Advancing Heart Failure Care Through Disease Management Programs: A Comprehensive Framework to Improve Outcomes
Abstract
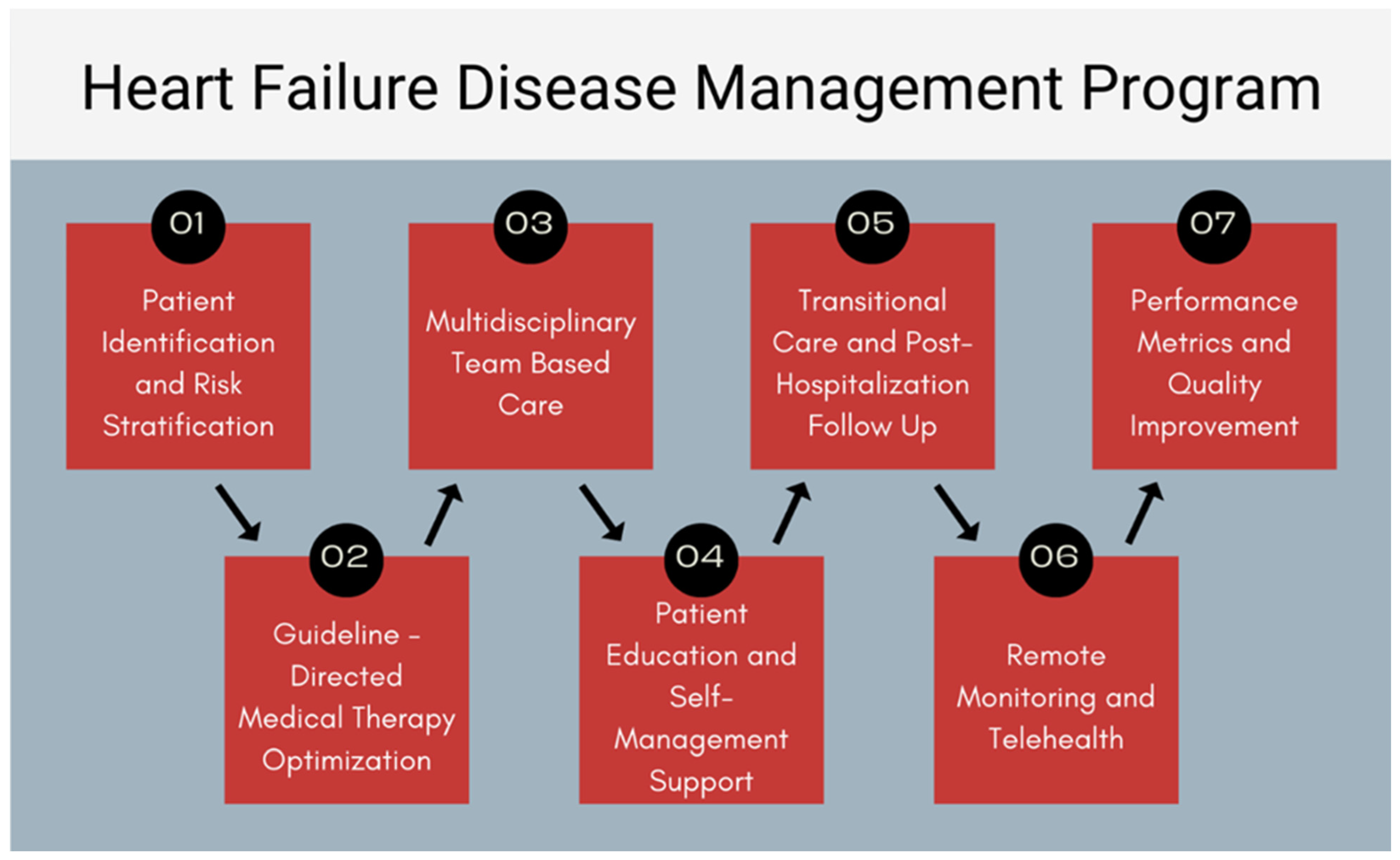
1. Introduction
2. Patient Identification and Risk Stratification
3. GDMT Implementation and Titration
- Angiotensin receptor–neprilysin inhibitors (ARNIs): sacubitril/valsartan demonstrated a 20% relative risk reduction in cardiovascular death or HF hospitalization compared to enalapril in the PARADIGM-HF trial [32].
- Beta-blockers (carvedilol, metoprolol succinate, and bisoprolol): these agents reduce all-cause mortality by approximately 30% [33].
- Mineralocorticoid receptor antagonists (MRAs): spironolactone and eplerenone significantly reduce mortality and HF hospitalizations [34].
- Sodium-glucose cotransporter 2 (SGLT2) inhibitors: dapagliflozin and empagliflozin have demonstrated reductions in HF hospitalization and cardiovascular death regardless of diabetes status [35].
4. Multidisciplinary Team-Based Care
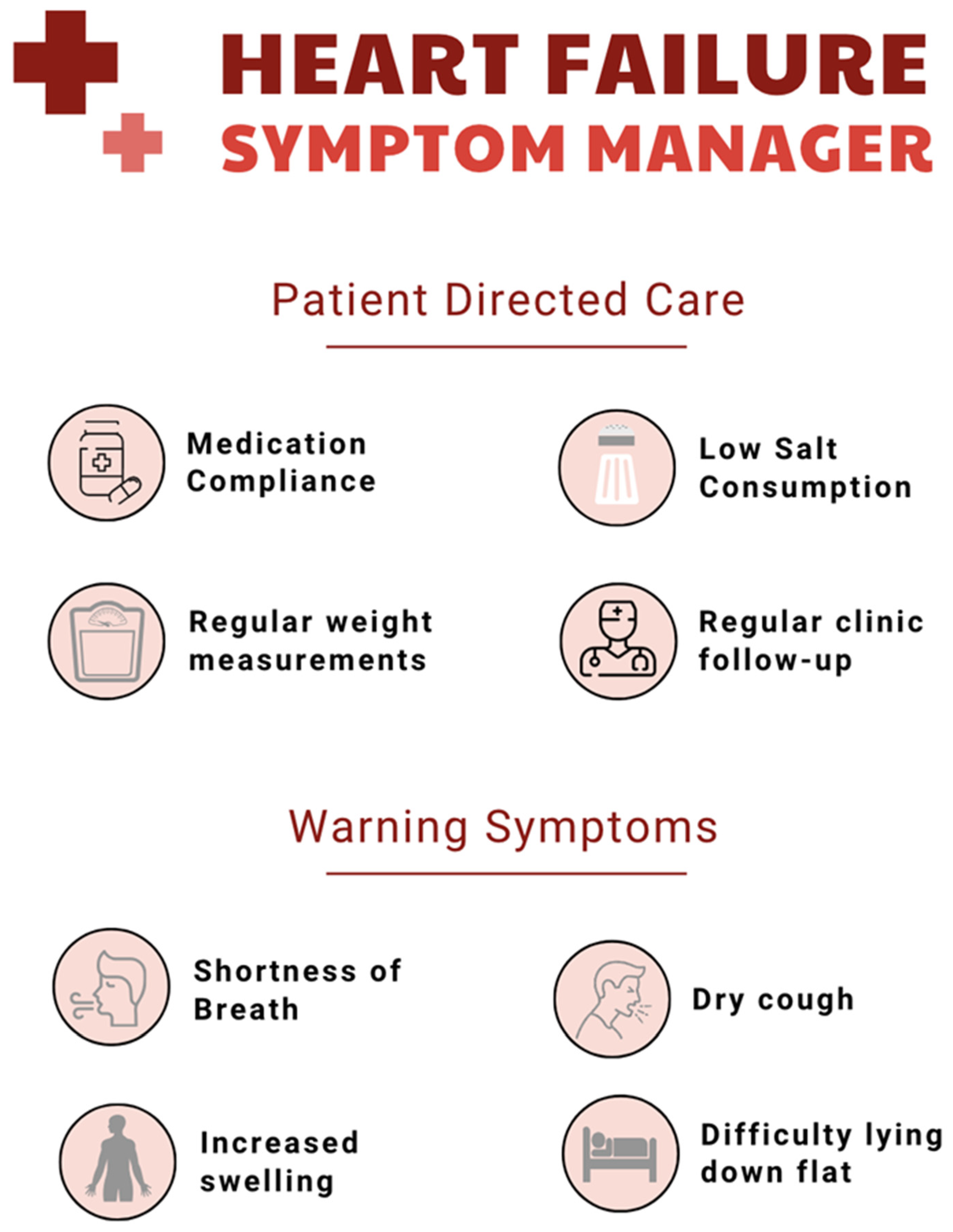
5. Patient Education and Self-Management Support
6. Transitional Care and Post-Hospitalization Follow-Up
7. Remote Monitoring and Telehealth
8. Performance Metrics and QI
9. Social Determinants of Health
10. Challenges and Limitations
11. Conclusions
12. Future Directions
- Integration of artificial intelligence (AI): AI-powered risk prediction models and ECG interpretation tools may enable real-time, personalized interventions.
- Expansion of virtual care: enhanced telemonitoring and remote titration clinics will increase access, especially for rural and underserved populations.
- Equity-driven customization: tailoring HF-DMPs to address disparities in access, health literacy, and social needs will be essential to achieving health equity.
- Outcomes-based research: large-scale studies are needed to evaluate the long-term impact of HF-DMPs on mortality, quality of life, and cost-effectiveness across various healthcare settings.
- National benchmarking: widespread adoption of performance metrics will allow for standardized comparisons and continuous quality improvement efforts.
- Rural health focus: investment in rural telehealth infrastructure, culturally sensitive patient education, and policy incentives will augment sustainable multidisciplinary care delivery.
Author Contributions
Funding
Conflicts of Interest
References
- Bozkurt, B.; Ahmad, T.; Alexander, K.; Baker, W.L.; Bosak, K.; Breathett, K.; Carter, S.; Drazner, M.H.; Dunlay, S.M.; Fonarow, G.C.; et al. HF STATS 2024: Heart Failure Epidemiology and Outcomes Statistics an Updated 2024 Report from the Heart Failure Society of America. J. Card. Fail. 2025, 31, 66–116. [Google Scholar] [CrossRef]
- Cooper, L.B.; Hernandez, A.F. Assessing the Quality and Comparative Effectiveness of Team-Based Care for HF: Who, What, Where, When, and How. Heart Fail. Clin. 2015, 11, 499–506. [Google Scholar] [CrossRef]
- Xia, J.; Brownell, N.K.; Fonarow, G.C.; Ziaeian, B. New Models for Heart Failure Care Delivery. Prog. Cardiovasc. Dis. 2024, 82, 70–89. [Google Scholar] [CrossRef]
- Abraham, W.T.; Adamson, P.B.; Bourge, R.C.; Aaron, M.F.; Costanzo, M.R.; Stevenson, L.W.; Strickland, W.; Neelagaru, S.; Raval, N.; Krueger, S.; et al. Wireless Pulmonary Artery Haemodynamic Monitoring in Chronic Heart Failure: A Randomised Controlled Trial. Lancet 2011, 377, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Van Grootven, B.; Jepma, P.; Rijpkema, C.; Verweij, L.; Leeflang, M.; Daams, J.; Deschodt, M.; Milisen, K.; Flamaing, J.; Buurman, B. Prediction Models for Hospital Readmissions in Patients with Heart Disease: A Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e047576. [Google Scholar] [CrossRef]
- Kao, D. Electronic Health Records and Heart Failure. Heart Fail. Clin. 2022, 18, 201–211. [Google Scholar] [CrossRef]
- Collins, G.S.; Moons, K.G.M.; Dhiman, P.; Riley, R.D.; Beam, A.L.; Van Calster, B.; Ghassemi, M.; Liu, X.; Reitsma, J.B.; van Smeden, M.; et al. TRIPOD+AI Statement: Updated Guidance for Reporting Clinical Prediction Models that Use Regression or Machine Learning Methods. BMJ 2024, 385, e078378. [Google Scholar] [CrossRef]
- Welsh, P.; Papacosta, O.; Ramsay, S.; Whincup, P.; McMurray, J.; Wannamethee, G.; Sattar, N. High-Sensitivity Troponin T and Incident Heart Failure in Older Men: British Regional Heart Study. J. Card. Fail. 2019, 25, 230–237. [Google Scholar] [CrossRef]
- Kelder, J.C.; Cowie, M.R.; McDonagh, T.A.; Hardman, S.M.C.; Grobbee, D.E.; Cost, B.; Hoes, A.W. Quantifying the Added Value of BNP in Suspected Heart Failure in General Practice: An Individual Patient Data Meta-Analysis. Heart 2011, 97, 959–963. [Google Scholar] [CrossRef]
- Hill, S.A.; Booth, R.A.; Santaguida, P.L.; Don-Wauchope, A.; Brown, J.A.; Oremus, M.; Ali, U.; Bustamam, A.; Sohel, N.; McKelvie, R.; et al. Use of BNP and NT-proBNP for the Diagnosis of Heart Failure in the Emergency Department: A Systematic Review of the Evidence. Heart Fail. Rev. 2014, 19, 421–438. [Google Scholar] [CrossRef]
- Rivera, A.S.; Sinha, A.; Ahmad, F.S.; Thorp, E.; Wilcox, J.E.; Lloyd-Jones, D.M.; Feinstein, M.J. Long-Term Trajectories of Left Ventricular Ejection Fraction in Patients with Chronic Inflammatory Diseases and Heart Failure: An Analysis of Electronic Health Records. Circ. Heart Fail. 2021, 14, e008478. [Google Scholar] [CrossRef]
- Nuzzi, V.; Raafs, A.; Manca, P.; Henkens, M.T.H.M.; Gregorio, C.; Boscutti, A.; Verdonschot, J.; Hazebroek, M.; Knackstedt, C.; Merlo, M.; et al. Left Atrial Reverse Remodeling in Dilated Cardiomyopathy. J. Am. Soc. Echocardiogr. 2023, 36, 154–162. [Google Scholar] [CrossRef]
- Rich, J.D.; Burns, J.; Freed, B.H.; Maurer, M.S.; Burkhoff, D.; Shah, S.J. Meta-Analysis Global Group in Chronic (MAGGIC) Heart Failure Risk Score: Validation of a Simple Tool for the Prediction of Morbidity and Mortality in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e009594. [Google Scholar] [CrossRef]
- Rajaguru, V.; Han, W.; Kim, T.H.; Shin, J.; Lee, S.G. LACE Index to Predict the High Risk of 30-Day Readmission: A Systematic Review and Meta-Analysis. J. Pers. Med. 2022, 12, 545. [Google Scholar] [CrossRef]
- Yu, S.; Farooq, F.; van Esbroeck, A.; Fung, G.; Anand, V.; Krishnapuram, B. Predicting Readmission Risk with Institution-Specific Prediction Models. Artif. Intell. Med. 2015, 65, 89–96. [Google Scholar] [CrossRef]
- Michaels, A.; Aurora, L.; Peterson, E.; Liu, B.; Pinto, Y.M.; Sabbah, H.N.; Williams, K.; Lanfear, D.E. Risk Prediction in Transition: MAGGIC Score Performance at Discharge and Incremental Utility of Natriuretic Peptides. J. Card. Fail. 2020, 26, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.L.; Cheon, O.; Manzano, J.-G.M.; Park, A.K.; Lin, H.Y.; Halm, J.K.; Baek, J.; Graviss, E.A.; Nguyen, D.T.; Kash, B.A.; et al. Comparison of LACE and HOSPITAL Readmission Risk Scores for CMS Target and Nontarget Conditions. Am. J. Med. Qual. 2022, 37, 299–306. [Google Scholar] [CrossRef]
- Tan, S.Y.; Sumner, J.; Wang, Y.; Yip, A.W. A Systematic Review of the Impacts of Remote Patient Monitoring (RPM) Interventions on Safety, Adherence, Quality-of-Life and Cost-Related Outcomes. NPJ Digit. Med. 2024, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Drazner, M.H.; Rame, J.E.; Stevenson, L.W.; Dries, D.L. Prognostic Importance of Elevated Jugular Venous Pressure and a Third Heart Sound in Patients with Heart Failure. N. Engl. J. Med. 2001, 345, 574–581. [Google Scholar] [CrossRef]
- Maier, S.K.G.; Paule, S.; Jung, W.; Koller, M.; Ventura, R.; Quesada, A.; Bordachar, P.; García-Fernández, F.J.; Schumacher, B.; Lobitz, N.; et al. Evaluation of Thoracic Impedance Trends for Implant-Based Remote Monitoring in Heart Failure Patients—Results from the (J-)HomeCARE-II Study. J. Electrocardiol. 2019, 53, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Koole, M.A.C.; Kauw, D.; Winter, M.M.; Dohmen, D.a.J.; Tulevski, I.I.; de Haan, R.; Somsen, G.A.; Schijven, M.P.; Robbers-Visser, D.; Mulder, B.J.M.; et al. First Real-World Experience with Mobile Health Telemonitoring in Adult Patients with Congenital Heart Disease. Neth. Heart J. 2019, 27, 30–37. [Google Scholar] [CrossRef]
- Chaudhry, S.I.; Mattera, J.A.; Curtis, J.P.; Spertus, J.A.; Herrin, J.; Lin, Z.; Phillips, C.O.; Hodshon, B.V.; Cooper, L.S.; Krumholz, H.M. Telemonitoring in Patients with Heart Failure. N. Engl. J. Med. 2010, 363, 2301–2309. [Google Scholar] [CrossRef]
- Abraham, W.T.; Stevenson, L.W.; Bourge, R.C.; Lindenfeld, J.A.; Bauman, J.G.; Adamson, P.B. CHAMPION Trial Study Group Sustained Efficacy of Pulmonary Artery Pressure to Guide Adjustment of Chronic Heart Failure Therapy: Complete Follow-up Results from the CHAMPION Randomised Trial. Lancet 2016, 387, 453–461. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, A.; Khanna, N.N.; Laird, J.R.; Nicolaides, A.; Faa, G.; Johri, A.M.; Mantella, L.E.; Fernandes, J.F.E.; Teji, J.S.; et al. Artificial Intelligence for Cardiovascular Disease Risk Assessment in Personalised Framework: A Scoping Review. eClinicalMedicine 2024, 73, 102660. [Google Scholar] [CrossRef] [PubMed]
- AI-Driven Technology in Heart Failure Detection and Diagnosis: A Review of the Advancement in Personalized Healthcare. Available online: https://www.mdpi.com/2073-8994/17/3/469 (accessed on 2 July 2025).
- Xie, Y.; Zhang, L.; Sun, W.; Zhu, Y.; Zhang, Z.; Chen, L.; Xie, M.; Zhang, L. Artificial Intelligence in Diagnosis of Heart Failure. J. Am. Heart Assoc. 2025, 14, e039511. [Google Scholar] [CrossRef]
- Croon, P.M.; Selder, J.L.; Allaart, C.P.; Bleijendaal, H.; Chamuleau, S.A.J.; Hofstra, L.; Išgum, I.; Ziesemer, K.A.; Winter, M.M. Current State of Artificial Intelligence-Based Algorithms for Hospital Admission Prediction in Patients with Heart Failure: A Scoping Review. Eur. Heart J. Digit. Health 2022, 3, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, L.S.; Aminorroaya, A.; Sangha, V.; Pedroso, A.F.; Asselbergs, F.W.; Brant, L.C.C.; Barreto, S.M.; Ribeiro, A.L.P.; Krumholz, H.M.; Oikonomou, E.K.; et al. Heart Failure Risk Stratification Using Artificial Intelligence Applied to Electrocardiogram Images: A Multinational Study. Eur. Heart J. 2025, 46, 1044–1053. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure with Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Effect of Metoprolol CR/XL in Chronic Heart Failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [CrossRef]
- Pitt, B.; Zannad, F.; Remme, W.J.; Cody, R.; Castaigne, A.; Perez, A.; Palensky, J.; Wittes, J. The Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999, 341, 709–717. [Google Scholar] [CrossRef]
- Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction|New England Journal of Medicine. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa1911303 (accessed on 30 May 2025).
- Tomasoni, D.; Adamo, M.; Bozkurt, B.; Heidenreich, P.; McDonagh, T.; Rosano, G.M.C.; Virani, S.A.; Zieroth, S.; Metra, M. Aiming at Harmony. Comparing and Contrasting International HFrEF Guidelines. Eur. Heart J. Suppl. 2022, 24, L20–L28. [Google Scholar] [CrossRef]
- Gislason, G.H.; Rasmussen, J.N.; Abildstrøm, S.Z.; Gadsbøll, N.; Buch, P.; Friberg, J.; Rasmussen, S.; Køber, L.; Stender, S.; Madsen, M.; et al. Long-Term Compliance with Beta-Blockers, Angiotensin-Converting Enzyme Inhibitors, and Statins after Acute Myocardial Infarction. Eur. Heart J. 2006, 27, 1153–1158. [Google Scholar] [CrossRef]
- Jaarsma, T.; Hill, L.; Bayes-Genis, A.; La Rocca, H.B.; Castiello, T.; Čelutkienė, J.; Marques-Sule, E.; Plymen, C.M.; Piper, S.E.; Riegel, B.; et al. Self-care of Heart Failure Patients: Practical Management Recommendations from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2021, 23, 157–174. [Google Scholar] [CrossRef]
- Dizdarevic-Hudic, L.; Halilovic, E.; Brkic, S.; Loncar, D.; Jahic, N.A.; Hudic, I.; Ibralic, A.M.; Suljic, Z.; Dizdarevic-Hudic, L.; Halilovic, E.; et al. The Impact of Patient Education on Rehospitalization Rate and Quality of Life in Heart Failure Patients. Int. J. Cardiovasc. Acad. 2025, 11, 6–14. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, Tolerability and Efficacy of up-Titration of Guideline-Directed Medical Therapies for Acute Heart Failure (STRONG-HF): A Multinational, Open-Label, Randomised, Trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Figtree, G.A.; Broadfoot, K.; Casadei, B.; Califf, R.; Crea, F.; Drummond, G.R.; Freedman, J.E.; Guzik, T.J.; Harrison, D.; Hausenloy, D.J.; et al. A Call to Action for New Global Approaches to Cardiovascular Disease Drug Solutions. Circulation 2021, 144, 159–169. [Google Scholar] [CrossRef]
- Patil, T.; Ali, S.; Kaur, A.; Akridge, M.; Eppes, D.; Paarlberg, J.; Parashar, A.; Jarmukli, N. Impact of Pharmacist-Led Heart Failure Clinic on Optimization of Guideline-Directed Medical Therapy (PHARM-HF). J. Cardiovasc. Transl. Res. 2022, 15, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Lynch, K.A.; Ganz, D.A.; Saliba, D.; Chang, D.S.; de Peralta, S.S. Improving Heart Failure Care and Guideline-Directed Medical Therapy through Proactive Remote Patient Monitoring-Home Telehealth and Pharmacy Integration. BMJ Open Qual. 2022, 11, e001901. [Google Scholar] [CrossRef]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Feltner, C.; Jones, C.D.; Cené, C.W.; Zheng, Z.-J.; Sueta, C.A.; Coker-Schwimmer, E.J.L.; Arvanitis, M.; Lohr, K.N.; Middleton, J.C.; Jonas, D.E. Transitional Care Interventions to Prevent Readmissions for Persons with Heart Failure: A Systematic Review and Meta-Analysis. Ann. Intern. Med. 2014, 160, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Bekelman, D.B.; Nowels, C.T.; Retrum, J.H.; Allen, L.A.; Shakar, S.; Hutt, E.; Heyborne, T.; Main, D.S.; Kutner, J.S. Giving Voice to Patients’ and Family Caregivers’ Needs in Chronic Heart Failure: Implications for Palliative Care Programs. J. Palliat. Med. 2011, 14, 1317–1324. [Google Scholar] [CrossRef]
- Allen, L.A.; Stevenson, L.W.; Grady, K.L.; Goldstein, N.E.; Matlock, D.D.; Arnold, R.M.; Cook, N.R.; Felker, G.M.; Francis, G.S.; Hauptman, P.J.; et al. Decision Making in Advanced Heart Failure a Scientific Statement from the American Heart Association. Circulation 2012, 125, 1928–1952. [Google Scholar] [CrossRef] [PubMed]
- Trends in 30- and 90-Day Readmission Rates for Heart Failure|Circulation: Heart Failure. Available online: https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.121.008335 (accessed on 2 July 2025).
- Heart Failure Disease Management: A Systematic Review of Effectiveness in Heart Failure with Preserved Ejection Fraction—PMC. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC7083420/ (accessed on 2 July 2025).
- Ong, M.K.; Romano, P.S.; Edgington, S.; Aronow, H.U.; Auerbach, A.D.; Black, J.T.; De Marco, T.; Escarce, J.J.; Evangelista, L.S.; Hanna, B.; et al. Effectiveness of Remote Patient Monitoring after Discharge of Hospitalized Patients with Heart Failure: The Better Effectiveness After Transition–Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern. Med. 2016, 176, 310–318. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Jacelon, C. Impact of Telehealth on Patient Self-Management of Heart Failure: A Review of Literature. J. Cardiovasc. Nurs. 2012, 27, 33–43. [Google Scholar] [CrossRef]
- Managing Sodium and Fluid Intake to Improve Heart Failure Outcomes—Mayo Clinic. Available online: https://www.mayoclinic.org/medical-professionals/transplant-medicine/news/managing-sodium-and-fluid-intake-to-improve-heart-failure-outcomes/mac-20572755 (accessed on 1 July 2025).
- Tight Fluid Restriction Not Needed in Chronic HF Patients: FRESH-UP|Tctmd.Com. Available online: https://www.tctmd.com/news/tight-fluid-restriction-not-needed-chronic-hf-patients-fresh (accessed on 1 July 2025).
- Herrmann, J.J.; Beckers-Wesche, F.; Baltussen, L.E.H.J.M.; Verdijk, M.H.I.; Bellersen, L.; Rocca, H.-P.B.; Jaarsma, T.; Pisters, R.; Wijk, S.S.; Rodwell, L.; et al. Fluid REStriction in Heart Failure vs Liberal Fluid UPtake: Rationale and Design of the Randomized FRESH-UP Study. J. Card. Fail. 2022, 28, 1522–1530. [Google Scholar] [CrossRef]
- Living with Heart Failure and Managing Advanced HF. Available online: https://www.heart.org/en/health-topics/heart-failure/living-with-heart-failure-and-managing-advanced-hf (accessed on 28 November 2024).
- Ha Dinh, T.T.; Bonner, A.; Clark, R.; Ramsbotham, J.; Hines, S. The Effectiveness of the Teach-Back Method on Adherence and Self-Management in Health Education for People with Chronic Disease: A Systematic Review. JBI Database Syst. Rev. Implement. Rep. 2016, 14, 210–247. [Google Scholar] [CrossRef]
- Talevski, J.; Shee, A.W.; Rasmussen, B.; Kemp, G.; Beauchamp, A. Teach-Back: A Systematic Review of Implementation and Impacts. PLoS ONE 2020, 15, e0231350. [Google Scholar] [CrossRef]
- CDC Guidance & Tools. Available online: https://www.cdc.gov/health-literacy/php/develop-materials/guidance-standards.html (accessed on 1 July 2025).
- CDCTobaccoFree How to Quit Smoking. Available online: https://www.cdc.gov/tobacco/campaign/tips/quit-smoking/index.html (accessed on 1 July 2025).
- Verma, V.; Zhang, M.; Bell, M.; Tarolli, K.; Donalson, E.; Vaughn, J.; Hickey, G.W. Outpatient Intravenous Diuretic Clinic: An Effective Strategy for Management of Volume Overload and Reducing Immediate Hospital Admissions. J. Clin. Med. Res. 2021, 13, 245–251. [Google Scholar] [CrossRef]
- Takahashi, E.A.; Schwamm, L.H.; Adeoye, O.M.; Alabi, O.; Jahangir, E.; Misra, S.; Still, C.H. An Overview of Telehealth in the Management of Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2022, 146, e558–e568. [Google Scholar] [CrossRef]
- Recommendations for the Implementation of Telehealth in Cardiovascular and Stroke Care: A Policy Statement from the American Heart Association|Circulation. Available online: https://www.ahajournals.org/doi/10.1161/cir.0000000000000475 (accessed on 1 July 2025).
- Scholte, N.T.B.; Gürgöze, M.T.; Aydin, D.; Theuns, D.A.M.J.; Manintveld, O.C.; Ronner, E.; Boersma, E.; de Boer, R.A.; van der Boon, R.M.A.; Brugts, J.J. Telemonitoring for Heart Failure: A Meta-Analysis. Eur. Heart. J. 2023, 44, 2911–2926. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.-A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of Telemedical Interventional Management in Patients with Heart Failure (TIM-HF2): A Randomised, Controlled, Parallel-Group, Unmasked Trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Radhoe, S.P.; Veenis, J.F.; Brugts, J.J. Invasive Devices and Sensors for Remote Care of Heart Failure Patients. Sensors 2021, 21, 2014. [Google Scholar] [CrossRef]
- Bourge, R.C.; Abraham, W.T.; Adamson, P.B.; Aaron, M.F.; Aranda, J.M.; Magalski, A.; Zile, M.R.; Smith, A.L.; Smart, F.W.; O’Shaughnessy, M.A.; et al. Randomized Controlled Trial of an Implantable Continuous Hemodynamic Monitor in Patients with Advanced Heart Failure: The COMPASS-HF Study. J. Am. Coll. Cardiol. 2008, 51, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.W.; Ross, H.J.; Rathman, L.D.; Boehmer, J.P. Remote Monitoring for Heart Failure Management at Home. J. Am. Coll. Cardiol. 2023, 81, 2272–2291. [Google Scholar] [CrossRef] [PubMed]
- Telehealth and Health Equity in Older Adults with Heart Failure: A Scientific Statement from the American Heart Association|Circulation: Cardiovascular Quality and Outcomes. Available online: https://www.ahajournals.org/doi/10.1161/HCQ.0000000000000123 (accessed on 1 July 2025).
- Home-Centers for Medicare & Medicaid Services|CMS. Available online: https://www.cms.gov/ (accessed on 1 July 2025).
- Vizient Inc.|Member-Driven Healthcare Performance Improvement. Available online: https://www.vizientinc.com/ (accessed on 1 July 2025).
- Green, C.P.; Porter, C.B.; Bresnahan, D.R.; Spertus, J.A. Development and Evaluation of the Kansas City Cardiomyopathy Questionnaire: A New Health Status Measure for Heart Failure. J. Am. Coll. Cardiol. 2000, 35, 1245–1255. [Google Scholar] [CrossRef]
- Homepage|PCORI. Available online: https://www.pcori.org/ (accessed on 1 July 2025).
- Provider Data Catalog. Available online: https://data.cms.gov/provider-data/?redirect=true (accessed on 1 July 2025).
- HFSA 2010 Comprehensive Heart Failure Practice Guideline—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20610207/ (accessed on 2 July 2025).
- UNOS|United Network for Organ Sharing|US Organ Transplantation. Available online: https://unos.org/ (accessed on 1 July 2025).
- Kavalieratos, D.; Gelfman, L.P.; Tycon, L.E.; Riegel, B.; Bekelman, D.B.; Ikejiani, D.Z.; Goldstein, N.; Kimmel, S.E.; Bakitas, M.A.; Arnold, R.M. Palliative Care in Heart Failure: Rationale, Evidence, and Future Priorities. J. Am. Coll. Cardiol. 2017, 70, 1919–1930. [Google Scholar] [CrossRef]
- Foroutan, F.; Rayner, D.G.; Ross, H.J.; Ehler, T.; Srivastava, A.; Shin, S.; Malik, A.; Benipal, H.; Yu, C.; Alexander, L.T.H.; et al. Global Comparison of Readmission Rates for Patients with Heart Failure. JACC 2023, 82, 430–444. [Google Scholar] [CrossRef]
- AlHabeeb, W. Heart Failure Disease Management Program: A Review. Medicine 2022, 101, e29805. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.A.; Ayodele, I.; Bress, A.P.; Sterling, M.R.; Pandey, A.; Derington, C.G.; Zheutlin, A.R.; Shah, K.S.; Greene, S.J.; Alhanti, B.; et al. Social Determinants of Health and Disparities in Guideline-Directed Medical Therapy Optimization for Heart Failure. Circ. Heart Fail. 2025, 18, e012357. [Google Scholar] [CrossRef] [PubMed]
- Addressing Social Determinants of Health in the Care of Patients with Heart Failure: A Scientific Statement From the American Heart Association|Circulation. Available online: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000767 (accessed on 2 July 2025).
- Pierce, J.B.; Ikeaba, U.; Peters, A.E.; DeVore, A.D.; Chiswell, K.; Allen, L.A.; Albert, N.M.; Yancy, C.W.; Fonarow, G.C.; Greene, S.J. Quality of Care and Outcomes Among Patients Hospitalized for Heart Failure in Rural vs Urban US Hospitals. JAMA Cardiol. 2023, 8, 376–385. [Google Scholar] [CrossRef]
- Lam, K.; Lu, A.D.; Shi, Y.; Covinsky, K.E. Assessing Telemedicine Unreadiness Among Older Adults in the United States during the COVID-19 Pandemic. JAMA Intern. Med. 2020, 180, 1389–1391. [Google Scholar] [CrossRef]
- Albert, M.A.; Churchwell, K.; Desai, N.; Johnson, J.C.; Johnson, M.N.; Khera, A.; Mieres, J.H.; Rodriguez, F.; Velarde, G.; Williams, D.R.; et al. Addressing Structural Racism Through Public Policy Advocacy: A Policy Statement from the American Heart Association. Circulation 2024, 149, e312–e329. [Google Scholar] [CrossRef] [PubMed]
| Category | Metric | Description | Organization | References |
|---|---|---|---|---|
| Process | Documentation of LVEF | Percent of HF patients with documented assessment of LVEF | AHA/ACC, CMS | [29,69] |
| Process | GDMT Prescription | ACEi/ARB/ARNI and beta-blocker prescribed in HFrEF | AHA/ACC, CMS | [29,55,69] |
| Process | Aldosterone Antagonist Use | For patients with LVEF ≤ 35%, if no contraindications | ACC/AHA/HFSA | [29,55,69] |
| Outcome | 30-Day Readmission Rate | Risk-adjusted rate of unplanned readmission after HF discharge | CMS Hospital Compare | [55,73] |
| Outcome | In-hospital Mortality | All-cause inpatient mortality in HF hospitalizations | CMS, Vizient | [69,70] |
| Outcome | Health Status/Quality of Life | Patient-reported outcomes using tools like KCCQ | PCORI | [71,72] |
| Structural | Presence of HF Program | Availability of multidisciplinary HF disease management team | HFSA | [74] |
| Structural | Access to Advanced HF Therapies | Transplant, LVAD, and palliative services | UNOS, HFSA | [29,75,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inam, M.; Sangrigoli, R.M.; Ruppert, L.; Saiganesh, P.; Hamad, E.A. Advancing Heart Failure Care Through Disease Management Programs: A Comprehensive Framework to Improve Outcomes. J. Cardiovasc. Dev. Dis. 2025, 12, 302. https://doi.org/10.3390/jcdd12080302
Inam M, Sangrigoli RM, Ruppert L, Saiganesh P, Hamad EA. Advancing Heart Failure Care Through Disease Management Programs: A Comprehensive Framework to Improve Outcomes. Journal of Cardiovascular Development and Disease. 2025; 12(8):302. https://doi.org/10.3390/jcdd12080302
Chicago/Turabian StyleInam, Maha, Robert M. Sangrigoli, Linda Ruppert, Pooja Saiganesh, and Eman A. Hamad. 2025. "Advancing Heart Failure Care Through Disease Management Programs: A Comprehensive Framework to Improve Outcomes" Journal of Cardiovascular Development and Disease 12, no. 8: 302. https://doi.org/10.3390/jcdd12080302
APA StyleInam, M., Sangrigoli, R. M., Ruppert, L., Saiganesh, P., & Hamad, E. A. (2025). Advancing Heart Failure Care Through Disease Management Programs: A Comprehensive Framework to Improve Outcomes. Journal of Cardiovascular Development and Disease, 12(8), 302. https://doi.org/10.3390/jcdd12080302







