Clinical Outcomes of Surgical Revascularization in Patients Presenting with Critical Limb Ischemia and Aortic Valve Stenosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Cardiac Evaluation
2.3. Surgical Details and Follow-Up Protocol
2.4. Definitions
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Clinical Profile, Anatomic Characteristics, and Surgical Details
3.2. Bypass Related Outcomes
3.3. Clinical Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review a + nd analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Manning, W.J. Asymptomatic aortic stenosis in the elderly: A clinical review. JAMA 2013, 310, 1490–1497. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef]
- Holmes, D.R., Jr.; Brennan, J.M.; Rumsfeld, J.S.; Dai, D.; O’Brien, S.M.; Vemulapalli, S.; Edwards, F.H.; Carroll, J.; Shahian, D.; Grover, F.; et al. Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA 2015, 313, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Malyar, N.M.; Kaier, K.; Freisinger, E.; Lüders, F.; Kaleschke, G.; Baumgartner, H.; Frankenstein, L.; Reinecke, H.; Reinöhl, J. Prevalence and impact of critical limb ischaemia on in-hospital outcome in transcatheter aortic valve implantation in Germany. EuroIntervention 2017, 13, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J. Vasc. Surg. 2019, 69, 3S–125S.e40. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev. Esp. Cardiol. 2022, 75, 524. [Google Scholar] [CrossRef] [PubMed]
- Stoner, M.C.; Calligaro, K.D.; Chaer, R.A.; Dietzek, A.M.; Farber, A.; Guzman, R.J.; Hamdan, A.D.; Landry, G.J.; Yamaguchi, D.J.; on behalf of the Society for Vascular Surgery. Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J. Vasc. Surg. 2016, 64, 1–21. [Google Scholar] [CrossRef]
- Ueshima, D.; Barioli, A.; Nai Fovino, L.; D’Amico, G.; Fabris, T.; Brener, S.J.; Tarantini, G. The impact of pre-existing peripheral artery disease on transcatheter aortic valve implantation outcomes: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2020, 95, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.B.; Elzeneini, M.; Neal, D.; Kamisetty, S.; Winchester, D.; Shah, S.K. Chronic Limb-Threatening Ischemia Is Associated with Higher Mortality and Limb Revascularization After Transcatheter Aortic Valve Replacement. Am. J. Cardiol. 2023, 207, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Romiti, M.; Albers, M.; Brochado-Neto, F.C.; Durazzo, A.E.; Pereira, C.A.; De Luccia, N. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J. Vasc. Surg. 2008, 47, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive summary; a report of the American College of Cardiology/American Heart Association Task Force on Practice. Circulation 2014, 129, 2440–2492. [Google Scholar] [CrossRef] [PubMed]
- Uhl, C.; Hock, C.; Ayx, I.; Zorger, N.; Steinbauer, M.; Töpel, I. Tibial and peroneal bypasses in octogenarians and nonoctogenarians with critical limb ischemia. J. Vasc. Surg. 2016, 63, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Morisaki, K.; Matsuda, D.; Guntani, A.; Aoyagi, T.; Kinoshita, G.; Yoshino, S.; Inoue, K.; Honma, K.; Yamaoka, T.; Mii, S.; et al. Treatment Outcomes in Octogenarians with Chronic Limb-Threatening Ischemia after Infrainguinal Bypass Surgery or Endovascular Therapy. Ann. Vasc. Surg. 2024, 106, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Molnár, A.Á.; Nádasy, G.L.; Dörnyei, G.; Patai, B.B.; Delfavero, J.; Fülöp, G.Á.; Kirkpatrick, A.C.; Ungvári, Z.; Merkely, B. The aging venous system: From varicosities to vascular cognitive impairment. Geroscience 2021, 43, 2761–2784. [Google Scholar] [CrossRef] [PubMed]
- Kvaslerud, A.B.; Santic, K.; Hussain, A.I.; Auensen, A.; Fiane, A.; Skulstad, H.; Aaberge, L.; Gullestad, L.; Broch, K. Outcomes in asymptomatic, severe aortic stenosis. PLoS ONE 2021, 16, e0249610. [Google Scholar] [CrossRef] [PubMed]
- Bellosta, R.; Piffaretti, G.; Bonardelli, S.; Castelli, P.; Chiesa, R.; Frigerio, D.; Lanza, G.; Pirrelli, S.; Rossi, G.; Trimarchi, S.; et al. Regional Survey in Lombardy, Northern Italy, on Vascular Surgery Intervention Outcomes During The COVID-19 Pandemic. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.M.; Kalbaugh, C.A.; Blackhurst, D.W.; Cass, A.L.; Trent, E.A.; Langan, E.M., 3rd; Youkey, J.R. Determinants of functional outcome after revascularization for critical limb ischemia: An analysis of 1000 consecutive vascular interventions. J. Vasc. Surg. 2006, 44, 747–755; discussion 755–756. [Google Scholar] [CrossRef] [PubMed][Green Version]
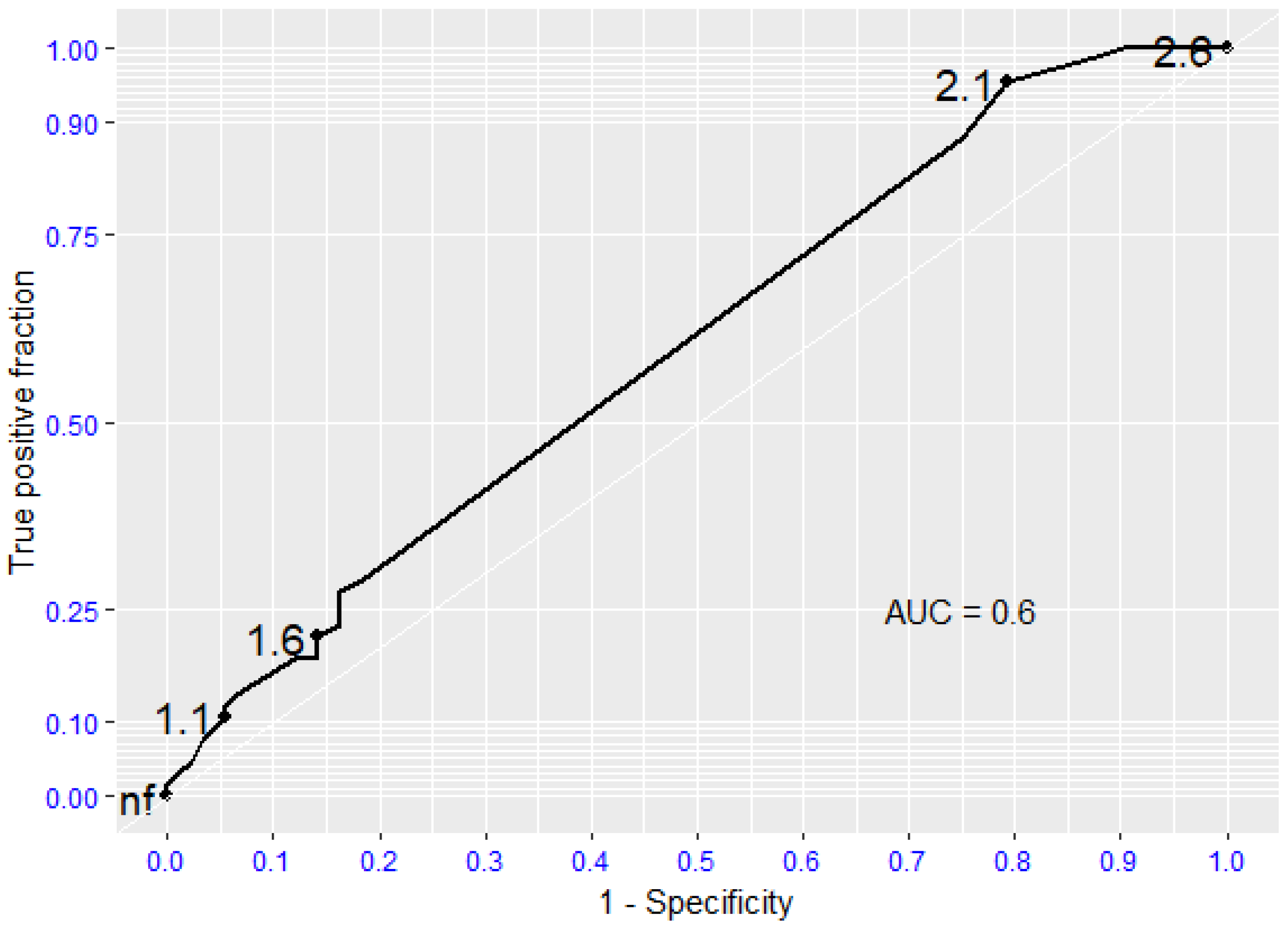
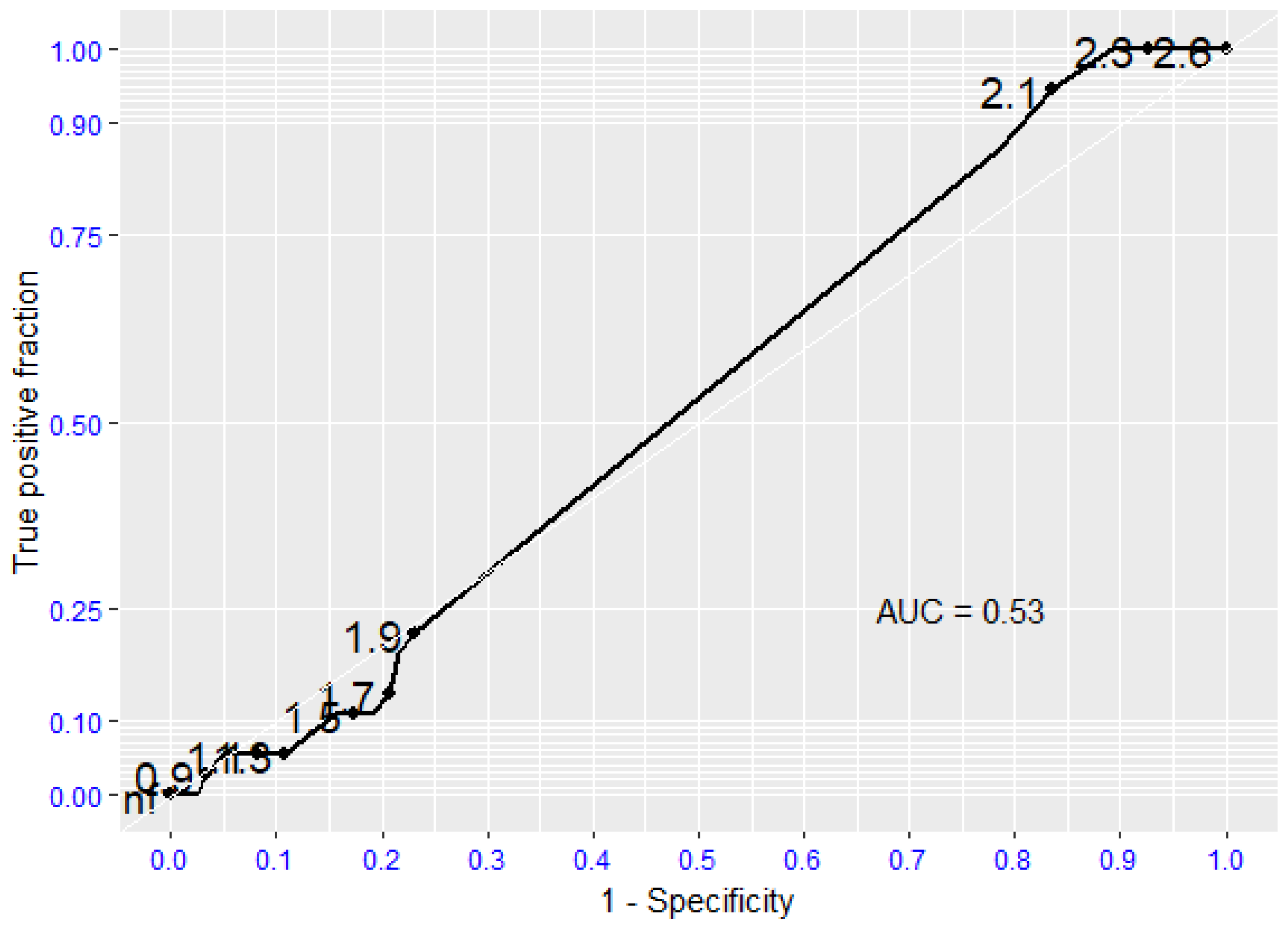
| Echo Parameters | Sclerosis | Mild AS | Moderate AS | Severe AS |
|---|---|---|---|---|
| Peak velocity, m/s | <2.5 | 2.5–3 | 3–4 | >4 |
| Mean gradient, mmhg | Normal | <20 | 20–40 | 40 |
| AVA, cm2 | Normal | ≥1.5 | 1–1.5 | <1 |
| Pre-Operatory Functional Status | |
|---|---|
| 0 | No impairment |
| 1 | Impaired, but able to carry out ADL without assistance |
| 2 | Needs some assistance to carry out ADL or ambulatory assistance |
| 3 | Requiring total assistance for ADL or nonambulatory |
| Variable | Level | Group B | Group A | p-Value |
|---|---|---|---|---|
| n = 133 | n = 25 | |||
| Sex (%) | Female | 39 (29.3) | 8 (32.0) | 0.976 |
| Male | 94 (70.7) | 17 (68.0) | ||
| Age (median, [IQR]) | 74.00 [65.00, 81.00] | 78.00 [76.00, 83.00] | 0.005 | |
| Vmax (median [IQR]) | 1.36 [1.17, 1.65] | 2.89 [2.60, 3.19] | <0.001 | |
| Mean Gp (median [IQR]) | 3.80 [2.90, 5.60] | 18.42 [13.16, 24.04] | <0.001 | |
| Weight, kg (median [IQR]) | 70.00 [58.00, 80.00] | 70.00 [55.00, 78.00] | 0.911 | |
| Height, m (median [IQR]) | 1.69 [1.62, 1.75] | 1.67 [1.64, 1.70] | 0.561 | |
| BMI (median [IQR]) | 24.69 [22.22, 26.67] | 24.80 [22.40, 27.66] | 0.971 | |
| Smoking habits (%) | No, past | 92 (69.2) | 19 (76.0) | 0.655 |
| Yes | 41 (30.8) | 6 (24.0) | ||
| Diabetes (%) | No | 73 (54.9) | 11 (44.0) | 0.434 |
| Yes | 60 (45.1) | 14 (56.0) | ||
| Hypertension (%) | No | 19 (14.3) | 2 (8.0) | 0.597 |
| Yes | 114 (85.7) | 23 (92.0) | ||
| Renal function impairment (%) | No impairment or mild | 116 (87.2) | 24 (96.0) | 0.355 |
| Severe or pre-terminal | 17 (12.8) | 1 (4.0) | ||
| Dislipidemia (%) | No | 34 (25.6) | 6 (24.0) | 1.000 |
| Yes | 99 (74.4) | 19 (76.0) | ||
| Cardiac status (%) | Asymptomatic or previous MI (>6 months) or silent | 86 (64.7) | 15 (60.0) | 0.827 |
| Recent MI (<6 mesi), arytmia, angina, reduction of EF | 47 (35.3) | 10 (40.0) | ||
| COPD (%) | No | 61 (45.9) | 11 (44.0) | 1.000 |
| Yes | 72 (54.1) | 14 (56.0) | ||
| Stroke (%) | No | 115 (86.5) | 23 (92.0) | 0.663 |
| Yes | 18 (13.5) | 2 (8.0) | ||
| Pre-operatory functional status (%) | No impairment | 39 (29.3) | 4 (16.0) | 0.479 |
| Impaired, but able to carry out ADL without assistance | 44 (33.1) | 8 (32.0) | ||
| Needs some assistance to carry out ADL or ambulatory assistance | 38 (28.6) | 10 (40.0) | ||
| Requiring total assistance for ADL or nonambulatory | 12 (9.0) | 3 (12.0) |
| Variable | Level | Group B | Group A | p-Value |
|---|---|---|---|---|
| Side n (%) | Right | 67 (50.4) | 8 (32.0) | 0.142 |
| Left | 66 (49.6) | 17 (68.0) | ||
| Rutherford Scale (%) | 3—Severe claudication | 10 (7.5) | 2 (8.0) | 0.157 |
| 4—Ischemic rest pain | 34 (25.6) | 4 (16.0) | ||
| 5—Minor tissue lost | 83 (62.4) | 15 (60.0) | ||
| 6—Major tissue lost | 6 (4.5) | 4 (16.0) | ||
| Level of revascularization n (%) | Above the knee | 26 (19.5) | 5 (20.0) | 1.000 |
| Below the knee | 107 (80.5) | 20 (80.0) | ||
| Graft material, prosthesis n (%) | No | 47 (35.3) | 9 (36.0) | 1.000 |
| Yes | 86 (64.7) | 16 (64.0) | ||
| Graft material, great saphenous vein n (%) | No | 64 (48.1) | 10 (40.0) | 0.597 |
| Yes | 69 (51.9) | 15 (60.0) |
| Variable | Level | Group B | Group A | p-Value |
|---|---|---|---|---|
| Bypass-related complications within 30 days n (%) | No | 91 (68.4) | 16 (64.0) | 0.841 |
| Yes | 42 (31.6) | 9 (36.0) | ||
| Patency, n (%) | No | 41 (31.5) | 9 (36.0) | 0.839 |
| Yes | 89 (68.5) | 16 (64.0) | ||
| Restenosis, n (%) | No | 87 (97.8) | 16 (100.0) | 1.000 |
| Yes | 2 (2.2) | 0 (0.0) | ||
| First complication after 30 days n (%) | No | 77 (57.9) | 12 (48.0) | 0.487 |
| Yes | 56 (42.1) | 13 (52.0) | ||
| Second complication after 30 days n (%) | No | 112 (84.2) | 21 (84.0) | 1.000 |
| Yes | 21 (15.8) | 4 (16.0) | ||
| Third complication after 30 days n (%) | No | 124 (93.2) | 21 (84.0) | 0.252 |
| Yes | 9 (6.8) | 4 (16.0) | ||
| Fourth complication after 30 days n (%) | No | 132 (99.2) | 22 (88.0) | 0.010 |
| Yes | 1 (0.8) | 3 (12.0) | ||
| Mean number of complications after 30 days [SD] | 0.65 [0.89] | 0.96 (1.34) | 0.429 | |
| Major amputation (%) | No | 111 (83.5) | 20 (80.0) | 0.895 |
| Yes | 22 (16.5) | 5 (20.0) | ||
| Time between intervention and major amputation in days (median, [IQR]) | 100.00 [64.50, 283.25] | 449.00 [432.00, 1144.00] | 0.016 |
| Univariate Logistic Model “Major Amputation” | |||
|---|---|---|---|
| Predictors | Odds Ratio | OR 95% CI | p-Value |
| AVA | 0.76 | (0.27, 2.49) | 0.638 |
| Age | 1.02 | (0.97, 1.07) | 0.420 |
| Complications within 30 days [yes vs. no] | 5.83 | (2.36, 14.57) | <0.001 |
| Functional status [Impaired, but able vs. not impaired] | 2.42 | (0.87, 25.67) | 0.109 |
| [Needs some assistance vs. not impaired] | 10.37 | (1.72, 45.82) | 0.016 |
| [Requiring total assistance vs. not impaired] | 6.67 | (1.91, 79.39) | 0.010 |
| Graft related complications [yes vs. no] | 2.74 | (1.17, 6.45) | 0.020 |
| Bypass length | 7.72 | (1.53, 140.68) | 0.049 |
| Cardiac status [recent MI vs. asymptomatic] | 2.65 | (1.14, 6.26) | 0.024 |
| Variable | Level | Group B | Group A | p-Value |
|---|---|---|---|---|
| Major Adverse Event (MAE) (%) | No | 79 (59.4) | 13 (52.0) | 0.142 |
| Cardiovascular event (MI/stroke/Sudden Cardiac death) | 33 (24.8) | 4 (16.0) | ||
| Death by other causes or unknown | 21 (15.8) | 8 (32.0) | ||
| Death, n (%) | No | 79 (59.4) | 13 (52.0) | 0.640 |
| Yes | 33 (24.8) | 12 (48.0) | ||
| Cardiovascular death, n (%) | No | 100 (75.2) | 21 (84.0) | 0.486 |
| Yes | 33 (24.8) | 4 (16.0) | ||
| Times between intervention and any MAE in days (median [IQR]) | 936 [238, 1478] | 792.50 [159.25, 1431.50] | 0.673 | |
| Time between intervention and death in days (mean; median [IQR]) | 961; 744.50 [244.50, 1475.25] | 893; 559 [165.25, 1431.50] | 0.630 | |
| Time between intervention and cardiovascular death in days (mean; median [IQR]) | 822; 341 [150, 1467] | 358; 181 [86.75, 452.25] | 0.221 | |
| Postoperative functional status, n (%) | No impairment | 58 (43.9) | 7 (29.2) | 0.157 |
| Impaired, but able to carry out ADL without assistance | 20 (15.2) | 7 (29.2) | ||
| Needs some assistance to carry out ADL or ambulatory assistance | 23 (17.4) | 2 (8.3) | ||
| Requiring total assistance for ADL or nonambulatory | 31 (23.5) | 8 (33.3) |
| Univariate Logistic Model for Variation of Functional Status | |||
|---|---|---|---|
| Predictors | Odds Ratio | OR 95% CI | p-Value |
| AVA | 0.45 | (0.16, 1.16) | 0.111 |
| Age | 1.08 | (1.04, 1.13) | <0.001 |
| Complications within 30 days [yes vs. no] | 3.52 | (1.43, 10.04) | 0.010 |
| Functional status [Impaired, but able vs. not impaired] | 3.02 | (1.28, 7.49) | 0.013 |
| [Needs some assistance vs. not impaired] | 10.62 | (6.09, 51.17) | <0.001 |
| [Requiring total assistance vs. not impaired] | 40.72 | (6.98, 782.41) | <0.001 |
| Graft related complications [yes vs. no] | 1.82 | (0.91, 3.77) | 0.095 |
| Bypass length | 2.52 | (1.13, 5.82) | 0.026 |
| Cardiac status [recent MI vs. asymptomatic] | 2.12 | (1.07, 4.30) | 0.033 |
| Rutherford scale | 2.24 | (1.38, 3.77) | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attisani, L.; Pucci, A.; Pegorer, M.A.; Luzzani, L.; Casali, F.; Luoni, G.; Tanagli, S.; Piffaretti, G.; Bellosta, R. Clinical Outcomes of Surgical Revascularization in Patients Presenting with Critical Limb Ischemia and Aortic Valve Stenosis. J. Cardiovasc. Dev. Dis. 2025, 12, 292. https://doi.org/10.3390/jcdd12080292
Attisani L, Pucci A, Pegorer MA, Luzzani L, Casali F, Luoni G, Tanagli S, Piffaretti G, Bellosta R. Clinical Outcomes of Surgical Revascularization in Patients Presenting with Critical Limb Ischemia and Aortic Valve Stenosis. Journal of Cardiovascular Development and Disease. 2025; 12(8):292. https://doi.org/10.3390/jcdd12080292
Chicago/Turabian StyleAttisani, Luca, Alessandro Pucci, Matteo A. Pegorer, Luca Luzzani, Francesco Casali, Giorgio Luoni, Stefano Tanagli, Gabriele Piffaretti, and Raffaello Bellosta. 2025. "Clinical Outcomes of Surgical Revascularization in Patients Presenting with Critical Limb Ischemia and Aortic Valve Stenosis" Journal of Cardiovascular Development and Disease 12, no. 8: 292. https://doi.org/10.3390/jcdd12080292
APA StyleAttisani, L., Pucci, A., Pegorer, M. A., Luzzani, L., Casali, F., Luoni, G., Tanagli, S., Piffaretti, G., & Bellosta, R. (2025). Clinical Outcomes of Surgical Revascularization in Patients Presenting with Critical Limb Ischemia and Aortic Valve Stenosis. Journal of Cardiovascular Development and Disease, 12(8), 292. https://doi.org/10.3390/jcdd12080292







