The Prognostic Implication of Left Atrial Strain Parameters with Conventional Left Atrial Parameters for the Prediction of Adverse Outcomes in Asian Patients with Hypertrophic Cardiomyopathy—An Echocardiographic Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiographic Analysis
2.3. Left Atrial Parameters
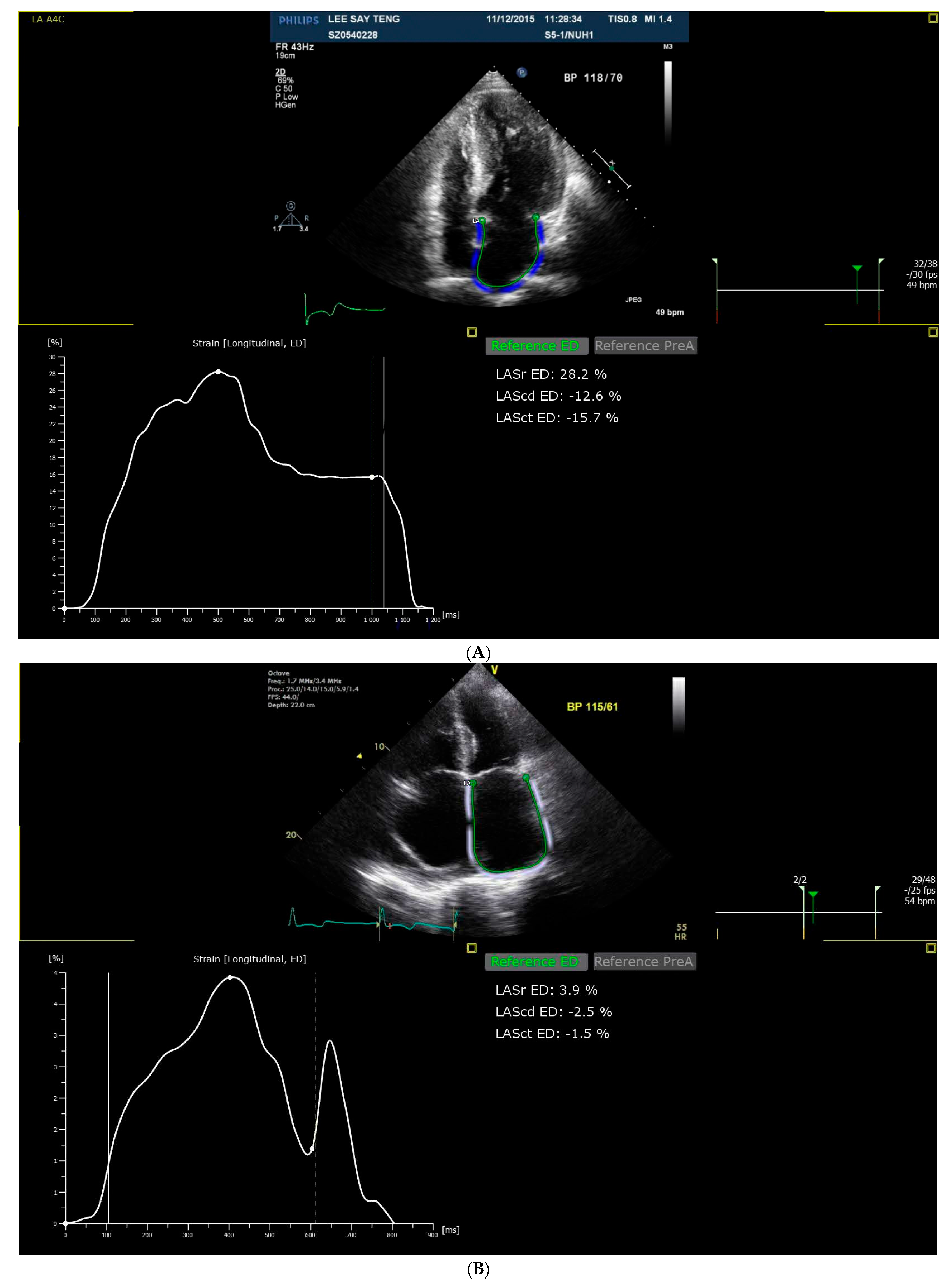
2.4. Strain Analysis
2.5. Follow-Up and Clinical Endpoints
2.6. Statistical Analysis
3. Results
3.1. Univariate Analysis
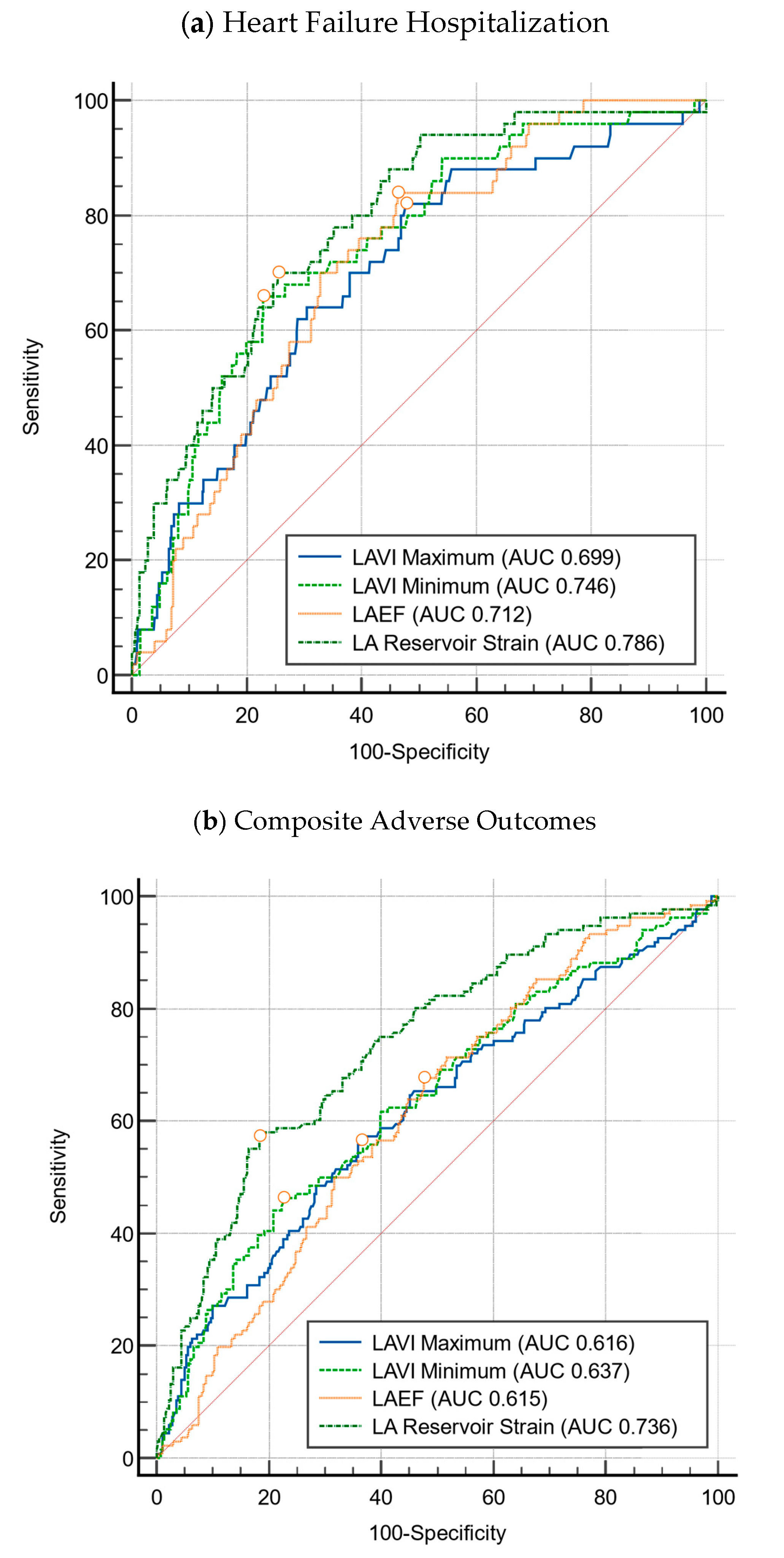
3.2. LA Strain
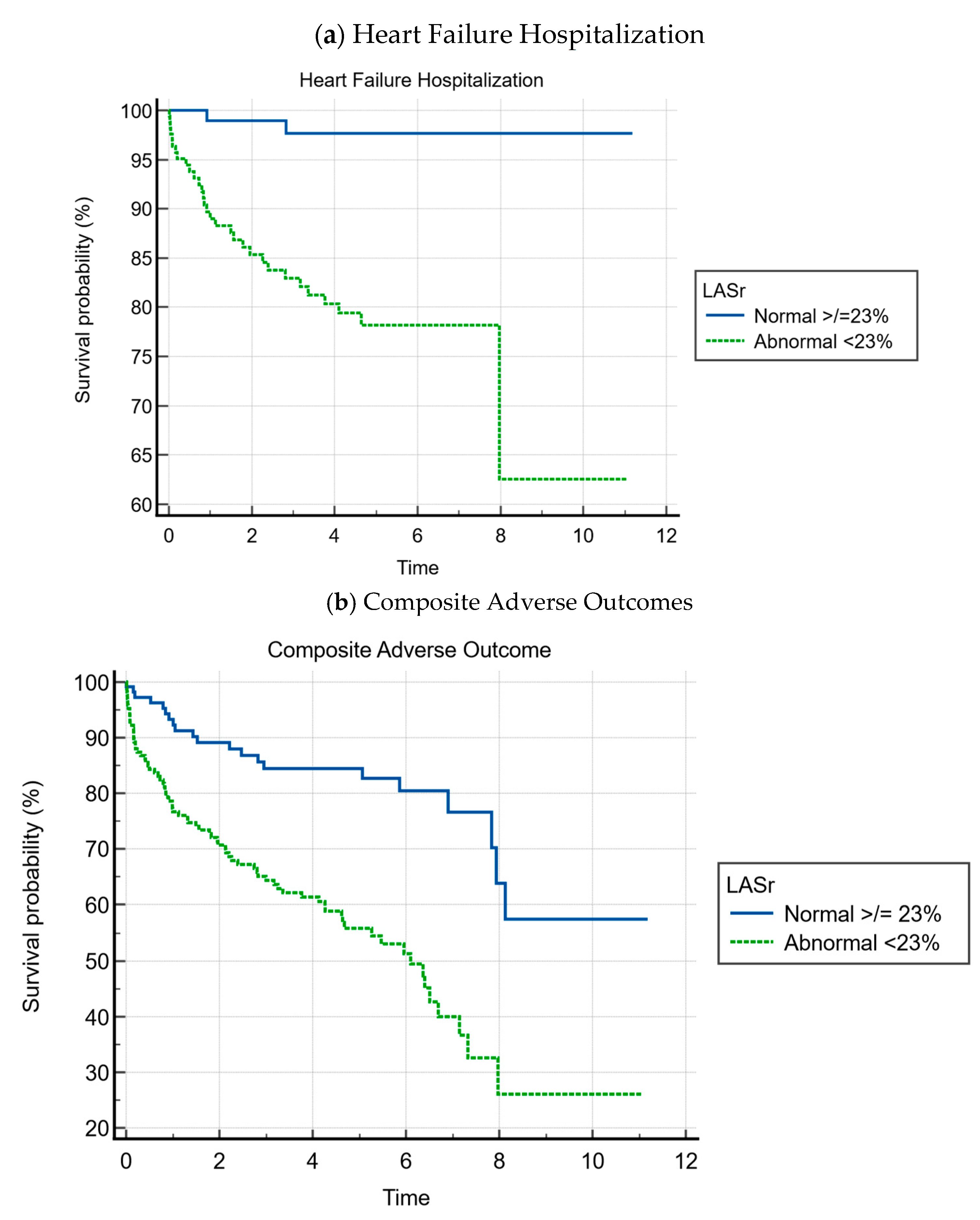
- Heart failure event-free survival was 81.8% for patients with abnormal LASr vs. 98.3% for patients with normal LASr (p < 0.001);
- Composite event-free survival was 55.2% for patients with abnormal LASr vs. 82.4% for patients with normal LASr (p < 0.001).
3.3. Multivariate Cox Regression Analysis
4. Discussion
4.1. Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCM | Hypertrophic cardiomyopathy |
| VT | Ventricular tachycardia |
| VF | Ventricular fibrillation |
| ICD | Implantable cardioverter defibrillator |
| LA | Left atrial |
| LV | Left ventricular |
| EF | Ejection fraction |
| LVOT | Left ventricular outflow tract |
| GLS | Global longitudinal strain |
| HFpEF | Heart failure with preserved ejection fraction |
| LVMI | Left ventricular mass index |
| LASr | Left atrial reservoir strain |
| LAScd | Left atrial conduit strain |
| LASct | Left atrial contraction strain |
| LAVImax | Maximum left atrial volume indexed to body surface area |
| LAVImin | Minimum left atrial volume indexed to body surface area |
| SGLT2i | Sodium-glucose cotransport inhibitor |
Appendix A
References
- Cirino, A.L.; Harris, S.; Lakdawala, N.K.; Michels, M.; Olivotto, I.; Day, S.M.; Abrams, D.J.; Charron, P.; Caleshu, C.; Semsarian, C.; et al. Role of Genetic Testing in Inherited Cardiovascular Disease: A Review. JAMA Cardiol. 2017, 2, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-F.; Hu, J.; Hu, J.; Nijjar, P.S.; Xu, J.; Shi, S.; Liang, D.; Liao, H.; Gao, J.; Lin, W.-H.; et al. Clinical Characteristics and Prognosis of Patients with Hypertrophic Cardiomyopathy and Heart Failure with Preserved Ejection Fraction. Clin. Res. Cardiol. 2024, 113, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Cecchi, F.; Poggesi, C.; Yacoub, M.H. Patterns of Disease Progression in Hypertrophic Cardiomyopathy: An Individualized Approach to Clinical Staging. Circ. Heart Fail. 2012, 5, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Haland, T.F.; Almaas, V.M.; Hasselberg, N.E.; Saberniak, J.; Leren, I.S.; Hopp, E.; Edvardsen, T.; Haugaa, K.H. Strain Echocardiography Is Related to Fibrosis and Ventricular Arrhythmias in Hypertrophic Cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 613–621. [Google Scholar] [CrossRef]
- Hohendanner, F.; Messroghli, D.; Bode, D.; Blaschke, F.; Parwani, A.; Boldt, L.; Heinzel, F.R. Atrial Remodelling in Heart Failure: Recent Developments and Relevance for Heart Failure with Preserved Ejection Fraction. ESC Heart Fail. 2018, 5, 211–221. [Google Scholar] [CrossRef]
- Melenovsky, V.; Hwang, S.-J.; Redfield, M.M.; Zakeri, R.; Lin, G.; Borlaug, B.A. Left Atrial Remodeling and Function in Advanced Heart Failure with Preserved or Reduced Ejection Fraction. Circ. Heart Fail. 2015, 8, 295–303. [Google Scholar] [CrossRef]
- Hoit, B.D. Left Atrial Size and Function. J. Am. Coll. Cardiol. 2014, 63, 493–505. [Google Scholar] [CrossRef]
- Thomas, L.; Marwick, T.H.; Popescu, B.A.; Donal, E.; Badano, L.P. Left Atrial Structure and Function, and Left Ventricular Diastolic Dysfunction. J. Am. Coll. Cardiol. 2019, 73, 1961–1977. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Pieske, B.; Butler, J.; Parissis, J.; Giamouzis, G.; Skoularigis, J.; Brutsaert, D.; Boudoulas, H. Global Left Atrial Failure in Heart Failure. Eur. J. Heart Fail. 2016, 18, 1307–1320. [Google Scholar] [CrossRef]
- Minami, Y.; Haruki, S.; Yashiro, B.; Suzuki, T.; Ashihara, K.; Hagiwara, N. Enlarged Left Atrium and Sudden Death Risk in Hypertrophic Cardiomyopathy Patients with or without Atrial Fibrillation. J. Cardiol. 2016, 68, 478–484. [Google Scholar] [CrossRef]
- Smiseth, O.A.; Torp, H.; Opdahl, A.; Haugaa, K.H.; Urheim, S. Myocardial Strain Imaging: How Useful Is It in Clinical Decision Making? Eur. Heart J. 2016, 37, 1196–1207. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidis, I.A.; Farmakis, D.; Papadopoulos, C.; Ikonomidis, I.; Parissis, J.; Rigopoulos, A.; Iliodromitis, E.K.; Kremastinos, D.T. Two-Dimensional Strain Analysis in Patients with Hypertrophic Cardiomyopathy and Normal Systolic Function: A 12-Month Follow-up Study. Am. Heart J. 2009, 158, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Debonnaire, P.; Joyce, E.; Hiemstra, Y.; Mertens, B.J.; Atsma, D.E.; Schalij, M.J.; Bax, J.J.; Delgado, V.; Marsan, N.A. Left Atrial Size and Function in Hypertrophic Cardiomyopathy Patients and Risk of New-Onset Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2017, 10, e004052. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, H.-K.; Rhee, T.-M.; Choi, Y.-J.; Hwang, I.-C.; Yoon, Y.E.; Park, J.-B.; Lee, S.-P.; Kim, Y.-J.; Cho, G.-Y. Left Atrial Reservoir Strain-Based Left Ventricular Diastolic Function Grading and Incident Heart Failure in Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2022, 15, e013556. [Google Scholar] [CrossRef]
- Ommen, S.R.; Ho, C.Y.; Asif, I.M.; Balaji, S.; Burke, M.A.; Day, S.M.; Dearani, J.A.; Epps, K.C.; Evanovich, L.; Ferrari, V.A.; et al. 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR Guideline for the Management of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2024, 83, 2324–2405. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Pathan, F.; Zainal Abidin, H.A.; Vo, Q.H.; Zhou, H.; D’Angelo, T.; Elen, E.; Negishi, K.; Puntmann, V.O.; Marwick, T.H.; Nagel, E. Left Atrial Strain: A Multi-Modality, Multi-Vendor Comparison Study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 102–110. [Google Scholar] [CrossRef]
- Singh, A.; Carvalho Singulane, C.; Miyoshi, T.; Prado, A.D.; Addetia, K.; Bellino, M.; Daimon, M.; Gutierrez Fajardo, P.; Kasliwal, R.R.; Kirkpatrick, J.N.; et al. Normal Values of Left Atrial Size and Function and the Impact of Age: Results of the World Alliance Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2022, 35, 154–164.e3. [Google Scholar] [CrossRef]
- Nyberg, J.; Jakobsen, E.O.; Østvik, A.; Holte, E.; Stølen, S.; Lovstakken, L.; Grenne, B.; Dalen, H. Echocardiographic Reference Ranges of Global Longitudinal Strain for All Cardiac Chambers Using Guideline-Directed Dedicated Views. JACC Cardiovasc. Imaging 2023, 16, 1516–1531. [Google Scholar] [CrossRef]
- IBM. SPSS Statistics 2020; IBM: Armonk, NY, USA, 2020. [Google Scholar]
- MedCalc Software Ltd. MedCalc Statistical Software 2020; MedCalc Software Ltd.: Ostend, Belgium, 2020. [Google Scholar]
- Li, J.; Fang, J.; Liu, Y.; Wei, X. Apical Hypertrophic Cardiomyopathy: Pathophysiology, Diagnosis and Management. Clin. Res. Cardiol. 2024, 113, 680–693. [Google Scholar] [CrossRef]
- Fumagalli, C.; Zocchi, C.; Ciabatti, M.; Milazzo, A.; Cappelli, F.; Fumagalli, S.; Pieroni, M.; Olivotto, I. From Atrial Fibrillation Management to Atrial Myopathy Assessment: The Evolving Concept of Left Atrium Disease in Hypertrophic Cardiomyopathy. Can. J. Cardiol. 2024, 40, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Guttmann, O.P.; Pavlou, M.; O’Mahony, C.; Monserrat, L.; Anastasakis, A.; Rapezzi, C.; Biagini, E.; Gimeno, J.R.; Limongelli, G.; Garcia-Pavia, P.; et al. Predictors of Atrial Fibrillation in Hypertrophic Cardiomyopathy. Heart 2017, 103, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Olivotto, I.; Hellawell, J.L.; Farzaneh-Far, R.; Blair, C.; Coppini, R.; Myers, J.; Belardinelli, L.; Maron, M.S. Novel Approach Targeting the Complex Pathophysiology of Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2016, 9, e002764. [Google Scholar] [CrossRef] [PubMed]
- Hiemstra, Y.L.; Debonnaire, P.; Bootsma, M.; Van Zwet, E.W.; Delgado, V.; Schalij, M.J.; Atsma, D.E.; Bax, J.J.; Marsan, N.A. Global Longitudinal Strain and Left Atrial Volume Index Provide Incremental Prognostic Value in Patients With Hypertrophic Cardiomyopathy. Circ. Cardiovasc. Imaging 2017, 10, e005706. [Google Scholar] [CrossRef]
- Shin, S.; Claggett, B.; Inciardi, R.M.; Santos, A.B.S.; Shah, S.J.; Zile, M.R.; Pfeffer, M.A.; Shah, A.M.; Solomon, S.D. Prognostic Value of Minimal Left Atrial Volume in Heart Failure With Preserved Ejection Fraction. JAHA 2021, 10, e019545. [Google Scholar] [CrossRef]
- Battel, O.; Newsome, K.; Izquierdo-Pretel, G. Global Longitudinal Strain as an Efficient Prognostic Tool in Hypertrophic Cardiomyopathy With Preserved Left Ventricular Ejection Fraction. Cureus 2022, 14, e30573. [Google Scholar] [CrossRef]
- Jia, F.; Chen, A.; Zhang, D.; Fang, L.; Chen, W. Prognostic Value of Left Atrial Strain in Heart Failure: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 9, 935103. [Google Scholar] [CrossRef]
- Tian, D.; Zhang, J.; He, Y.; Xiong, Z.; Zhao, M.; Hu, S.; Song, Q.; Li, Z. Predictive Value of Left Atrial Strain Analysis in Adverse Clinical Events in Patients with Hypertrophic Cardiomyopathy: A CMR Study. BMC Cardiovasc. Disord. 2023, 23, 42. [Google Scholar] [CrossRef]
- Tayal, B.; Malahfji, M.; Buergler, J.M.; Shah, D.J.; Nagueh, S.F. Hemodynamic Determinants of Left Atrial Strain in Patients with Hypertrophic Cardiomyopathy: A Combined Echocardiography and CMR Study. PLoS ONE 2021, 16, e0245934. [Google Scholar] [CrossRef]
- Inoue, K.; Khan, F.H.; Remme, E.W.; Ohte, N.; García-Izquierdo, E.; Chetrit, M.; Moñivas-Palomero, V.; Mingo-Santos, S.; Andersen, Ø.S.; Gude, E.; et al. Determinants of Left Atrial Reservoir and Pump Strain and Use of Atrial Strain for Evaluation of Left Ventricular Filling Pressure. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 61–70. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. J. Echocardiogr. 2016, 17, 1321–1360. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left Atrial Strain and Compliance in the Diagnostic Evaluation of Heart Failure with Preserved Ejection Fraction. Eur. J. Heart Fail. 2019, 21, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Owens, A.; Wolski, K.; Geske, J.B.; Saberi, S.; Wang, A.; Sherrid, M.; Cremer, P.C.; Lakdawala, N.K.; Tower-Rader, A.; et al. Mavacamten in Patients With Hypertrophic Cardiomyopathy Referred for Septal Reduction: Week 56 Results From the VALOR-HCM Randomized Clinical Trial. JAMA Cardiol. 2023, 8, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Nissen, S.E.; Abraham, T.; Olivotto, I.; Garcia-Pavia, P.; Lopes, R.D.; Verheyen, N.; Wever-Pinzon, O.; Wolski, K.; Jaber, W.; et al. Mavacamten in Symptomatic Nonobstructive Hypertrophic Cardiomyopathy. JACC Heart Fail. 2025, 13, 358–370. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Udelson, J.E.; Maron, M.S. Clinical Spectrum and Management of Heart Failure in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2018, 6, 353–363. [Google Scholar] [CrossRef]
- Li, T.Y.; Yeo, S.; Ngiam, N.J.; Lee, C.-H.; Low, T.T.; Lim, Y.-C.; Evangelista, L.K.M.; Lee, E.C.; Sari, N.Y.; Yeo, T.-C.; et al. Effects of Sex on Clinical Outcomes of Hypertrophic Cardiomyopathy in Singapore. Ann. Acad. Med. Singap. 2023, 52, 348–355. [Google Scholar] [CrossRef]
- Van Oosterhout, R.E.M.; De Boer, A.R.; Maas, A.H.E.M.; Rutten, F.H.; Bots, M.L.; Peters, S.A.E. Sex Differences in Symptom Presentation in Acute Coronary Syndromes: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2020, 9, e014733. [Google Scholar] [CrossRef]
- Riedinger, M.S.; Dracup, K.A.; Brecht, M.L.; Padilla, G.; Sarna, L.; Ganz, P.A. Quality of Life in Patients with Heart Failure: Do Gender Differences Exist? Heart Lung 2001, 30, 105–116. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V. Sex and Gender Differences in Heart Failure. Int. J. Heart Fail. 2020, 2, 157–181. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Cara, M.D.; Nicolosi, G.L.; Eusebio, A.; Bordonali, M.; Santalucia, P.; Lombardo, M. Rapid Risk Stratification of Acute Ischemic Stroke Patients in the Emergency Department: The Incremental Prognostic Role of Left Atrial Reservoir Strain. J. Stroke Cerebrovasc. Dis. 2021, 30, 106100. [Google Scholar] [CrossRef]
- Brabham, D.; Muniz, A. Abstract 203: Inter-Rater Reliability Of Myocardial Strain Measurements In Comparison With Ejection Fraction In A Rural, Clinical Setting Using Speckle Tracking Echocardiography. Circ. Cardiovasc. Qual. Outcomes 2022, 15, A203. [Google Scholar] [CrossRef]
- Mandoli, G.E.; Pastore, M.C.; Procopio, M.C.; Pica, A.; Vigna, M.; Benfari, G.; Diviggiano, E.E.; Martini, L.; Lunghetti, S.; Focardi, M.; et al. Unveiling the Reliability of Left Atrial Strain Measurement: A Dedicated Speckle Tracking Software Perspective in Controls and Cases. Eur. Heart J. Imaging Methods Pract. 2024, 2, qyae061. [Google Scholar] [CrossRef]
| Variables | Overall (n = 291) | Heart Failure Hospitalization (n = 33) | Free from Heart Failure (n = 258) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| Baseline Demographics | |||||
| Age (years) | 58.75 ± 16.79 | 70.58 ± 16.79 | 57.24 ± 16.68 | 1.06 (1.03–1.09) | <0.001 |
| Female, n (%) | 82 (28.2%) | 19 (57.6%) | 63 (24.4%) | 4.20 (1.99–8.86) | <0.001 |
| BMI | 25.30 ± 4.48 | 24.59 ± 4.19 | 25.39 ± 4.52 | 0.96 (0.88–1.04) | 0.330 |
| Ethnicity | - | 0.549 | |||
| Chinese | 194 (66.7%) | 24 (72.7%) | 170 (65.9%) | ||
| Malay | 53 (18.2%) | 5 (15.2%) | 48 (18.6%) | ||
| Indian | 35 (12.0%) | 4 (12.1%) | 31 (12.0%) | ||
| Other | 9 (3.1%) | 0 (0.0%) | 9 (3.5%) | ||
| Comorbidities | |||||
| Hypertension | 131 (45.0%) | 22 (67.6%) | 109 (42.2%) | 2.73 (1.27–5.87) | 0.009 |
| Hyperlipidemia | 117 (40.2%) | 17 (51.5%) | 100 (38.8%) | 1.68 (0.81–3.47) | 0.188 |
| Diabetes Mellitus | 47 (16.2%) | 10 (30.3%) | 37 (14.3%) | 2.60 (1.14–5.90) | 0.040 |
| Ischemic Heart Disease | 80 (27.5%) | 16 (48.5%) | 64 (24.8%) | 2.85 (1.36–5.97) | 0.007 |
| Atrial Fibrillation | 51 (17.5%) | 11 (21.6%) | 40 (15.5%) | 2.73 (1.23–6.06) | 0.025 |
| Chronic Kidney Disease | 3 (1.0%) | 0 (0.0%) | 3 (1.2%) | - | >0.999 |
| Previous Stroke | 31 (10.7%) | 6 (18.2%) | 25 (9.7%) | 2.07 (0.78–5.50) | 0.139 |
| Medication Use | |||||
| Antiplatelet | 87 (29.9%) | 15 (45.5%) | 72 (27.9%) | 2.15 (1.03–4.50) | 0.045 |
| Oral Anticoagulation | 58 (20.0%) | 16 (48.5%) | 42 (16.3%) | 4.98 (2.33–10.65) | 0.001 |
| Beta Blockers | 189 (64.9%) | 27 (81.8%) | 162 (62.8%) | 2.67 (1.06–6.69) | 0.033 |
| ACE-I/ARB/ARNI | 82 (28.2%) | 14 (42.4%) | 68 (26.4%) | 2.06 (0.98–4.33) | 0.065 |
| Calcium Channel Blockers | 58 (19.9%) | 8 (24.2%) | 50 (19.4%) | 1.33 (0.57–3.13) | 0.493 |
| Diuretics | 49 (16.8%) | 15 (45.5%) | 34 (13.2%) | 5.49 (2.53–11.91) | <0.001 |
| Oral Antiarrhythmics | 17 (5.8%) | 6 (18.2%) | 11 (4.3%) | 4.99 (1.71–14.56) | 0.007 |
| Type of HCM | |||||
| Sigmoid Septum | 52 (17.9%) | 7 (21.2%) | 45 (17.4%) | - | 0.610 |
| Reverse Curvature | 44 (15.1%) | 2 (6.1%) | 42 (16.3%) | ||
| Apical | 105 (36.1%) | 14 (42.4%) | 91 (35.3%) | ||
| Concentric | 85 (29.2%) | 9 (27.3%) | 76 (29.5%) | ||
| Mid Cavity | 1 (0.3%) | 0 (0.0%) | 1 (0.4%) | ||
| Other | 4 (1.4%) | 1 (3.0%) | 3 (1.2%) | ||
| Echocardiographic Parameters | |||||
| LVEF (%) | 64.89 ± 11.58 | 61.33 ± 12.21 | 65.34 ± 11.44 | 0.98 (0.95–1.01) | 0.049 |
| LVEF < 50% | 19 (6.5%) | 6 (18.2%) | 13 (5.0%) | 4.19 (1.47–11.92) | 0.019 |
| LVMI (g/m2) | 138.20 ± 46.94 | 153.67 ± 47.74 | 136.22 ± 46.56 | 1.01 (1.01–1.02) | 0.033 |
| Maximal Wall Thickness (mm) | 20.47 ± 4.59 | 20.64 ± 4.51 | 20.41 ± 4.60 | 1.02 (0.95–1.11) | 0.558 |
| MWT >/= 30 | 14 (4.8%) | 1 (3.0%) | 13 (5.0%) | 0.59 (0.08–4.65) | 0.497 |
| LVOT gradient (mmHg) >/= 30 | 48 (16.5%) | 6 (18.2%) | 42 (16.3%) | 1.14 (0.45–2.94) | 0.808 |
| More than Moderate MR | 18 (6.2%) | 5 (15.2%) | 13 (5.0%) | 3.37 (1.12–10.14) | 0.040 |
| LV E/e’ | 15.65 ± 8.40 | 20.64 ± 10.39 | 15.02 ± 7.92 | 1.08 (1.03–1.12) | <0.001 |
| Elevated Filling Pressure | 154 (52.9%) | 24 (72.7%) | 130 (50.4%) | 2.63 (1.18–5.87) | 0.462 |
| LAVImax (mL/m2) | 42.28 ± 20.92 | 61.75 ± 29.28 | 39.79 ± 18.11 | 1.04 (1.02–1.06) | 0.001 |
| LAVImin (mL/m2) | 22.39 ± 16.70 | 38.26 ± 23.81 | 20.36 ± 14.41 | 1.05 (1.03–1.07) | <0.001 |
| LAEF (%) | 50.9 ± 14.60 | 40.5% ± 11.6% | 52.2 ± 14.4 | 0.01 (0.01–0.06) | <0.001 |
| Strain Parameters | |||||
| LASr ED | 21.19 ± 12.11 | 11.12 ± 8.05 | 22.48 ± 11.95 | 0.89 (0.85–0.93) | <0.001 |
| LASr ED <23 | 172 (59.1%) | 31 (93.9%) | 141 (54.7%) | 12.86 (3.02–54.87) | <0.001 |
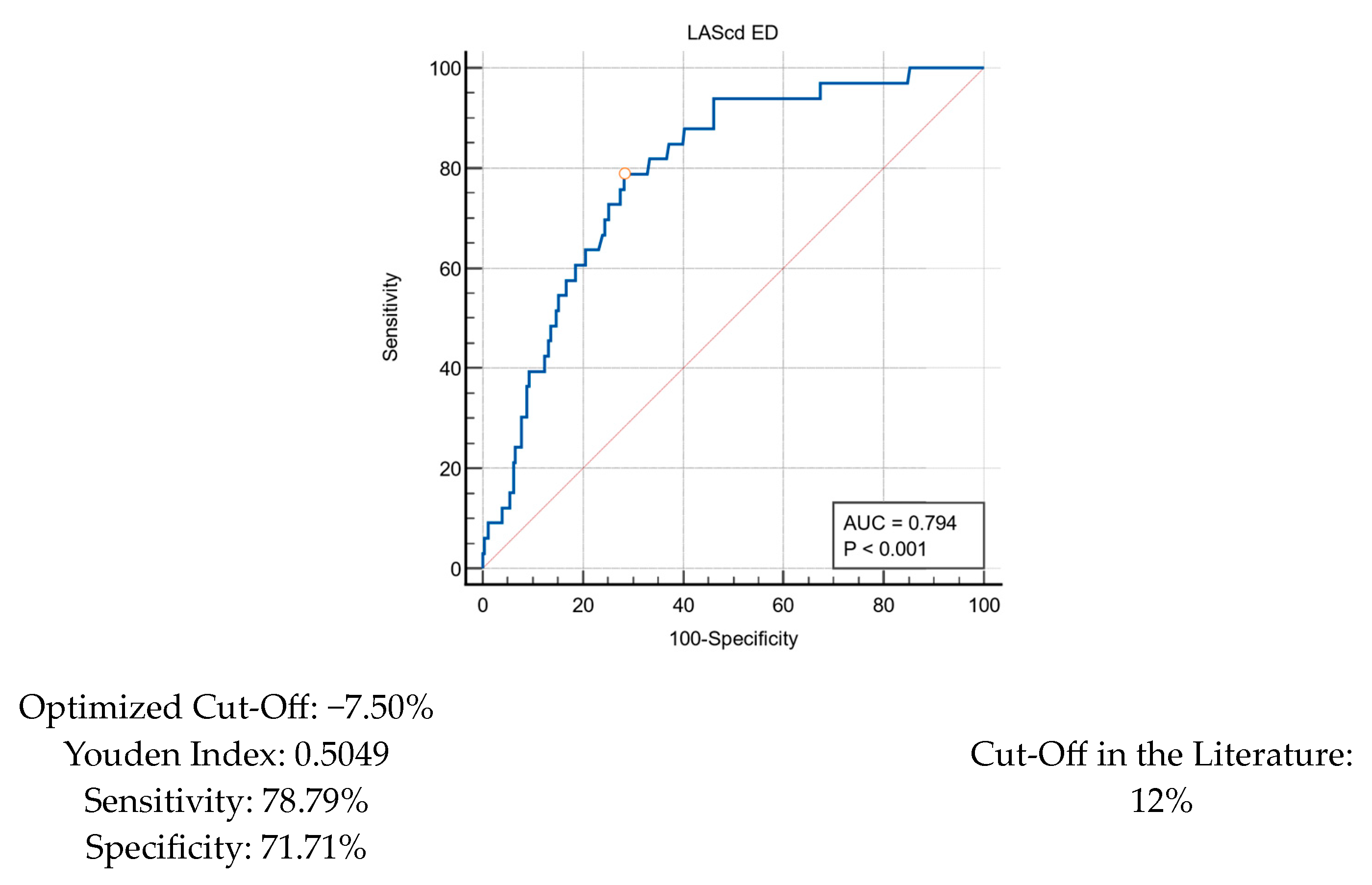
| LAScd ED | −11.83 ± 8.20 | −5.60 ± 4.29 | −12.62 ± 8.24 | 1.25 (1.13–1.37) | <0.001 |
| LAScd ED < 12 | 176 (60.5%) | 31 (93.9%) | 145 (56.2%) | 12.08 (2.83–51.54) | <0.001 |
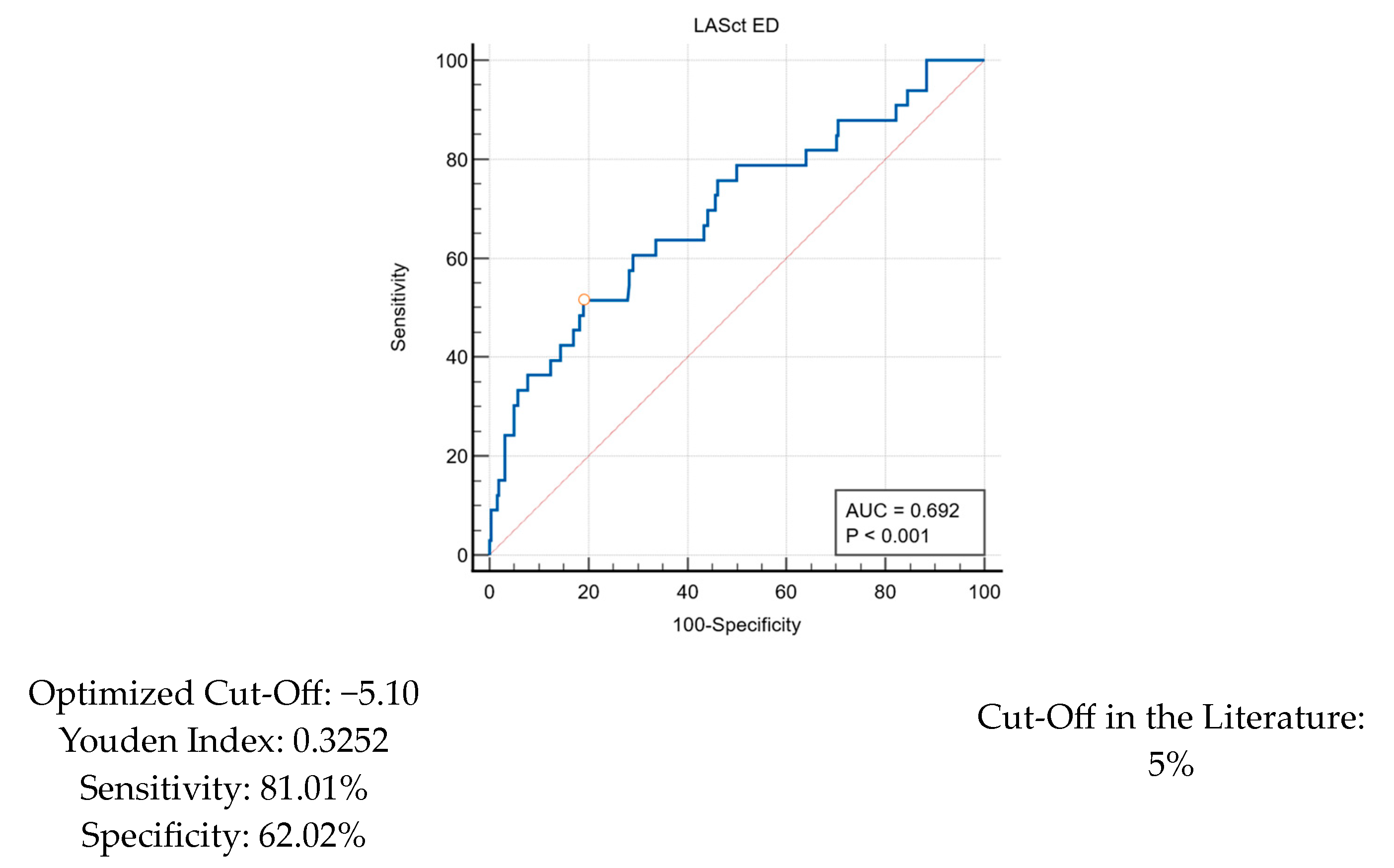
| LASct ED | −9.33 ± 6.25 | −5.31 ± 6.29 | −9.85 ± 6.07 | 1.15 (1.07–1.23) | <0.001 |
| LASct ED < 5 | 65 (22.3%) | 16 (48.5%) | 49 (19.0%) | 4.01 (1.90–8.50) | <0.001 |
| LVGLS | −12.92 ± 4.16 | −9.47 ± 3.17 | −13.36 ± 4.07 | 1.32 (1.17–1.47) | <0.001 |
| Variables | Overall (n = 291) | Heart Failure Hospitalization (n = 33) | Free from Heart Failure (n = 258) | Hazard Ratio (95% CI) | p-Value |
|---|---|---|---|---|---|
| Baseline Demographics | |||||
| Age (years) | 58.75 ± 16.79 | 68.29 ± 15.72 | 53.91 ± 15.20 | 1.07 (1.05–1.09) | <0.001 |
| Male, n (%) | 209 (71.8%) | 60 (61.2%) | 149 (77.2%) | 2.15 (1.27–3.63) | 0.010 |
| BMI | 25.30 ± 4.48 | 24.99 ± 4.97 | 24.45 ± 4.21 | 0.98 (0.92–1.03) | 0.404 |
| Ethnicity | 0.65 (0.45–1.29) | 0.224 | |||
| Chinese | 194 (66.7%) | 73 (74.5%) | 121 (62.7%) | ||
| Malay | 53 (18.2%) | 17 (17.3%) | 36 (18.7%) | ||
| Indian | 35 (12.0%) | 8 (8.2%) | 27 (14.0%) | ||
| Other | 9 (3.1%) | 0 (0.0%) | 9 (4.7%) | ||
| Comorbidities | |||||
| Hypertension | 131 (45.0%) | 61 (62.2%) | 70 (36.3%) | 2.90 (1.75–4.79) | 0.001 |
| Hyperlipidemia | 117 (40.2%) | 49 (50.0%) | 68 (35.2%) | 1.84 (1.12–3.01) | 0.017 |
| Diabetes Mellitus | 47 (16.2%) | 25 (25.5%) | 22 (11.4%) | 2.66 (1.41–5.02) | 0.005 |
| Ischemic Heart Disease | 80 (27.5%) | 36 (36.7%) | 44 (22.8%) | 1.97 (1.16–3.34) | 0.013 |
| Atrial Fibrillation | 51 (17.5%) | 24 (24.5%) | 27 (14.0%) | 1.99 (1.08–3.69) | 0.034 |
| Chronic Kidney Disease | 3 (1.0%) | 2 (2.0%) | 1 (0.5%) | 4.00 (0.37–44.67) | 0.277 |
| Previous Stroke | 31 (10.7%) | 22 (7.6%) | 9 (4.7%) | 5.92 (2.61–13.44) | <0.001 |
| Medication Use | |||||
| Antiplatelet | 87 (29.9%) | 43 (43.9%) | 44 (22.8%) | 2.65 (1.57–4.46) | <0.001 |
| Oral Anticoagulation | 58 (20.0%) | 29 (29.6%) | 29 (15.0%) | 2.48 (1.37–4.47) | 0.003 |
| Beta Blockers | 189 (64.9%) | 66 (67.3%) | 123 (65.1%) | 1.17 (0.70–1.96) | 0.604 |
| ACE-I/ARB/ARNI | 82 (28.3%) | 32 (32.7%) | 50 (25.9%) | 1.39 (0.82–2.36) | 0.338 |
| Calcium Channel Blockers | 58 (19.9%) | 27 (27.6%) | 31 (16.1%) | 1.99 (1.11–3.57) | 0.045 |
| Diuretics | 49 (16.8%) | 32 (65.3%) | 17 (8.8%) | 5.02 (2.61–9.64) | <0.001 |
| Oral Antiarrhythmics | 17 (5.8%) | 8 (8.2%) | 9 (4.7%) | 1.82 (0.68–4.87) | 0.201 |
| Type of HCM | |||||
| Sigmoid Septum | 52 (17.9%) | 22 (22.4%) | 30 (15.5%) | - | 0.402 |
| Reverse Curvature | 44 (15.1%) | 10 (10.2%) | 34 (17.6%) | ||
| Apical | 105 (36.1%) | 36 (36.7%) | 69 (35.8%) | ||
| Concentric | 85 (29.2%) | 28 (28.6%) | 57 (29.5%) | ||
| Mid Cavity | 1 (0.3%) | 0 (0.0%) | 1 (0.5%) | ||
| Other | 4 (1.4%) | 2 (2.0%) | 2 (1.0%) | ||
| Echocardiographic Parameters | |||||
| LVEF (%) | 64.89 ± 11.58 | 63.44 ± 12.66 | 65.62 ±10.95 | 0.98 (0.96–1.01) | 0.069 |
| LVEF < 50% | 20 (6.9%) | 10 (10.2%) | 9 (4.7%) | 2.32 (0.91–5.92) | 0.055 |
| LVMI (g/m2) | 138.20 ± 46.94 | 151.66 ± 52.30 | 131.36 ± 42.50 | 1.01 (1.01–1.02) | <0.001 |
| Maximal Wall Thickness (mm) | 20.47 ± 4.59 | 20.93 ± 4.76 | 20.24 ± 4.49 | 1.04 (0.98–1.09) | 0.566 |
| MWT >/= 30 | 14 (4.8%) | 5 (5.1%) | 9 (4.7%) | 1.10 (.036–3.37) | 0.571 |
| LVOT Gradient (mmHg) >/= 30 | 48 (16.5%) | 19 (19.4%) | 29 (15.0%) | 1.36 (0.72–2.57) | 0.269 |
| More than Moderate MR | 18 (6.2%) | 9 (9.2%) | 9 (4.7%) | 2.07 (0.79–5.39) | 0.201 |
| LV E/e’ | 15.65 ± 8.40 | 17.81 ± 9.91 | 14.57 ± 7.32 | 1.05 (1.01–1.08) | 0.004 |
| Elevated Filling Pressure | 154 (52.9%) | 65 (66.3%) | 89 (46.1%) | 2.30 (1.39–3.83) | 0.001 |
| LAVImax (mL/m2) | 42.28 ± 20.92 | 49.47 ± 24.87 | 38.63 ± 17.57 | 1.02 (1.01–1.04) | 0.024 |
| LAVImin (mL/m2) | 22.39 ± 16.70 | 28.28 ± 19.77 | 19.40 ± 14.03 | 1.03 (1.02–1.05) | <0.001 |
| LAEF (%) | 50.9 ± 14.60 | 46.6% ± 13.4% | 53.2% ± 14.7% | 0.05 (0.01–0.26) | <0.001 |
| Strain Parameters | |||||
| LASr ED | 21.19 ± 12.11 | 14.92 ± 10.11 | 24.37 ± 11.81 | 0.92 (0.89–0.95) | <0.001 |
| LASr ED < 23 | 172 (59.1%) | 77 (78.6%) | 95 (49.2%) | 3.78 (2.16–6.62) | <0.001 |
| LAScd ED | −11.83 ± 8.20 | −7.60 ± 5.83 | −13.97 ± 8.40 | 1.16 (1.10–1.22) | <0.001 |
| LAScd ED < 12 | 176 (60.5%) | 83 (84.7%) | 93 (48.2%) | 5.95 (3.21–11.04) | <0.001 |
| LASct ED | −9.33 ± 6.25 | −7.25 ± 6.14 | −10.39 ± 6.06 | 1.09 (1.04–1.14) | <0.001 |
| LASct ED < 5 | 65 (22.3%) | 35 (35.7%) | 30 (15.5%) | 3.02 (1.71–5.33) | <0.001 |
| LVGLS | −12.92 ± 4.16 | −11.02 ± 3.72 | −13.88 ± 4.04 | 1.21 (1.13–1.29) | <0.001 |
| (a) Heart Failure Hospitalization | |||
| Multivariable Models | Adjusted HR (95% CI) | p-value | C-statistic (95% CI) |
| Baseline Model + LAVImax | 1.025 (1.006–1.043) | 0.009 | 0.808 (0.738–0.879) |
| Baseline Model + LAVImin | 1.030 (1.009–1.052) | 0.005 | 0.818 (0.749–0.887) |
| Baseline Model + LAEF | 0.068 (0.004–1.038) | 0.053 | 0.816 (0.750–0.883) |
| Baseline Model + LACI | 1.890 (1.020–3.883) | 0.043 | 0.809 (0.739–0.878) |
| Baseline Model + LACI, cut-off > 40% | 7.91 (2.871–11.784) | <0.001 | 0.854 (0.804–0.905) |
| Baseline Model + LASr ED | 0.877 (0.829–0.928) | <0.001 | 0.861 (0.807–0.915) |
| Baseline Model + LASr ED, cut-off of 23 | 7.07 (1.55–32.67) | 0.012 | 0.833 (0.776–0.889) |
| Baseline Model + LAScd ED | 1.229 (1.106–1.366) | 0.0001 | 0.844 (0.784–0.905) |
| Baseline Model + LAScd ED, cut-off of 12 | 6.95 (1.58–31.03) | 0.010 | 0.833 (0.778–0.887) |
| Baseline Model + LASct ED | 1.180 (1.086–1.281) | 0.0001 | 0.838 (0.770–0.905) |
| Baseline Model + LASct ED, cut-off of 5 | 3.30 (1.59–6.88) | 0.001 | 0.839 (0.781–0.896) |
| (b) Composite Adverse Outcomes | |||
| Multivariable Models | Adjusted HR (95% CI) | p-value | C-statistic (95% CI) |
| Baseline Model + LAVImax | 1.006 (0.995–1.018) | 0.294 | 0.743 (0.691–0.796) |
| Baseline Model + LAVImin | 1.011 (0.997–1.025) | 0.139 | 0.746 (0.694–0.799) |
| Baseline Model + LAEF | 0.469 (0.098–2.243) | 0.343 | 0.744 (0.691–0.796) |
| Baseline Model + LACI | 1.890 (1.020–3.883) | 0.043 | 0.809 (0.739–0.878) |
| Baseline Model + LACI, cut-off > 40% | 2.062 (1.323–3.213) | 0.0014 | 0.761 (0.710–0.811) |
| Baseline Model + LASr ED | 0.936 (0.912–0.961) | <0.0001 | 0.774 (0.725–0.824) |
| Baseline Model + LASr ED, cut-off of 23 | 2.01 (1.56–3.48) | 0.013 | 0.752 (0.701–0.802) |
| Baseline Model + LAScd ED | 1.110 (1.059–1.164) | <0.0001 | 0.766 (0.716–0.816) |
| Baseline Model + LAScd ED, cut-off of 12 | 3.31 (1.84–6.97) | <0.001 | 0.764 (0.715–0.812) |
| Baseline Model + LASct ED | 1.083 (1.034–1.128) | 0.0001 | 0.763 (0.711–0.814) |
| Baseline Model + LASct ED, cut-off of 5 | 2.47 (1.57–3.88) | <0.001 | 0.764 (0.712–0.915) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seah, A.; Li, T.Y.W.; Sari, N.Y.; Lee, C.-H.; Yeo, T.-C.; Yip, J.W.L.; Lim, Y.C.; Poh, K.-K.; Kong, W.K.F.; Lin, W.; et al. The Prognostic Implication of Left Atrial Strain Parameters with Conventional Left Atrial Parameters for the Prediction of Adverse Outcomes in Asian Patients with Hypertrophic Cardiomyopathy—An Echocardiographic Study. J. Cardiovasc. Dev. Dis. 2025, 12, 261. https://doi.org/10.3390/jcdd12070261
Seah A, Li TYW, Sari NY, Lee C-H, Yeo T-C, Yip JWL, Lim YC, Poh K-K, Kong WKF, Lin W, et al. The Prognostic Implication of Left Atrial Strain Parameters with Conventional Left Atrial Parameters for the Prediction of Adverse Outcomes in Asian Patients with Hypertrophic Cardiomyopathy—An Echocardiographic Study. Journal of Cardiovascular Development and Disease. 2025; 12(7):261. https://doi.org/10.3390/jcdd12070261
Chicago/Turabian StyleSeah, Andre, Tony Y. W. Li, Novi Yanti Sari, Chi-Hang Lee, Tiong-Cheng Yeo, James W. L. Yip, Yoke Ching Lim, Kian-Keong Poh, William K. F. Kong, Weiqin Lin, and et al. 2025. "The Prognostic Implication of Left Atrial Strain Parameters with Conventional Left Atrial Parameters for the Prediction of Adverse Outcomes in Asian Patients with Hypertrophic Cardiomyopathy—An Echocardiographic Study" Journal of Cardiovascular Development and Disease 12, no. 7: 261. https://doi.org/10.3390/jcdd12070261
APA StyleSeah, A., Li, T. Y. W., Sari, N. Y., Lee, C.-H., Yeo, T.-C., Yip, J. W. L., Lim, Y. C., Poh, K.-K., Kong, W. K. F., Lin, W., Sia, C.-H., & Wong, R. C. C. (2025). The Prognostic Implication of Left Atrial Strain Parameters with Conventional Left Atrial Parameters for the Prediction of Adverse Outcomes in Asian Patients with Hypertrophic Cardiomyopathy—An Echocardiographic Study. Journal of Cardiovascular Development and Disease, 12(7), 261. https://doi.org/10.3390/jcdd12070261






