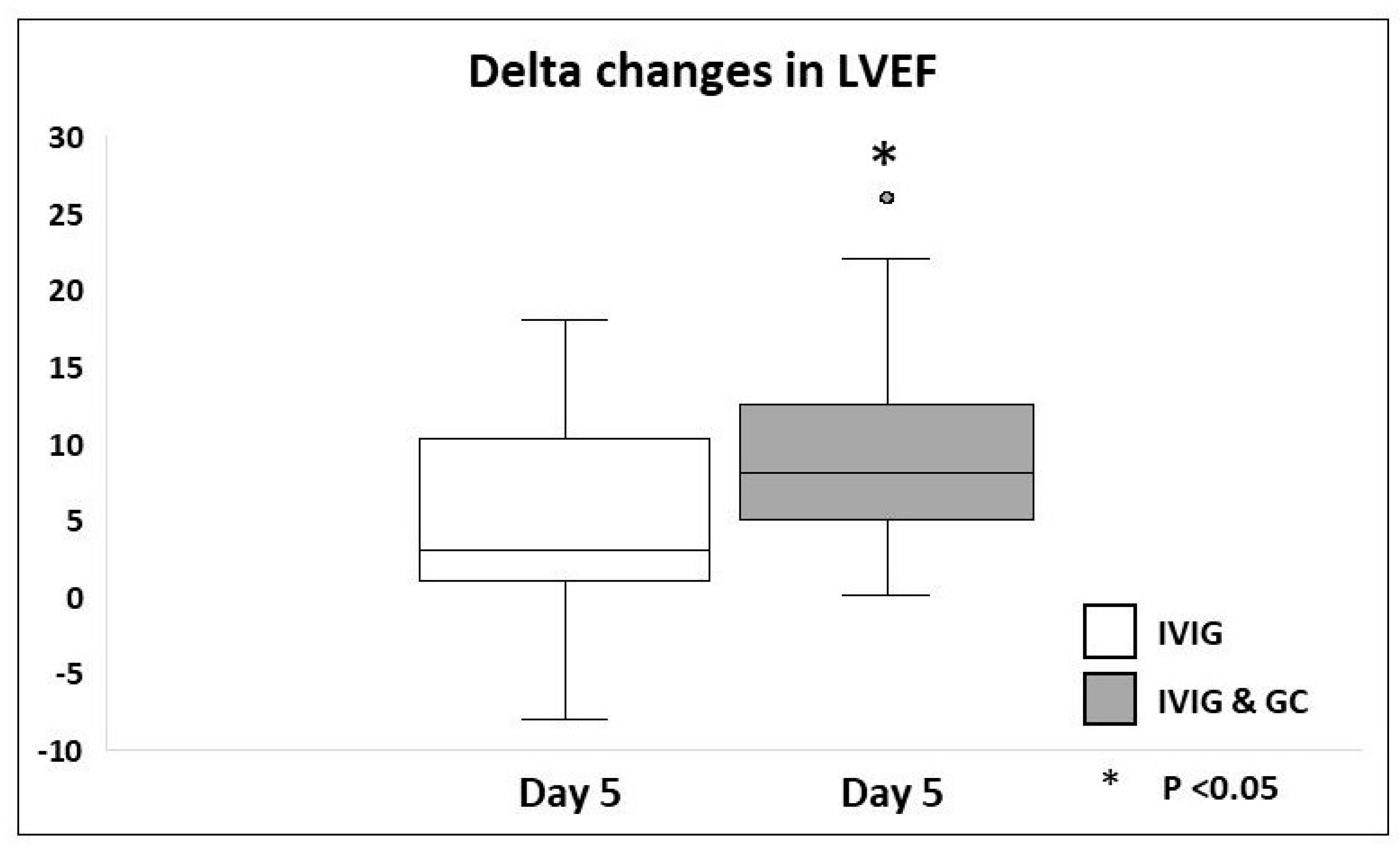
| Echocardiographic results | ||||
| Delta ↑ in LVEF % | ||||
| Day 0 to day 4–5 | 3 [1, 9.5] | 8 [5, 12] | 3.84 [0.55, 8.23] | 0.032 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dachepally, R.; Sarkis, R.; DonaireGarcia, A.; Kovvuri, M.; Jayasimha, K.; Chaturvedi, A.; Ali, A.; Panupattanapong, S.; Latifi, S.; Agarwal, H. Comparison of Immunomodulatory Therapies for Cardiovascular Clinical and Inflammatory Markers Outcomes in Mild to Moderately Ill Hospitalized Multisystem Inflammatory Syndrome in Children Patients. J. Cardiovasc. Dev. Dis. 2025, 12, 324. https://doi.org/10.3390/jcdd12090324
Dachepally R, Sarkis R, DonaireGarcia A, Kovvuri M, Jayasimha K, Chaturvedi A, Ali A, Panupattanapong S, Latifi S, Agarwal H. Comparison of Immunomodulatory Therapies for Cardiovascular Clinical and Inflammatory Markers Outcomes in Mild to Moderately Ill Hospitalized Multisystem Inflammatory Syndrome in Children Patients. Journal of Cardiovascular Development and Disease. 2025; 12(9):324. https://doi.org/10.3390/jcdd12090324
Chicago/Turabian StyleDachepally, Rashmitha, Reem Sarkis, Alvaro DonaireGarcia, Meghana Kovvuri, Karunya Jayasimha, Adrija Chaturvedi, Amr Ali, Sirada Panupattanapong, Samir Latifi, and Hemant Agarwal. 2025. "Comparison of Immunomodulatory Therapies for Cardiovascular Clinical and Inflammatory Markers Outcomes in Mild to Moderately Ill Hospitalized Multisystem Inflammatory Syndrome in Children Patients" Journal of Cardiovascular Development and Disease 12, no. 9: 324. https://doi.org/10.3390/jcdd12090324
APA StyleDachepally, R., Sarkis, R., DonaireGarcia, A., Kovvuri, M., Jayasimha, K., Chaturvedi, A., Ali, A., Panupattanapong, S., Latifi, S., & Agarwal, H. (2025). Comparison of Immunomodulatory Therapies for Cardiovascular Clinical and Inflammatory Markers Outcomes in Mild to Moderately Ill Hospitalized Multisystem Inflammatory Syndrome in Children Patients. Journal of Cardiovascular Development and Disease, 12(9), 324. https://doi.org/10.3390/jcdd12090324







