Decreased Aortic Elasticity in Noncompaction Cardiomyopathy Compared to Dilated Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
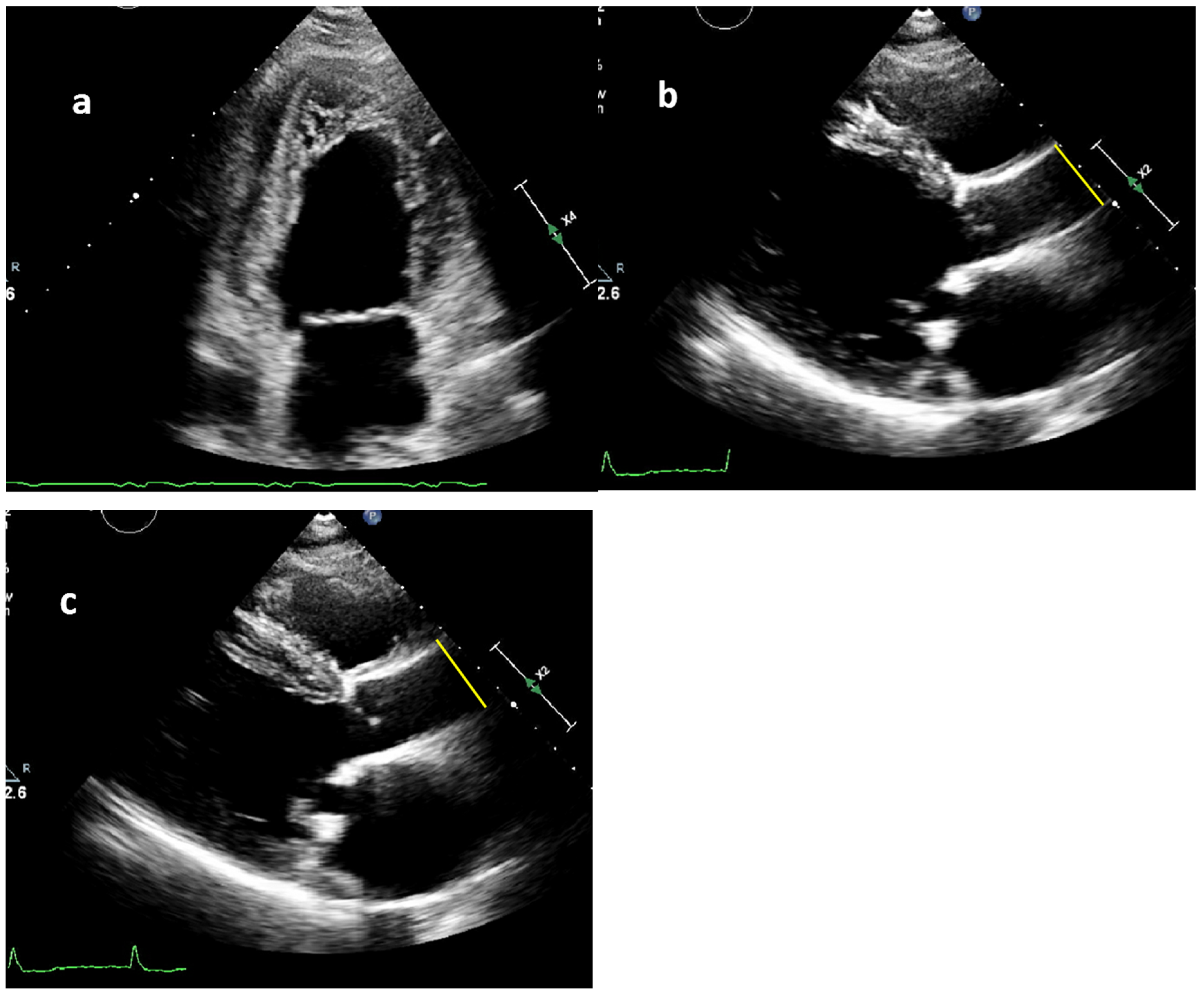
2.2. Measurement of Blood Pressure and Aortic Diameters
2.3. Aortic Stiffness Index
2.4. Statistics
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASI | Aortic stiffness index |
| DCM | Dilated cardiomyopathy |
| HF | Heart failure |
| ICD | Implantable cardioverter–defibrillator |
| LVAD | Left ventricular assist device |
| LV | Left ventricle |
| LVEF | Left ventricular ejection fraction |
| NCCM | Noncompaction cardiomyopathy |
| PLAX | Parasternal long-axis view |
| SCD | Sudden cardiac death |
| TTE | Transthoracic echocardiography |
References
- London, G.M.; Cohn, J.N. Prognostic application of arterial stiffness: Task forces. Am. J. Hypertens. 2002, 15, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Sciatti, E.; Vizzardi, E.; Bonadei, I.; Fabbricatore, D.; Prati, F.; Pagnoni, M.; Metra, M. Prognostic evaluation of the elastic properties of the ascending aorta in dilated cardiomyopathy. Eur. J. Clin. Investig. 2018, 48, e12950. [Google Scholar] [CrossRef] [PubMed]
- Cecelja, M.; Chowienczyk, P. Role of arterial stiffness in cardiovascular disease. JRSM Cardiovasc. Dis. 2012, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Sasayama, S.; Yagi, S.; Asakawa, T.; Hirai, T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc. Res. 1987, 21, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Boonyasirinant, T.; Rajiah, P.; Setser, R.M.; Lieber, M.L.; Lever, H.M.; Desai, M.Y.; Flamm, S.D. Aortic stiffness is increased in hypertrophic cardiomyopathy with myocardial fibrosis: Novel insights in vascular function from magnetic resonance imaging. J. Am. Coll. Cardiol. 2009, 54, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Vizzardi, E.; Caretta, G.; Bonadei, I.; Rovetta, R.; Sciatti, E.; Pezzali, N.; Lombardi, C.M.; Quinzani, F.; Salghetti, F.; D’Aloia, A.; et al. Echocardiographic elastic properties of ascending aorta and their relationship with exercise capacity in patients with non-ischemic dilated cardiomyopathy. Int. J. Cardiol. Heart Vessel. 2014, 3, 78–81. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, B.M.; Caliskan, K.; Soliman, O.I.; Kauer, F.; van der Zwaan, H.B.; Vletter, W.B.; van Vark, L.C.; Cate, F.J.T.; Geleijnse, M.L. Diagnostic value of rigid body rotation in noncompaction cardiomyopathy. J. Am. Soc. Echocardiogr. 2011, 24, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.; Khandheria, B.K.; Libhaber, E.; Maharaj, N.; Dos Santos, C.; Matioda, H.; Essop, M.R. Left ventricular twist in left ventricular noncompaction. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.L.; Tajik, A.J.; Wilansky, S.; Steidley, D.E.; Mookadam, F. Isolated noncompaction of the left ventricular myocardium in adults: A systematic overview. J. Card. Fail. 2011, 17, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, P.M.; Polovina, M.; Bauersachs, J.; Arad, M.; Ben Gal, T.; Lund, L.H.; Felix, S.B.; Arbustini, E.; Caforio, A.L.P.; Farmakis, D.; et al. Heart failure in cardiomyopathies: A position paper from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Jenni, R.; Oechslin, E.; Schneider, J.; Jost, C.A.; Kaufmann, P.A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: A step towards classification as a distinct cardiomyopathy. Heart 2001, 86, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; Blom, N.A.; De Boer, R.A.; et al. 2023 ESC Guidelines for the management of cardiomyopathies: Developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, A.; Flachskampf, F.A.; Erbel, R.; Antonini-Canterin, F.; Vlachopoulos, C.; Rocchi, G.; Sicari, R.; Nihoyannopoulos, P.; Zamorano, J.; European Association of Echocardiography. Echocardiography in aortic diseases: EAE recommendations for clinical practice. Eur. J. Echocardiogr. 2010, 11, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Patrianakos, A.P.; Parthenakis, F.I.; Nyktari, E.; Malliaraki, N.; Karakitsos, D.N.; Vardas, P.E. Central aortic stiffness in patients with nonischemic dilated cardiomyopathy: Relationship with neurohumoral activation. J. Card. Fail. 2009, 15, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Bonapace, S.; Rossi, A.; Cicoira, M.; Golia, G.; Zanolla, L.; Franceschini, L.; Conte, L.; Marino, P.; Zardini, P.; Vassanelli, C. Aortic stiffness correlates with an increased extracellular matrix turnover in patients with dilated cardiomyopathy. Am. Heart J. 2006, 152, 93.e1–93.e6. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Caliskan, K.; Geleijnse, M.L.; Soliman, O.I.; Anwar, A.M.; ten Cate, F.J. Alterations in aortic elasticity in noncompaction cardiomyopathy. Int. J. Cardiovasc. Imaging 2008, 24, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Jo, S.H. Arterial Stiffness and Heart Failure With Preserved Ejection Fraction. J. Korean Med. Sci. 2024, 39, e195. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Lombardo, M.; Baravelli, M.; Vincenti, A.; Rigamonti, E.; Vanoli, E.; Nicolosi, G.L.; Sommese, C.; Anzà, C. AnatoMy and physIopathoLogy of the heArt in a ceNtenarian cOhort (MILANO study). Am. Heart J. 2018, 205, 12–20. [Google Scholar] [CrossRef] [PubMed]
| Variable | DCM (n = 60) | NCCM (n = 60) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age at echo, years | 49 ± 16 | 49 ± 16 | 0.870 |
| Age at presentation, years | 37 ± 14 | 40 ± 16 | 0.286 |
| Males | 33 (55) | 33 (55) | |
| Comorbidities | |||
| Hypertension | 17 (28) | 13 (22) | 0.527 |
| Diabetes | 14 (23) | 1 (2) | 0.001 |
| Hypercholesterolemia | 4 (7) | 6 (10) | 0.741 |
| Coronary artery disease | 8 (13) | 5 (8) | 0.557 |
| Ventricular arrhythmia | 11 (18) | 10 (17) | 1.000 |
| Heart failure hospitalization | 15 (25) | 11 (18) | 0.506 |
| Stroke | 2 (3) | 3 (5) | 1.00 |
| CAD | 0 (0) | 1 (2) | 1.00 |
| Stroke | 2 (3) | 3 (5) | 1.00 |
| Primary presentation | |||
| Heart failure | 24 (40) | 18 (30) | 0.339 |
| Physical Examination | |||
| Height, m | 1.76 ± 10 | 1.76 ± 10 | 0.785 |
| Weight, kg | 82 ± 18 | 81 ± 17 | 0.626 |
| Systolic BP, mmHg | 117 ± 18 | 128 ± 16 | 0.001 |
| Diastolic BP, mmHg | 70 ± 10 | 77 ± 9 | 0.001 |
| Medication | |||
| Beta-receptor antagonist | 57 (95) | 44 (73) | 0.003 |
| ACE-inhibitor/ARB | 54 (90) | 39 (65) | 0.002 |
| Diuretics | 44 (73) | 14 (23) | <0.001 |
| Aldosterone receptor antagonist | 38 (63) | 13 (22) | <0.001 |
| Devices, | |||
| ICD implantation | 41 (70) | 28 (48) | 0.025 |
| Appropriate therapy | 8 (20) | 5 (19) | 1.000 |
| Inappropriate therapy | 2 (5) | 3 (11) | 0.628 |
| Variable | DCM (n = 60) | NCCM (n = 60) | p-Value |
|---|---|---|---|
| LA diameter, mm | 41 ± 8 | 39 ± 7 | 0.241 |
| LVED diameter, mm | 60 ± 11 | 58 ± 9 | 0.427 |
| LVES diameter, mm | 47 ± 16 | 44 ± 13 | 0.397 |
| LVEF, % | 34 ± 12 | 42 ± 11 | 0.004 |
| Aortic regurgitations | 3 (7) | 6 (11) | 0.623 |
| Systolic ascending aortic diameter, mm | 30 ± 5 | 31 ± 5 | 0.883 |
| Diastolic ascending aortic diameter, mm | 28 ± 4 | 29 ± 5 | 0.483 |
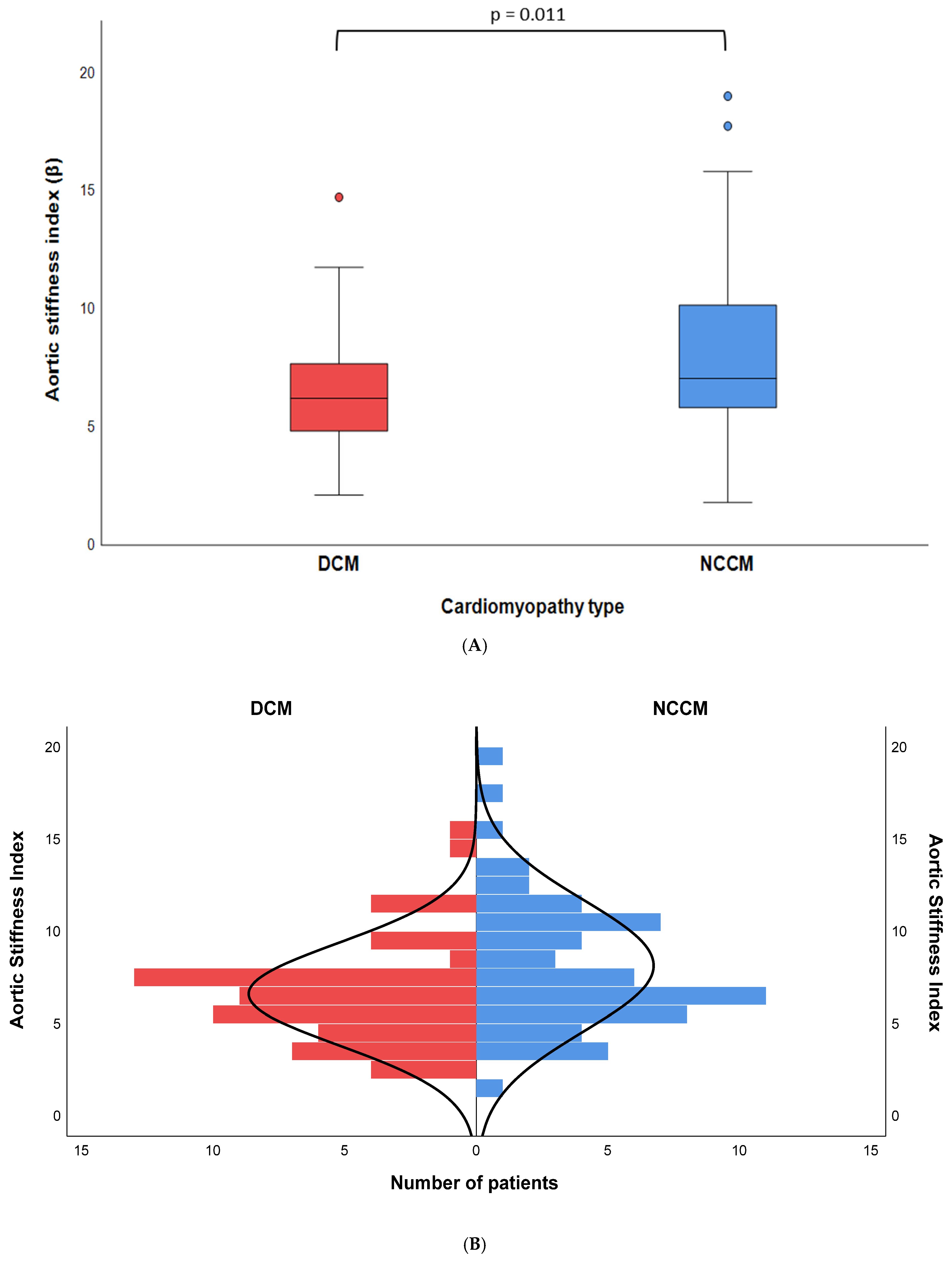
| Aortic stiffness index # | 6.2 [4.8–7.7] | 7.0 [5.8–10.2] | 0.011 |
| Model 1 Corrected for Age and Sex | Model 2 Corrected for Clinical Characteristics | Model 3 Corrected for Clinical Characteristics and Heart Failure Medication | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables: | β | CI 95% | p-Value | β | CI 95% | p-Value | β | CI 95% | p-Value |
| Noncompaction CMP | 1.714 | 0.651–2.778 | 0.002 | 1.667 | 0.220–3.114 | 0.025 | 1.771 | 0.253–3.289 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tukker, M.; Mohamedhoesein, S.; Kaya, E.; Schinkel, A.F.L.; Caliskan, K. Decreased Aortic Elasticity in Noncompaction Cardiomyopathy Compared to Dilated Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2025, 12, 303. https://doi.org/10.3390/jcdd12080303
Tukker M, Mohamedhoesein S, Kaya E, Schinkel AFL, Caliskan K. Decreased Aortic Elasticity in Noncompaction Cardiomyopathy Compared to Dilated Cardiomyopathy. Journal of Cardiovascular Development and Disease. 2025; 12(8):303. https://doi.org/10.3390/jcdd12080303
Chicago/Turabian StyleTukker, Martijn, Sharida Mohamedhoesein, Emrah Kaya, Arend F.L. Schinkel, and Kadir Caliskan. 2025. "Decreased Aortic Elasticity in Noncompaction Cardiomyopathy Compared to Dilated Cardiomyopathy" Journal of Cardiovascular Development and Disease 12, no. 8: 303. https://doi.org/10.3390/jcdd12080303
APA StyleTukker, M., Mohamedhoesein, S., Kaya, E., Schinkel, A. F. L., & Caliskan, K. (2025). Decreased Aortic Elasticity in Noncompaction Cardiomyopathy Compared to Dilated Cardiomyopathy. Journal of Cardiovascular Development and Disease, 12(8), 303. https://doi.org/10.3390/jcdd12080303






