The Role of Non-HDL Cholesterol and Apolipoprotein B in Cardiovascular Disease: A Comprehensive Review
Abstract
1. Introduction
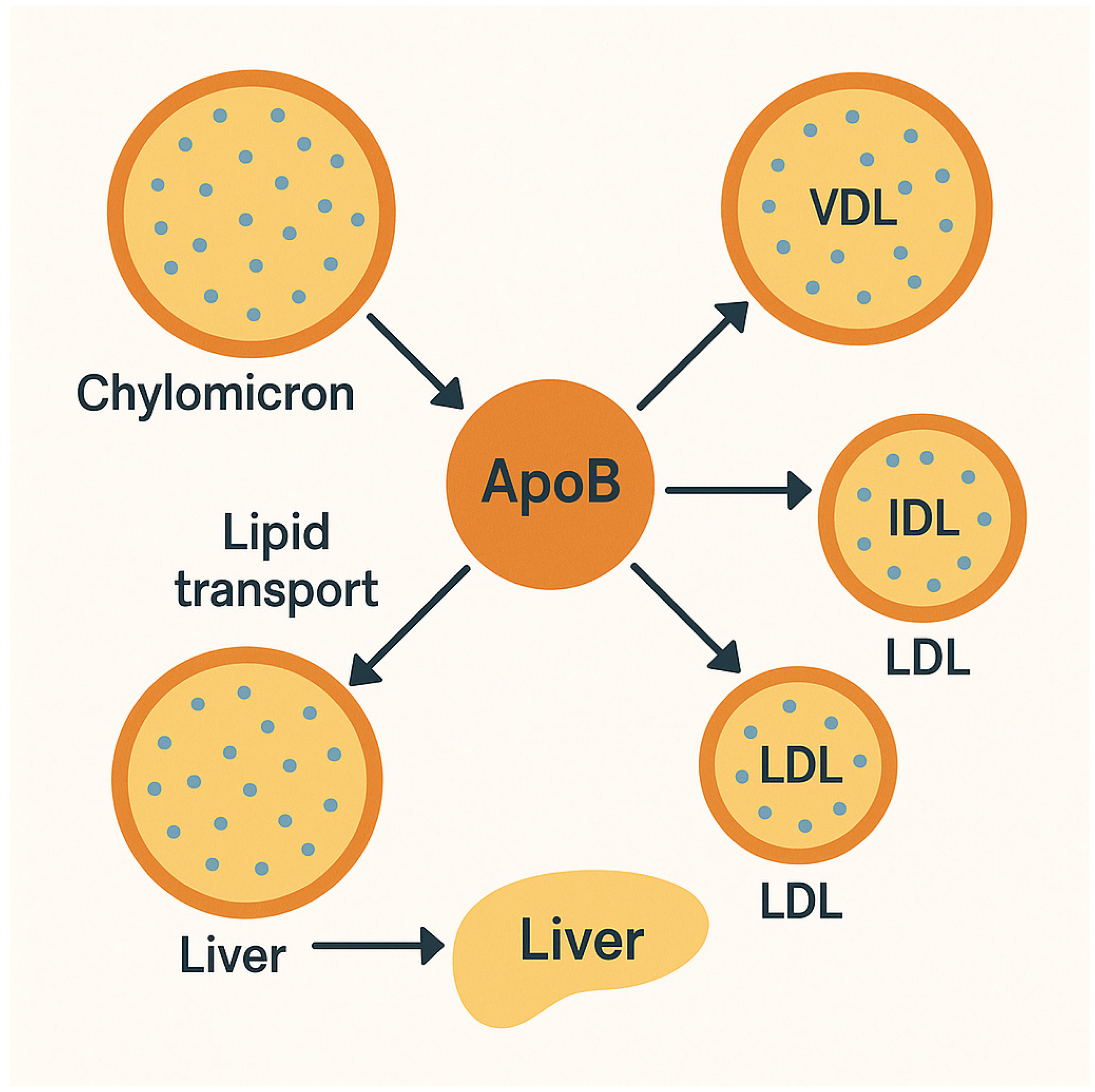
2. Pathophysiology: Non-HDL-C and Apo B and Their Role in Atherosclerosis
3. Familial Hypercholesterolemia and Genotype-Independent Atherosclerosis
4. Clinical Significance in Diabetes Mellitus
5. Implications for Hypertriglyceridemia and Obesity
6. Clinical Evidence Supporting Non-HDL-C and Apo B
7. Genetic and Biomarker Insights Supporting the Use of Non-HDL-C and Apo B in CV Prevention
8. The Role of Non-HDL Cholesterol and Apo B in Chronic Kidney Disease and Pediatric Populations
9. Sex Differences in the Use of Non-HDL Cholesterol and Apo B for CV Prevention
10. The Role of High-Density Lipoprotein
11. Current Guideline Recommendations
12. Therapeutic Implications for Decreasing Non-HDL-C and Apo B
13. Emerging Technologies and Implementation
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AACE | American Association of Clinical Endocrinologists |
| AAP | American Society of Pediatrics |
| ACC | American College of Cardiology |
| ADA | American Diabetes Association |
| AHA | American Heart Association |
| AI | Artificial intelligence |
| ASCVD | Atherosclerotic cardiovascular disease |
| Apo B | Apolipoprotein B |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| CETP | Cholesteryl ester transfer protein |
| CKD | Chronic kidney disease |
| ESC | European Society of Cardiology |
| GWAS | Genome-wide association studies |
| IDL | Intermediate density lipoprotein |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| Lp(a) | Lipoprotein A |
| LDL-C | Low-density lipoprotein-C |
| NHLBI | National Heart Lung Blood Institute |
| NMR | Nuclear magnetic resonance |
| Non-HDL- | C Non-high-density lipoprotein |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9 |
| TG | Triglyceride |
| T2DM | Type 2 diabetes mellitus |
| VLDL | Very low-density lipoprotein |
References
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Simes, R.J.; Durrington, P.; Hitman, G.A.; Welch, K.M.A.; et al. Association of LDL Cholesterol, Non-HDL Cholesterol, and Apolipoprotein B Levels with Risk of Cardiovascular Events among Patients Treated with Statins: A Meta-Analysis. JAMA 2012, 307, 1302–1309. [Google Scholar] [CrossRef]
- Silverman, M.G.; Ference, B.A.; Im, K.; Wiviott, S.D.; Giugliano, R.P.; Grundy, S.M.; Braunwald, E.; Sabatine, M.S. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-Analysis. JAMA 2016, 316, 1289–1297. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2017, 23, 1–87. [Google Scholar] [CrossRef] [PubMed]
- Kastelein, J.J.P.; van der Steeg, W.A.; Holme, I.; Gaffney, M.; Cater, N.B.; Barter, P.; Deedwania, P.; Olsson, A.G.; Boekholdt, S.M.; Demicco, D.A.; et al. Lipids, Apolipoproteins, and Their Ratios in Relation to Cardiovascular Events with Statin Treatment. Circulation 2008, 117, 3002–3009. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Islam, S.; Yusuf, S.; McQueen, M.J. Discordance Analysis of Apolipoprotein B and Non-High Density Lipoprotein Cholesterol as Markers of Cardiovascular Risk in the INTERHEART Study. Atherosclerosis 2012, 225, 444–449. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Sabatine, M.S.; Giugliano, R.P.; Keech, A.C.; Honarpour, N.; Wiviott, S.D.; Murphy, S.A.; Kuder, J.F.; Wang, H.; Liu, T.; Wasserman, S.M.; et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N. Engl. J. Med. 2017, 376, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Lincoff, A.M.; Brennan, D.; Ray, K.K.; Mason, D.; Kastelein, J.J.P.; Thompson, P.D.; Libby, P.; Cho, L.; Plutzky, J.; et al. Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. N. Engl. J. Med. 2023, 388, 1353–1364. [Google Scholar] [CrossRef]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef]
- Ip, S.; Lichtenstein, A.H.; Chung, M.; Lau, J.; Balk, E.M. Systematic Review: Association of Low-Density Lipoprotein Subfractions with Cardiovascular Outcomes. Ann. Intern. Med. 2009, 150, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Gusev, E.; Sarapultsev, A. Atherosclerosis and inflammation: Insights from the theory of general pathological processes. Int. J. Mol. Sci. 2023, 24, 7910. [Google Scholar] [CrossRef] [PubMed]
- Sniderman, A.D.; Williams, K.; Contois, J.H.; Monroe, H.M.; McQueen, M.J.; de Graaf, J.; Furberg, C.D. A Meta-Analysis of Low-Density Lipoprotein Cholesterol, Non-High-Density Lipoprotein Cholesterol, and Apolipoprotein B as Markers of Cardiovascular Risk. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 337–345. [Google Scholar] [CrossRef]
- Tsimikas, S. Lipoprotein(a): Diagnosis, prognosis, and emerging therapies. J. Am. Coll. Cardiol. 2017, 69, 692–711. [Google Scholar] [CrossRef] [PubMed]
- Galkina, E.; Ley, K. Immune and inflammatory mechanisms of atherosclerosis. Annu. Rev. Immunol. 2009, 27, 165–197. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Nordestgaard, B.G.; Varbo, A.; Benn, M.; Tybjaerg-Hansen, A.; Langsted, A. Remnant cholesterol as a causal risk factor for ischemic heart disease. J. Am. Coll. Cardiol. 2016, 67, 2238–2246. [Google Scholar] [CrossRef]
- Writing Committee; Lloyd-Jones, D.M.; Morris, P.B.; Ballantyne, C.M.; Birtcher, K.K.; Covington, A.M.; DePalma, S.M.; Minissian, M.B.; Orringer, C.E.; Smith, S.C.; et al. 2022 ACC Expert Consensus Decision Pathway on the Role of Nonstatin Therapies for LDL-Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 80, 1366–1418. [Google Scholar] [CrossRef]
- Johansen, M.Ø.; Nielsen, S.F.; Afzal, S.; Vedel-Krogh, S.; Davey Smith, G.; Nordestgaard, B.G. Very Low-Density Lipoprotein Cholesterol May Mediate a Substantial Component of the Effect of Obesity on Myocardial Infarction Risk: The Copenhagen General Population Study. Clin. Chem. 2021, 67, 276–287. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration; Lewington, S.; Whitlock, G.; Clarke, R.; Sherliker, P.; Emberson, J.; Halsey, J.; Qizilbash, N.; Peto, R.; Collins, R. Blood Cholesterol and Vascular Mortality by Age, Sex, and Blood Pressure: A Meta-Analysis of Individual Data from 61 Prospective Studies with 55,000 Vascular Deaths. Lancet Lond. Engl. 2007, 370, 1829–1839. [Google Scholar] [CrossRef]
- Clarke, R.; Peden, J.F.; Hopewell, J.C.; Kyriakou, T.; Goel, A.; Heath, S.C.; Parish, S.; Barlera, S.; Franzosi, M.G.; Rust, S.; et al. Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N. Engl. J. Med. 2009, 361, 2518–2528. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, C.; Dewan, A.; Orsoni, A.; Merki, E.; Miller, E.R.; Shin, M.J.; Binder, C.J.; Hörkkö, S.; Krauss, R.M.; Chapman, M.J.; et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 2008, 49, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Borén, J.; Chapman, M.J.; Krauss, R.M.; Packard, C.J.; Bentzon, J.F.; Binder, C.J.; Daemen, M.J.; Demer, L.L.; Hegele, R.A.; Nicholls, S.J.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease: Pathophysiological, Genetic, and Therapeutic Insights: A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2020, 41, 2313–2330. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef]
- Goldberg, A.C.; Hopkins, P.N.; Toth, P.P.; Ballantyne, C.M.; Rader, D.J.; Robinson, J.G.; Daniels, S.R.; Gidding, S.S.; De Ferranti, S.D.; Ito, M.K.; et al. Familial hypercholesterolemia: Screening, diagnosis and management of pediatric and adult patients. J. Clin. Lipidol. 2011, 5 (Suppl. S3), S1–S8. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-Density Lipoproteins Cause Atherosclerotic Cardiovascular Disease. 1. Evidence from Genetic, Epidemiologic, and Clinical Studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Danesh, J.; Erqou, S.; Walker, M.; Thompson, S.G.; Tipping, R.; Ford, C.; Pressel, S.; Walldius, G.; Jungner, I.; et al. The Emerging Risk Factors Collaboration: Analysis of Individual Data on Lipid, Inflammatory and Other Markers in over 1.1 Million Participants in 104 Prospective Studies of Cardiovascular Diseases. Eur. J. Epidemiol. 2007, 22, 839–869. [Google Scholar] [CrossRef]
- McQueen, M.J.; Hawken, S.; Wang, X.; Ounpuu, S.; Sniderman, A.; Probstfield, J.; Steyn, K.; Sanderson, J.E.; Hasani, M.; Volkova, E.; et al. Lipids, Lipoproteins, and Apolipoproteins as Risk Markers of Myocardial Infarction in 52 Countries (the INTERHEART Study): A Case-Control Study. Lancet Lond. Engl. 2008, 372, 224–233. [Google Scholar] [CrossRef]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of Potentially Modifiable Risk Factors Associated with Myocardial Infarction in 52 Countries (the INTERHEART Study): Case-Control Study. Lancet Lond. Engl. 2004, 364, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T.G.; Sanderson, E.; Palmer, T.M.; Ala-Korpela, M.; Ference, B.A.; Davey Smith, G.; Holmes, M.V. Evaluating the Relationship between Circulating Lipoprotein Lipids and Apolipoproteins with Risk of Coronary Heart Disease: A Multivariable Mendelian Randomisation Analysis. PLoS Med. 2020, 17, e1003062. [Google Scholar] [CrossRef] [PubMed]
- Emerging Risk Factors Collaboration; Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; et al. Major Lipids, Apolipoproteins, and Risk of Vascular Disease. JAMA 2009, 302, 1993–2000. [Google Scholar] [CrossRef] [PubMed]
- Pencina, M.J.; D’Agostino, R.B.; Zdrojewski, T.; Williams, K.; Thanassoulis, G.; Furberg, C.D.; Peterson, E.D.; Vasan, R.S.; Sniderman, A.D. Apolipoprotein B Improves Risk Assessment of Future Coronary Heart Disease in the Framingham Heart Study beyond LDL-C and Non-HDL-C. Eur. J. Prev. Cardiol. 2015, 22, 1321–1327. [Google Scholar] [CrossRef]
- Safian, R.D.; Textor, S.C. Renal-artery stenosis. N. Engl. J. Med. 2001, 344, 431–442. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Heimbürger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong as-sociation between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int. 2005, 55, 1899–1911. [Google Scholar] [CrossRef]
- Vaziri, N.D. Disorders of Lipid Metabolism in Nephrotic Syndrome: Mechanisms and Consequences. Kidney Int. 2016, 90, 41–52. [Google Scholar] [CrossRef]
- Wanner, C.; Tonelli, M. Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members KDIGO Clinical Practice Guideline for Lipid Management in CKD: Summary of Recommendation Statements and Clinical Approach to the Patient. Kidney Int. 2014, 85, 1303–1309. [Google Scholar] [CrossRef]
- Strong, J.P.; Malcom, G.T.; McMahan, C.A.; Tracy, R.E.; Newman, W.P.; Herderick, E.E.; Cornhill, J.F. Prevalence and extent of atherosclerosis in adolescents and young adults: Implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA 1999, 281, 727–735. [Google Scholar] [CrossRef]
- Daniels, S.R.; Greer, F.R. Committee on Nutrition Lipid Screening and Cardiovascular Health in Childhood. Pediatrics 2008, 122, 198–208. [Google Scholar] [CrossRef]
- Gidding, S.S.; Rana, J.S.; Prendergast, C.; McGill, H.; Carr, J.J.; Liu, K.; Colangelo, L.A.; Loria, C.M.; Lima, J.; Terry, J.G.; et al. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Risk Score in Young Adults Predicts Coronary Artery and Abdominal Aorta Calcium in Middle Age: The CARDIA Study. Circulation 2016, 133, 139–146. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. National Heart, Lung, and Blood Institute Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents: Summary Report. Pediatrics 2011, 128 (Suppl. 5), S213–S256. [Google Scholar] [CrossRef] [PubMed]
- de Ferranti, S.D.; Steinberger, J.; Ameduri, R.; Baker, A.; Gooding, H.; Kelly, A.S.; Mietus-Snyder, M.; Mitsnefes, M.M.; Peterson, A.L.; St-Pierre, J.; et al. Cardiovascular Risk Reduction in High-Risk Pediatric Patients: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e603–e634. [Google Scholar] [CrossRef]
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of Inflammation in the Pathogenesis of Atherosclerosis and Therapeutic Interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Rifai, N.; Cook, N.R.; Bradwin, G.; Buring, J.E. Non-HDL Cholesterol, Apolipoproteins A-I and B100, Standard Lipid Measures, Lipid Ratios, and CRP as Risk Factors for Cardiovascular Disease in Women. JAMA 2005, 294, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Crawford, S.L.; Chae, C.U.; Everson-Rose, S.A.; Sowers, M.F.; Sternfeld, B.; Sutton-Tyrrell, K. Are Changes in Cardiovascular Disease Risk Factors in Midlife Women Due to Chronological Aging or to the Menopausal Transition? J. Am. Coll. Cardiol. 2009, 54, 2366–2373. [Google Scholar] [CrossRef]
- Sniderman, A.D.; Thanassoulis, G.; Glavinovic, T.; Navar, A.M.; Pencina, M.; Catapano, A.; Ference, B.A. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019, 4, 1287–1295. [Google Scholar] [CrossRef]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T.; et al. Plasma HDL Cholesterol and Risk of Myocardial Infarction: A Mendelian Randomisation Study. Lancet Lond. Engl. 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Rizvi, A.A.; Stoian, A.P.; Janez, A.; Rizzo, M. Lipoproteins and Cardiovascular Disease: An Update on the Clinical Significance of Atherogenic Small, Dense LDL and New Therapeutical Options. Biomedicines 2021, 9, 1579. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and Safety of More Intensive Lowering of LDL Cholesterol: A Meta-Analysis of Data from 170,000 Participants in 26 Randomised Trials. Lancet Lond. Engl. 2010, 376, 1670–1681. [Google Scholar] [CrossRef]
- Cannon, C.P.; Blazing, M.A.; Giugliano, R.P.; McCagg, A.; White, J.A.; Theroux, P.; Darius, H.; Lewis, B.S.; Ophuis, T.O.; Jukema, J.W.; et al. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N. Engl. J. Med. 2015, 372, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
| Condition/Risk Category | Non-HDL-C Target (mg/dL) | Apo B Target (mg/dL) | Guideline Source |
|---|---|---|---|
| Very High CV Risk (e.g., T2DM + organ damage) | <85 | <65 | 2023 ESC Guidelines |
| High CV Risk (e.g., T2DM without complications) | <100 | <80 | 2023 ESC Guidelines |
| Extreme Risk (e.g., progressive ASCVD) | LDL-C + 25 = ~< 80 | <70 | 2017 AACE/ACE Guidelines |
| High Risk (e.g., diabetes + ≥1 ASCVD risk factor) | LDL-C + 30 = ~< 100 | <80 | 2017 AACE/ACE Guidelines |
| General Primary Prevention | Not specified | Not routinely measured | 2019 ACC/AHA Guidelines (defer formal targets) |
| Hypertriglyceridemia (secondary marker) | Individualized | Measure ApoB | 2023 ESC (emphasizes use in complex dyslipidemia) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsi, V.; Argyriou, N.; Fragoulis, C.; Tsioufis, K. The Role of Non-HDL Cholesterol and Apolipoprotein B in Cardiovascular Disease: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2025, 12, 256. https://doi.org/10.3390/jcdd12070256
Katsi V, Argyriou N, Fragoulis C, Tsioufis K. The Role of Non-HDL Cholesterol and Apolipoprotein B in Cardiovascular Disease: A Comprehensive Review. Journal of Cardiovascular Development and Disease. 2025; 12(7):256. https://doi.org/10.3390/jcdd12070256
Chicago/Turabian StyleKatsi, Vasiliki, Nikolaos Argyriou, Christos Fragoulis, and Konstantinos Tsioufis. 2025. "The Role of Non-HDL Cholesterol and Apolipoprotein B in Cardiovascular Disease: A Comprehensive Review" Journal of Cardiovascular Development and Disease 12, no. 7: 256. https://doi.org/10.3390/jcdd12070256
APA StyleKatsi, V., Argyriou, N., Fragoulis, C., & Tsioufis, K. (2025). The Role of Non-HDL Cholesterol and Apolipoprotein B in Cardiovascular Disease: A Comprehensive Review. Journal of Cardiovascular Development and Disease, 12(7), 256. https://doi.org/10.3390/jcdd12070256






