Markers in Acute Coronary Syndrome: Distal Coronary Embolism at Percutaneous Coronary Intervention
Abstract
1. Introduction
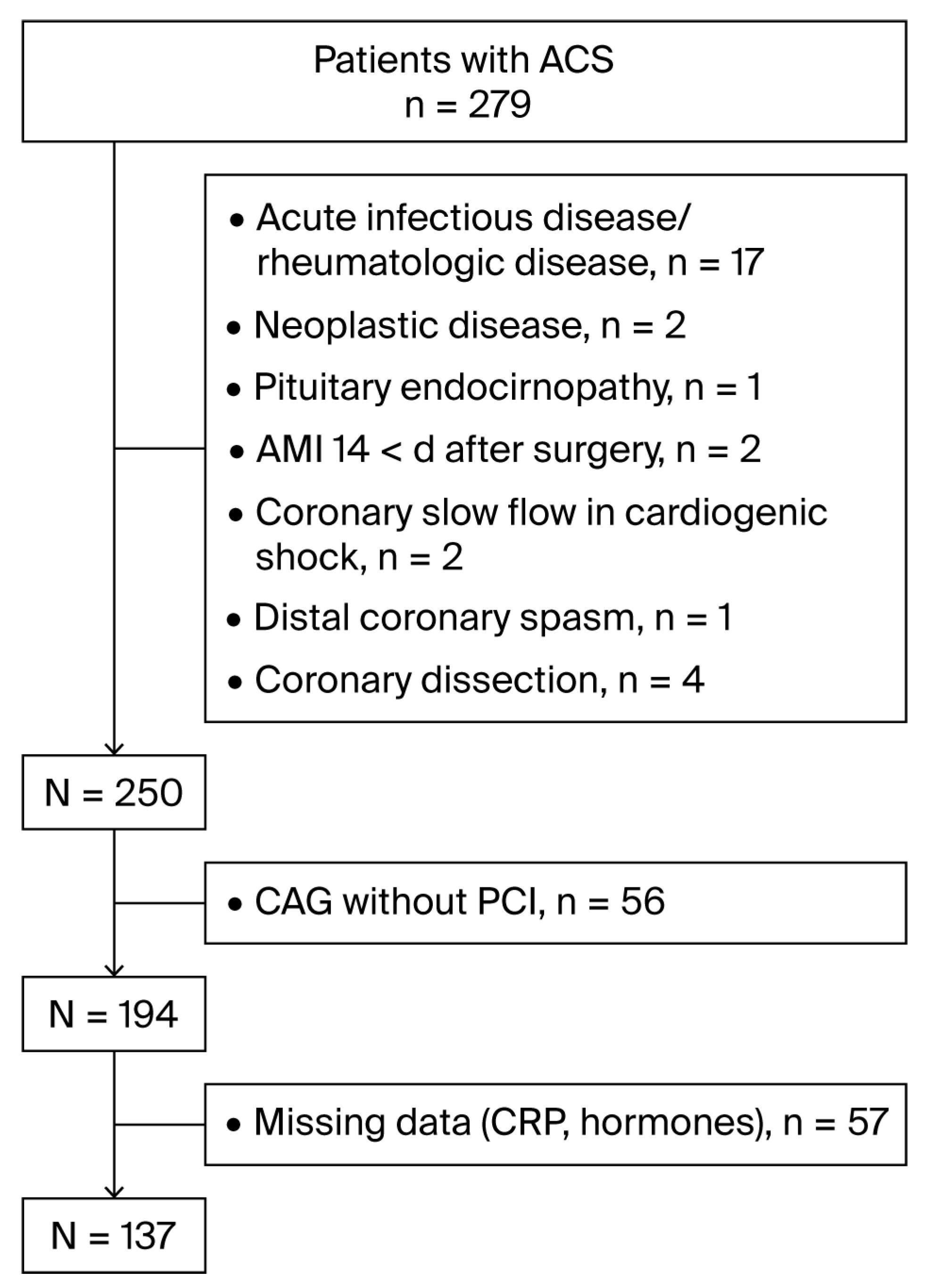
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Assessment
2.3. PCI Procedure
2.4. Echocardiography
2.5. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACS | Acute coronary syndrome |
| AMI | Acute myocardial infarction |
| STEMI | Acute myocardial infarction with persisting ST elevation |
| NSTEMI | Acute myocardial infarction with non-persisting ST elevation |
| CAD | Coronary artery disease |
| CAG | Coronary angiography |
| IRA | Infarct-related artery |
| PCI | Percutaneous coronary intervention |
| TIMI | Thrombolysis in myocardial infarction |
| MBG | Myocardial blush grade |
| GFR | Glomerular filtration rate |
| CK | Creatine kinase |
| CPK-MB | Muscle–brain fraction of CK |
| hs TnT | High-sensitivity troponin T |
| E2 | Total 17β-estradiol |
| T | Total testosterone |
| DHEA-S | Dehydroepiandrosterone sulfate |
| ACTH | Adrenocorticotropic hormone |
| CRP | C-reactive protein |
| oxLDL | Oxidized low-density lipoproteins |
| HDL | High-density lipoprotein |
| LDL | Low-density lipoprotein |
| TG | Triglyceride |
| oxLDL | Oxidized low-density lipoprotein |
| PLT | Platelets |
| WBC | White blood cell count |
| EF | Ejection fraction |
| GNT | Glyceryl trinitrate |
Appendix A
| Patient | Age, Sex | CAD Type | IRA | TIMI Flow Grade | MBG | Intracoronary Vasodilator | Stent Type | EF, % |
|---|---|---|---|---|---|---|---|---|
| 1 | 63 y, man | 1-vessel | LADprox | 3 | 2 | Verapamil; GTN 400 µg | BMS | 42 |
| 2 | 70 y, man | 3-vessel | RCAprox | 3 | - | Eptifibatide 14 mg + 6 mg GTN 400 µg; nitroprusside | BMS | 49 |
| 3 | 78 y, man | 2-vessel | LADmid | 3 | - | Verapamil; Eptifibatide 14 mg + 6 mg; GTN Nitroprusside | BMS | 64 |
| 4 | 48 y, man | 2-vessel | LADprox | 3 | Verapamil; GTN | BMS | 50 | |
| 5 | 59 y, man | 2-vessel | RCAprox | 3 | - | GTN 100 µg | BMS | 48 |
| 6 | 63 y, man | 3-vessel | LAD | 2-3 | - | GTN 100 µg | BMS | 54 |
| 7 | 64 y, man | 1-vessel | LAD | 2-3 | - | Verapamil 200 µg | BMS | 48 |
| 8 | 57 y, man | 1-vessel | LADmid | 3 | - | Verapamil 100 µg; GTN 300 µg | DES | 59 |
| 9 | 72 y, woman | 1-vessel | RCAprox | 3 | - | None | BMS | 48 |
| 10 | 65 y, woman | 1-vessel | LADprox | 3 | - | Verapamil; GTN; Nitroprusside | BMS | 48 |
| 11 | 75 y, woman | 3-vessel | RCA | 3 | 2–3 | GTN 100 µg | BMS | 51 |
| 12 | 75 y, woman | 1-vessel | LADprox | 3 | - | GTN 200 µg; Eptifibatide 4 mg | DES | 66 |
| 13 | 85 y, woman | 1-vessel | RCAprox | 3 | - | Nitroprusside | BMS | 58 |
References
- Dong-Bao, L.; Qi, H.; Zhi, L.; Shan, W.; Wei-Ying, J. Predictors and short-term prognosis of angiographically detected distal embolization after emergency percutaneous coronary intervention for ST-elevation acute myocardial infarction. Clin. Res. Cardiol. 2009, 98, 773–779. [Google Scholar] [CrossRef]
- Henriques, J.P.; Zijlstra, F.; Ottervanger, J.P.; Van’t Hof, A.W.; Hoorntie, J.C.A.; Suryapranata, H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur. Heart J. 2002, 23, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Verburg, A.; Van’t Hof, A.W.; Berg, J.T.; Kereiakes, D.J.; Coller, B.S.; Gibson, C.M. Current and future roles of glycoprotein IIb-IIIa inhibitors in primary angioplasty for ST-segment elevation myocardial infarction. Biomedicines 2024, 12, 2023. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Gibson, C.M.; Bekkandi, F.; Noc, M.; Maioli, M.; Zorman, S.; Zeymer, U.; Gabriel, H.M.; Emre, A.; Cutlip, D.; et al. Impact of distal embolization on myocardial perfusion and survival among patients undergoing primary angioplasty with glycoprotein IIb-IIIa inhibitors: Insights from the EGYPT cooperation. J. Thromb. Thrombolysis 2010, 30, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Tanboga, I.H.; Topcu, S.; Aksakal, E.; Kalkan, K.; Sevimli, S.; Acikel, M. Determinants of angiographic thrombus burden in patients with ST-segment elevation myocardial infarction. Clin. Appl. Thromb. Hemost. 2014, 20, 716–722. [Google Scholar] [CrossRef]
- Shah, G.A.; Malik, T.; Farooqi, S.; Ahmed, S.; Abid, K. Frequency and impact of slow flow/no flow in primary percutaneous coronary intervention. J. Pak. Med. Assoc. 2021, 71, 2548–2553. [Google Scholar] [CrossRef]
- Bae, J.H.; Kwon, T.G.; Hyun, D.W.; Rihal, C.S.; Lerman, A. Predictors of slow flow during primary percutaneous coronary intervention: An intravascular ultrasound-virtual histology study. Heart 2008, 94, 1559–1564. [Google Scholar] [CrossRef]
- Lim, S.; Cha, J.J.; Hong, S.J.; Kim, J.H.; Joo, H.J.; Park, J.H.; Yu, C.W.; Ahn, T.H.; Lim, D.-S. Association between high lipid burden of target lesion and slow TIMI flow in coronary interventions. J. Clin. Med. 2022, 11, 5401. [Google Scholar] [CrossRef]
- Kai, T.; Oka, S.; Hoshino, K.; Watanabe, K.; Nakamura, J.; Abe, M.; Watanabe, A. Renal dysfunction as a predictor of slow-flow/no-reflow phenomenon and impaired ST segment resolution after percutaneous coronary intervention in ST-elevation myocardial infarction with initial Thrombolysis in Myocardial Infarction Grade 0. Circ. J. 2021, 85, 1770–1778. [Google Scholar] [CrossRef]
- Savic, L.; Mrdovic, I.; Asanin, M.; Stankovic, S.; Lasica, R.; Krlianac, G.; Rajic, D.; Simic, D. The impact of kidney function on the slow-flow/no-reflow phenomenon in patients treated with primary percutaneous coronary intervention: Registry analysis. J. Interv. Cardiol. 2022, 2022, 5815274. [Google Scholar] [CrossRef]
- Iijima, R.; Shinji, H.; Ikeda, N.; Itaya, H.; Makino, K.; Funatsu, A.; Yokouchi, I.; Komatsu, H.; Ito, N.; Nuruki, H.; et al. Comparison of coronary arterial finding by intravascular ultrasound in patients with “transient no-reflow” versus “reflow” during percutaneous coronary intervention in acute coronary syndrome. Am. J. Cardiol. 2006, 97, 29–33. [Google Scholar] [CrossRef]
- Kuntz, R.E.; Rogers, C.; Baim, D.S. Percutaneous coronary intervention–induced emboli during primary PCI for STEMI: Too little, too much, or too late? Am. Heart J. 2005, 150, 4–6. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Mouliou, D.S. C-reactive protein: Pathophysiology, diagnosis, false test results and a novel diagnostic algorithm for clinicians. Dis. 2023, 11, 132. [Google Scholar] [CrossRef] [PubMed]
- Cirrincione, L.R.; Crews, B.O.; Dickenson, J.A.; Krasowski, M.D.; Rongitch, J.; Imoborek, K.L.; Goldstein, Z.; Greene, D.N. Oral estrogen leads to falsely low concentrations of estradiol in a common immunoassay. Endocr. Connect. 2022, 11, e210550. [Google Scholar] [CrossRef] [PubMed]
- Fraley, A.E.; Tsimikas, S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr. Opin. Lipidol. 2006, 17, 502–509. [Google Scholar] [CrossRef]
- Rampidis, G.P.; Benetos, G.; Benz, D.C.; Giannopoulo, A.A.; Buechel, R.R. A guide for Gensini Score calculation. Atherosclerosis 2019, 287, 181–183. [Google Scholar] [CrossRef]
- The TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. N. Engl. J. Med. 1985, 312, 932–936. [Google Scholar] [CrossRef]
- van’t Hof, A.W.; Liem, A.; Suryapranata, H.; Hoomtie, J.C.A.; de Boer, M.J.; Zijlstra, F. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation 1998, 97, 2302–2306. [Google Scholar] [CrossRef]
- Ungi, T.; Sasi, V.; Ungi, I.; Forster, T.; Palkó, A.; Nemes, A. Comparison of two visual angiographic perfusion grades in acute myocardial infarction. Ups. J. Med. Sci. 2009, 114, 149–153. [Google Scholar] [CrossRef]
- Gibson, C.M.; Cannon, C.P.; Murphy, S.A.; Marble, S.J.; Barron, H.V.; Braunwald, E. TIMI Study group Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation 2002, 105, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.; Karagouis, L.A.; Becker, L.C.; Sorensen, S.G.; Menlove, R.L. Ventriculographic, enzymatic, and electrocardiographic evidence from the TEAM-3 Study. Circulation 1993, 87, 1829–1839. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sanspnetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic relevance of type 4a myocardial infarction and periprocedural myocardial injury in patients with non-ST-segment-elevation myocardial infarction. Circulation 2025, 151, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.C.; Tucker, K.L.; Rodriguez, F.; Porneala, B.C.; Meigs, J.B.; López, L. Cardiovascular risk factors and dehydroepiandrosterone sulfate among latinos in the Boston Puerto Rican Health Study. J. Endocr. Soc. 2019, 3, 291–303. [Google Scholar] [CrossRef]
- Lennartsson, A.; Kushnir, M.M.; Bergguist, J.; Jonsdottir, I.H. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol. Psychol. 2012, 90, 143–149. [Google Scholar] [CrossRef]
- Anni, N.S.; Jung, S.J.; Shim, J.-S.; Jeon, Y.W.; Lee, G.B.; Kim, H.C. Stressful life events and serum triglyceride levels: The cardiovascular and metabolic diseases etiology research center cohort in Korea. Epidemiol. Health 2021, 43, e2021042. [Google Scholar] [CrossRef]
- Alayón, A.N.; Arizal, N.O.; Correa, M.N.; Toro, J.L.; Rojas, F.H. Cortisol, cardiovascular risk, and anxiety in full-time workers in Cartagena, Colombia, 2023. Front. Psychiatry 2025, 16, 1491987. [Google Scholar] [CrossRef]
- Semerdzhieva, N.E.; Tsakova, A.D.; Lozanova, V.V. Sex-Specific impact of 17β-estradiol and testosterone levels on inflammation and injury in acute myocardial infarction—Preliminary results. Biomedicines 2025, 13, 1466. [Google Scholar] [CrossRef]
- Ma, Y.; Fan, X.; Han, J.; Cheng, Y.; Zhao, J.; Fang, W.; Gao, L. Critical illness and sex hormones: Response and impact of the hypothalamic–pituitary–gonadal axis. Ther. Adv. Endocrinol. Metab. 2025, 16, 1–20. [Google Scholar] [CrossRef]
- Bae, J.H.; Bassenge, E.; Kim, K.B.; Kim, Y.N.; Kim, K.S.; Lee, H.J.; Moon, K.C.; Lee, M.S.; Park, K.Y.; Schwemmer, M. Postprandial hypertriglyceridemia impairs endothelial function by enhanced oxidant stress. Atherosclerosis 2001, 155, 517–523. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Chen, J.Y.; Chen, P.Y.; on behalf of TRUST Investigators. The relationship between fasting triglyceride level and prevalence and severity of angiographic coronary artery disease in 16,650 patients from the TRUST study in the statins era. Eur. Heart J. 2013, 34 (Suppl. 1), P1550. [Google Scholar] [CrossRef]
- Kaplan, S.; Kaplan, T.S.; Kiris, A.; Gedikli, O. Impact of initial platelet count on baseline angiographic finding and end-points in ST-elevation myocardial infarction referred for primary percutaneous coronary intervention. Int. J. Clin. Exp. Med. 2014, 7, 1064–1070. [Google Scholar] [PubMed]
- Stone, G.W.; Maehara, A.; Muller, J.E.; Rizik, D.G.; Shunk, K.A.; Ben-Yehuda, O.; Genereux, P.; Dressler, O.; Parvataneni, R.; Madden, S.; et al. Plaque Characterization to inform the prediction and prevention of periprocedural myocardial infarction during percutaneous coronary intervention: The CANARY Trial (Coronary Assessment by Near-infrared of Atherosclerotic Rupture-prone Yellow). JACC Cardiovasc. Interv. 2015, 8, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wei, X.; Chen, W.; Wan, D.; Han, W.; Liu, H. Effects of early PCSK9 inhibitor application on inflammation levels and microcirculatory function after PCI in patients with NSTE-ACS. Am. J. Transl. Res. 2023, 15, 3586–3596. [Google Scholar] [PubMed]
- Osorio, A.; Gutierrez, M.A.; Ortega, E.; Ruiz-Requena, E. Dehydroepiandrosterone sulfate and growth axis hormones in patients with ischemic heart disease. Horm. Res. 2002, 57, 165–169. [Google Scholar] [CrossRef]
- Ruiz Salmerón, R.J.; Arbol, J.L.; Torrededia, J.; Muñoz, J.R.; Luque, A.L.; Irlés, J.R.; Ruiz Requena, M.E.; Bolaños, J. Dehydroepiandrosterone sulfate and lipids in acute myocardial infarct. Rev. Clin. Esp. 1992, 190, 398–402. [Google Scholar]
- Dimopoulou, I.; Stamoulis, K.; Lyberopoulos, P.; Alevizopoulou, P.; Theodorakopoulou, M.; Orfanos, S.; Tzanela, M.; Kopterides, P.; Lignos, M.; Papadomichelakis, E.; et al. Cortisol and dehydroepiandrosterone sulphate levels in ICU patients upon admission correlate with severity of disease. Crit. Care 2006, 10 (Suppl. 1), P261. [Google Scholar] [CrossRef]
- Carvalho, L.S.F.; Panzoldo, N.; Santos, S.N.; Modolo, R.; Almeida, B.; Quinaglia, E.; Silva, J.C.; Silva, J.C.; Nadruz, W., Jr.; Faria, E.C.; et al. HDL levels and oxidizability during myocardial infarction are associated with reduced endothelial-mediated vasodilation and nitric oxide bioavailability. Atherosclerosis 2014, 237, 840–846. [Google Scholar] [CrossRef]
- Shechter, M.; Merz, C.N.; Paul-Labrador, M.J.; Kaul, S. Blood glucose and platelet dependent thrombosis in patients with coronary artery disease. J. Am. Coll. Cardiol. 2000, 35, 300–307. [Google Scholar] [CrossRef]
- Yu, B.; Mo, Y.; Hu, X.; Wang, W.; Liu, J.; Jin, J.; Lun, Z.; Luo Bu, C.R.; Dong, H.; Zhou, Y. Triglyceride-glucose index is associated with quantitative flow ratio in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Front. Cardiovasc. Med. 2022, 9, 1002030. [Google Scholar] [CrossRef]
- Bilgin, M.; Akkaya, E.; Dokuyucu, R. Prognostic value of triglyceride glucose index in ST-elevation myocardial infarction: A key predictor of mortality and thrombus burden. Diagnostics 2024, 14, 2261. [Google Scholar] [CrossRef] [PubMed]
- Sezer, M.; Royen, N.; Umman, B.; Bugra, Z.; Bulluck, H.; Hausenloy, D.J.; Umman, S. Coronary microvascular injury in reperfused acute myocardial infarction: A view from an integrative perspective. J. Am. Heart Assoc. 2018, 7, e009949. [Google Scholar] [CrossRef] [PubMed]
- De Luca, G.; Gibson, C.M.; Bellandi, F.; Noc, M.; Dudek, D.; Zeymer, U.; Arntz, H.; Cutlip, D.; Maioli, M.; Zorman, S.; et al. Diabetes mellitus is associated with distal embolization, impaired myocardial perfusion, and higher mortality in patients with ST-segment elevation myocardial infarction treated with primary angioplasty and glycoprotein IIb-IIIa inhibitors. Atherosclerosis 2009, 207, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Cherneva, Z.V.; Denchev, S.V.; Gospodinova, M.V.; Tsakova, A.; Cherneva, R.V. Inflammatory cytokines at admission-independent prognostic markers in patients with acute coronary syndrome and hyperglycaemia. Acute Card. Care 2012, 14, 13–19. [Google Scholar] [CrossRef]
| Myocardial Blush | Angiographic Contrast | |
|---|---|---|
| Grades | Angiographic contrast progression | Washout phase |
| MBG 0 | No myocardial blush | No myocardial blush |
| MBG 1 | Slow entry, failure to exit the microvasculature | Dye staining—present on the next injection |
| MBG 2 | Delayed entry into the microvasculature | Dye staining—only minimally diminished in intensity |
| MBG 3 | Ground-glass appearance (“blush”) or opacification of the myocardium in the distribution of the culprit lesion | Opacification is cleared normally. Only mildly/moderately persistent [18] |
| Variable | Mean ± SD |
|---|---|
| Age, years | 64.5 ± 12.3 |
| Sex (men/women) | 87 (64%)/50 (36%) |
| Hypertension | 134 (96.4%) |
| Diabetes mellitus | 43 (30.9%) |
| BMI, kg/m2 | 28.5 ± 5.0 |
| Weight, kg | 83.9 ± 16.1 |
| Distal coronary embolism, n (%) | 13 (9.4%) |
| STEMI, n (%) | 109 (78.4%) |
| NSTEMI, n (%) | 16 (11.5%) |
| Unstable angina, n (%) | 14 (10.1%) |
| 1-vessel CAD, n (%) | 45 (32.4%) |
| 2-vessel CAD, n (%) | 52 (37.4%) |
| 3-vessel CAD, n (%) | 42 (30.2%) |
| Syntax score | 14.9 ± 8.2 |
| Gensini score | 46.7±38.9 |
| Prior AMI, n (%) | 15 (10.8%) |
| Atrial fibrillation, n (%) | 18 (12.9%) |
| Statin, n (%) | 13 (10.4%) |
| E2, pmol/L | 134.2 ± 93.3 |
| E2/T | 0.6 ± 1.2 |
| DHEA-S, µmol/L | 3.2 ± 2.3 |
| GFR, ml/min/m2 | 73.8 ± 66.2 |
| PLT × 109/L | 248.2 ± 68.4 |
| Glucose, mmol/L | 10.9 ± 13.8 |
| WBC × 109/L | 10.2 ± 3.5 |
| CRP, mg/L | 33.9 ± 77.7 |
| Total cholesterol, mmol/L | 5.2 ± 1.3 |
| HDL, mmol/L | 1.3 ± 0.5 |
| LDL, mmol/L | 3.1 ± 1.2 |
| TG, mmol/L | 1.6 ± 0.7 |
| oxLDL, ng/mL | 9.4 ± 6.5 |
| Left ventricular EF, % | 53.3 ± 10.9 |
| CK, U/L | 865.9 ± 1238.5 |
| CK-MB, U/L | 93.5 ± 135.8 |
| hsTnT, ng/mL | 2.1 ± 2.9 |
| Variable | Uncomplicated PCI | Distal Coronary Embolism | p | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| Age, years | 63.3 ± 12.2 | 63.6 ± 10.1 | 0.844 | 0.993 | 0.948–1.041 | 0.782 |
| Male patients | 80 (64%) | 9 (64.3%) | NS | |||
| Diabetes mellitus | 13 (2.6%) | 6 (1.2%) | NS | |||
| Statin | 12 (10.7%) | 1 (7.8%) | NS | |||
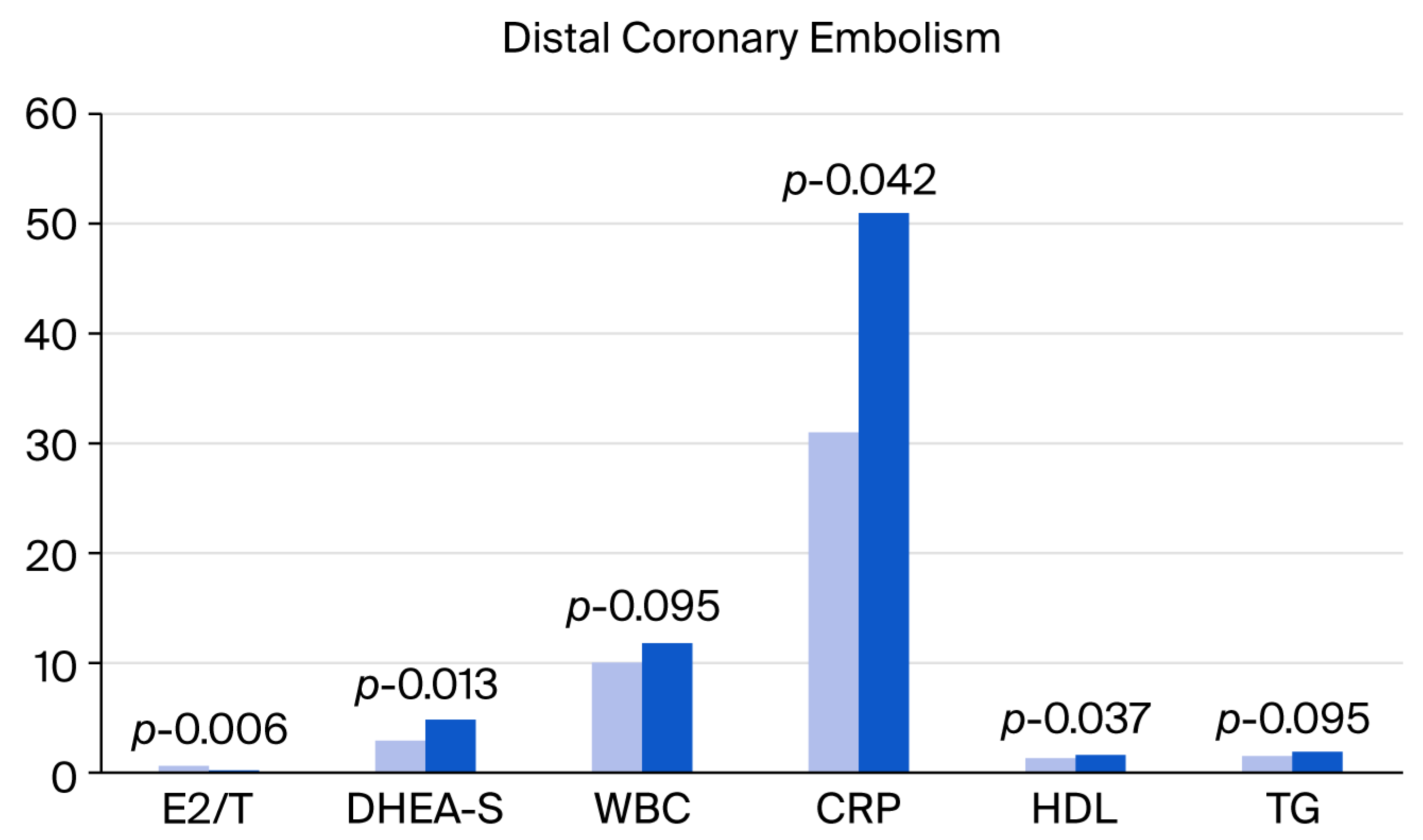
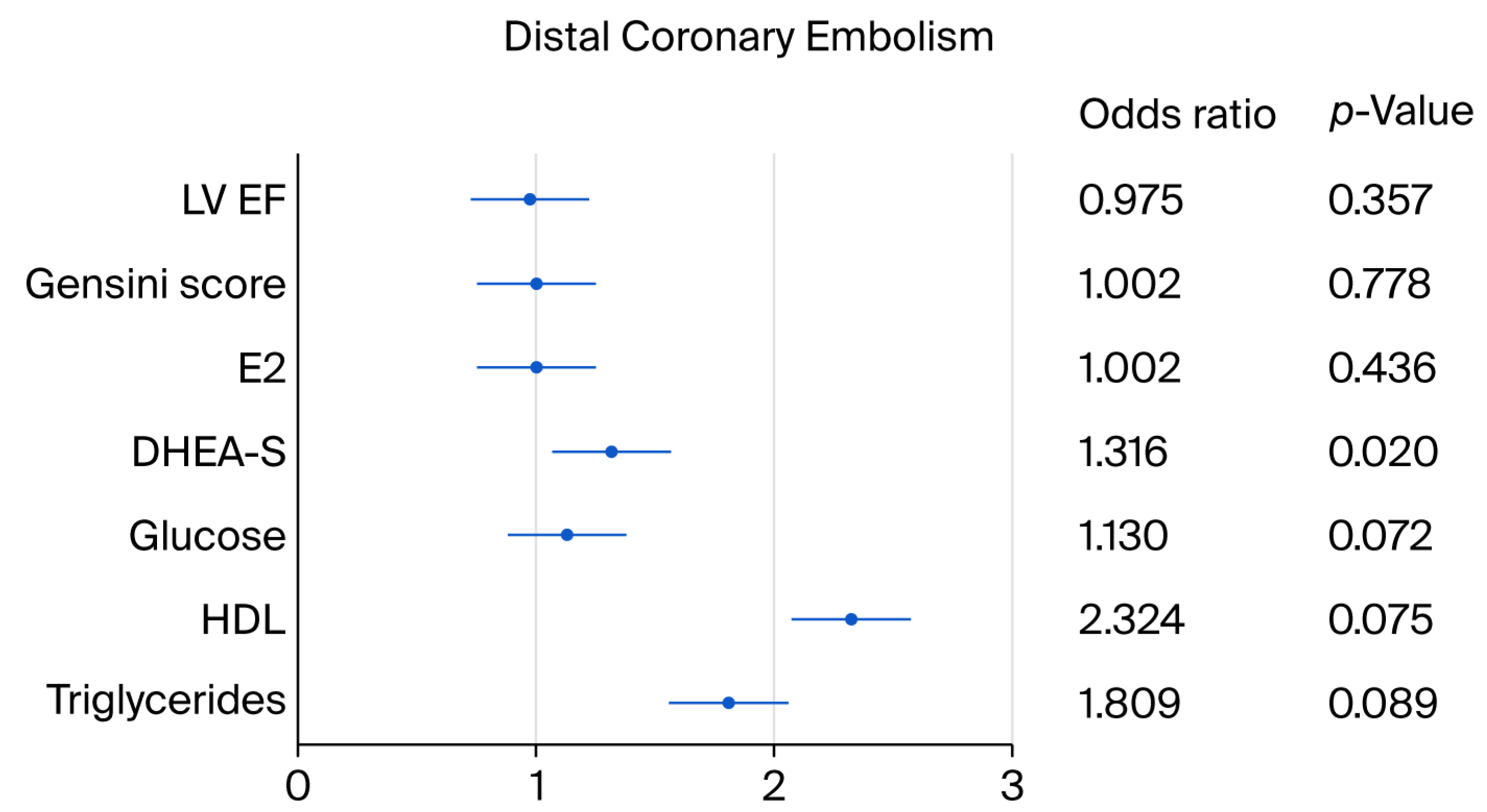
| E2 | 131.9 ± 96.8 | 155.2 ± 59.7 | 0.077 ** | 1.002 | 0.997–1.008 | 0.436 |
| E2/T | 0.6 ± 1.3 | 0.2 ± 0.2 | 0.006 * | 0.303 | 0.045–2.055 | 0.222 |
| DHEA-S | 2.9 ± 2.2 | 4.8 ± 2.6 | 0.013 * | 1.316 | 1.044–1.659 | 0.020 * |
| BMI | 28.4 ± 51.1 | 28.1 ± 3.2 | 0.825 | 0.980 | 0.862–1.116 | 0.750 |
| GFR, ml/min/m2 | 74.7 ± 69.4 | 65.2 ± 22.5 | 0.419 | 0.993 | 0.970–1.018 | 0.595 |
| PLT | 250.8 ± 69.3 | 225.7 ± 57.7 | 0.212 | 0.990 | 0.980–1.000 | 0.210 |
| WBC | 10.1 ± 3.3 | 11.8 ± 4.8 | 0.095 ** | 1.127 | 0.976–1.016 | 0.775 |
| CRP | 31.9 ± 79.7 | 51.8 ± 56.5 | 0.042 * | 1.002 | 0.997–1.007 | 0.412 |
| Glucose | 8.1 ± 3.5 | 10.0 ± 3.5 | 0.064 ** | 1.130 | 0.990–1.300 | 0.072 ** |
| TG-glucose index | 4.9 ± 0.3 | 5.0 ± 0.4 | 0.445 | 1.940 | 0.360–10.480 | 0.443 |
| Total cholesterol | 5.3 ± 1.3 | 5.1 ± 1.6 | 0.706 | 0.920 | 0.572–1.480 | 0.730 |
| HDL | 1.3 ± 0.4 | 1.6 ± 1.0 | 0.037 * | 2.326 | 0.918–5.897 | 0.075 ** |
| LDL | 3.1 ± 1.2 | 2.9 ± 1.2 | 0.667 | 0.896 | 0.544–1.475 | 0.665 |
| TG | 1.5 ± 0.7 | 1.9 ± 1.1 | 0.095 ** | 1.809 | 0.913–3.583 | 0.089 ** |
| TG/HDL | 1.3 ± 0.7 | 1.6 ± 1.2 | 0.820 | 1.390 | 0.760–2.540 | 0.284 |
| oxLDL | 9.6 ± 6.6 | 7.8 ± 6.2 | 0.447 | 0.954 | 0.822–1.107 | 0.533 |
| SYNTAX score | 15.0 ± 8.4 | 14.5 ± 5.8 | 0.835 | 0.992 | 0.925–1.065 | 0.834 |
| Gensini score | 46.4 ± 39.1 | 49.6 ± 39.4 | 0.776 | 1.002 | 0.989–1.016 | 0.778 |
| LV EF% | 53.3 ± 10.7 | 50.7 ± 9.5 | 0.215 | 0.975 | 0.925–1.029 | 0.357 |
| DHEA-S | ||||||
|---|---|---|---|---|---|---|
| Variable | Quartile 1 | Quartile 4 | p | OR | 95% CI | p |
| Age | 71.3 ± 9.5 | 56.2 ± 10.9 | 0.0001 * | 0.880 | 0.827–0.955 | 0.001 * |
| E2 | 112.2 ± 141.6 | 168.8 ± 79.9 | <0.0001 * | 1.010 | 1.000–1.010 | 0.109 |
| E2/T | 0.9 ± 1.6 | 0.2 ± 0.3 | 0.016 * | 0.140 | 0.018–0.713 | 0.020 * |
| BMI | 28.5 ± 5.4 | 30.2 ± 4.4 | 0.236 | 1.087 | 0.944–1.252 | 0.232 |
| Weight | 84.4 ± 17.5 | 89.3 ± 16.3 | 0.468 | 1.031 | 0.993–1.071 | 0.109 |
| GFR | 57.4 ± 17.4 | 79.9 ± 30.5 | 0.003 * | 1.056 | 1.013–1.102 | 0.002 * |
| WBC | 9.3 ± 3.1 | 10.3 ± 3.0 | 0.254 | 1.062 | 0.873–1.291 | 0.549 |
| CRP | 18.3 ± 24.4 | 35.7 ± 43.8 | 0.022 * | 1.022 | 0.994–1.049 | 0.064 ** |
| Total cholesterol | 5.1 ± 1.4 | 5.3 ± 1.3 | 0.548 | 1.140 | 0.750–1.750 | 0.542 |
| HDL | 1.4 ± 0.4 | 1.5 ± 0.8 | 0.606 | 1.290 | 0.500–3.340 | 0.604 |
| LDL | 3.0 ± 1.3 | 3.2 ± 1.2 | 0.739 | 1.080 | 0.690–1.610 | 0.734 |
| TG | 1.5 ± 0.6 | 1.9 ± 0.9 | 0.034 * | 2.320 | 1.018–5.270 | 0.045 * |
| TG/HDL | 1.3 ± 1.0 | 1.6 ± 0.9 | 0.187 | 1.350 | 0.730–2.500 | 0.334 |
| oxLDL | 1.3 ± 1.0 | 1.3 ± 1.0 | 0.265 | 0.939 | 0.855–1.031 | 0.186 |
| Gluc | 8.3 ± 3.7 | 9.3 ± 3.7 | 0.386 | 1.070 | 0.920–1.250 | 0.380 |
| TG-Glucose index | 4.9 ± 0.3 | 5 ± 0.3 | 0.082 ** | 5.580 | 0.780–39.99 | 0.087 ** |
| Syntax score | 14.5 ± 7.8 | 15.5 ± 6.6 | 0.618 | 1.001 | 0.922–1.086 | 0.989 |
| Gensini score | 58.1 ± 63.1 | 53.3 ± 34.4 | 0.734 | 0.996 | 0.984–1.008 | 0.489 |
| LV EF | 54.1 ± 12.6 | 52.7 ± 9.9 | 0.654 | 0.979 | 0.927–1.033 | 0.431 |
| CK | 378.6 ± 615.2 | 1440.2 ± 1379.5 | 0.001 * | 1.001 | 1.000–1.002 | 0.011 * |
| CK-MB | 39.7 ± 56.9 | 169.8 ± 161.2 | 0.001 * | 1.013 | 1.003–1.022 | 0.010 * |
| hsTnT | 0.4 ± 0.7 | 4.3 ± 3.8 | <0.0001 * | 3.048 | 1.423–6.527 | 0.004 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semerdzhieva, N.E.; Dimitrov, S.; Tsakova, A.; Gospodinova, M.; Atanasov, P.; Lozanova, V. Markers in Acute Coronary Syndrome: Distal Coronary Embolism at Percutaneous Coronary Intervention. J. Cardiovasc. Dev. Dis. 2025, 12, 315. https://doi.org/10.3390/jcdd12080315
Semerdzhieva NE, Dimitrov S, Tsakova A, Gospodinova M, Atanasov P, Lozanova V. Markers in Acute Coronary Syndrome: Distal Coronary Embolism at Percutaneous Coronary Intervention. Journal of Cardiovascular Development and Disease. 2025; 12(8):315. https://doi.org/10.3390/jcdd12080315
Chicago/Turabian StyleSemerdzhieva, Niya Emilova, Simeon Dimitrov, Adelina Tsakova, Mariana Gospodinova, Petar Atanasov, and Vesela Lozanova. 2025. "Markers in Acute Coronary Syndrome: Distal Coronary Embolism at Percutaneous Coronary Intervention" Journal of Cardiovascular Development and Disease 12, no. 8: 315. https://doi.org/10.3390/jcdd12080315
APA StyleSemerdzhieva, N. E., Dimitrov, S., Tsakova, A., Gospodinova, M., Atanasov, P., & Lozanova, V. (2025). Markers in Acute Coronary Syndrome: Distal Coronary Embolism at Percutaneous Coronary Intervention. Journal of Cardiovascular Development and Disease, 12(8), 315. https://doi.org/10.3390/jcdd12080315






