Short-Term Outcomes of Partial Upper Ministernotomy for Aortic Valve Replacement Within the Learning Curve Context
Abstract
1. Introduction
2. Material and Methods
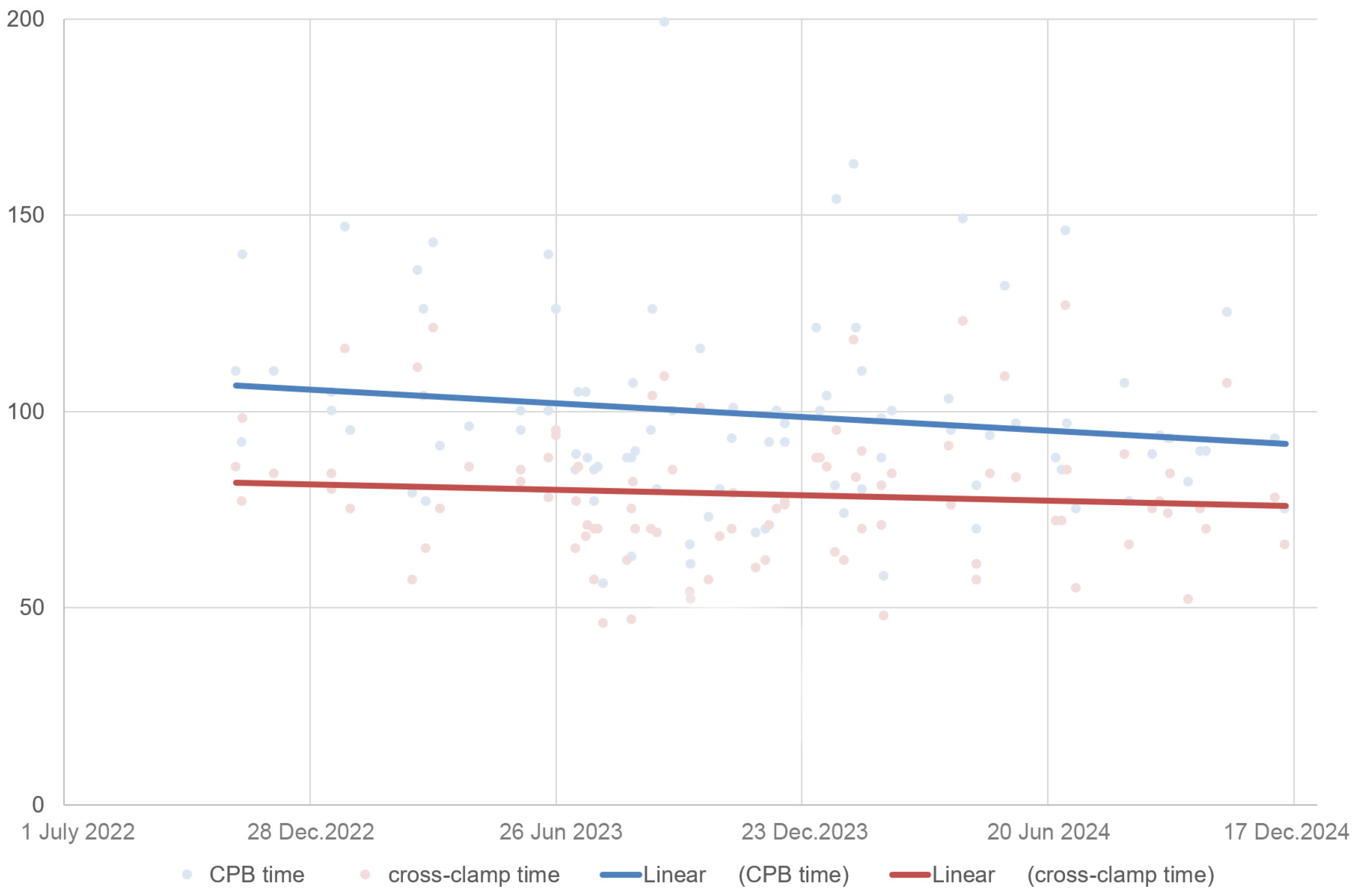
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| AS | aortic stenosis |
| AVB | atrioventricular block |
| AVR | aortic valve replacement |
| BMI | body mass index |
| BSA | body surface area |
| CAPD | chronic obstructive pulmonary disease |
| CPB | cardiopulmonary bypass |
| DHS | Department of Heart Surgery of the East Slovak Institute of Cardiovascular Diseases and the Faculty of Medicine at Pavol Jozef Šafárik University in Košice |
| DM | diabetes mellitus |
| iAVA | indexed aortic valve area |
| ICU | intensive care unit |
| iEOA | indexed effective orifice area |
| IHD | ischaemic heart disease |
| LVEF | left ventricle ejection fraction |
| MS | median sternotomy |
| POAF | paroxysmal postoperative atrial fibrillation |
| PPOAF | persistent postoperative atrial fibrillation |
| PUMS | partial upper ministernotomy |
| RAMT | right anterolateral minithoracotomy |
References
- Daeter, E.J.; de Beaufort, H.W.L.; Roefs, M.M.; van Boven, W.J.P.; van Veghel, D.; van der Kaaij, N.P. Cardiothoracic Surgery Registration Committee of the Netherlands Heart, R. First-time surgical aortic valve replacement: Nationwide trends and outcomes from The Netherlands Heart Registration. Eur. J. Cardio-Thorac. Surg. 2024, 65, ezae177. [Google Scholar] [CrossRef] [PubMed]
- Toporcer, T.; Lukacin, S.; Kraus, A.; Homola, M.; Beres, A.; Trebisovsky, M.; Radotzy, D.; Rohn, V.; Kolesar, A. Bioprostheses and Mechanical Prostheses for Aortic Valve Replacement in Patients Aged 50 to 65 Years Offer Similar Long-Term Survival Rates. J. Cardiovasc. Dev. Dis. 2025, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Traxler, D.; Krotka, P.; Laggner, M.; Mildner, M.; Graf, A.; Reichardt, B.; Wendt, R.; Auer, J.; Moser, B.; Mascherbauer, J.; et al. Mechanical aortic valve prostheses offer a survival benefit in 50-65 year olds: AUTHEARTVISIT study. Eur. J. Clin. Investig. 2022, 52, e13736. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiary, F.; Salamate, S.; Amer, M.; Sirat, S.; Bayram, A.; Doss, M.; El-Sayed Ahmad, A. Comparison of Right Anterior Mini-Thoracotomy Versus Partial Upper Sternotomy in Aortic Valve Replacement. Adv. Ther. 2022, 39, 4266–4284. [Google Scholar] [CrossRef]
- Bakhtiary, F.; El-Sayed Ahmad, A.; Amer, M.; Salamate, S.; Sirat, S.; Borger, M.A. Video-Assisted Minimally Invasive Aortic Valve Replacement Through Right Anterior Minithoracotomy for All Comers With Aortic Valve Disease. Innovations 2021, 16, 169–174. [Google Scholar] [CrossRef]
- Ghoreishi, M.; Thourani, V.H.; Badhwar, V.; Massad, M.; Svensson, L.; Taylor, B.S.; Pasrija, C.; Gammie, J.S.; Jacobs, J.P.; Cox, M.; et al. Less-Invasive Aortic Valve Replacement: Trends and Outcomes From The Society of Thoracic Surgeons Database. Ann. Thorac. Surg. 2021, 111, 1216–1223. [Google Scholar] [CrossRef]
- Leviner, D.B.; Ronai, T.; Abraham, D.; Eliad, H.; Schwartz, N.; Sharoni, E. Minimal Learning Curve for Minimally Invasive Aortic Valve Replacement. Thorac. Cardiovasc. Surg. 2024, 73, 296–303. [Google Scholar] [CrossRef]
- Karadzha, A.; Sharifulin, R.; Khrushchev, S.; Afanasyev, A.; Sapegin, A.; Zheleznev, S.; Chernyavsky, A.; Bogachev-Prokophiev, A. Minimally invasive versus conventional methods for aortic root surgery: Choosing the right approach. Asian Cardiovasc. Thorac. Ann. 2024, 32, 285–293. [Google Scholar] [CrossRef]
- Tagliafierro, M.; Chauvette, V.; Bouhout, I.; Levesque, S.; Guertin, M.C.; Lamarche, Y.; Poirier, N.; Bernier, P.L.; Cartier, R.; El-Hamamsy, I.; et al. Learning curve of the Ross procedure after more than 650 interventions: A single-center, retrospective analysis. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2025, 67, ezaf071. [Google Scholar] [CrossRef]
- Gennai, S.; Leone, N.; Bartolotti, L.A.; Andreoli, F.; Migliari, M.; Silingardi, R. Comprehensive Learning Curve Analysis of a Long-Term Experience With Thoracic Endovascular Aneurysm Repair. J. Endovasc. Ther. Off. J. Int. Soc. Endovasc. Spec. 2024, 31, 901–909. [Google Scholar] [CrossRef]
- Verevkin, A.; Dashkevich, A.; Gadelkarim, I.; Shaqu, R.; Otto, W.; Sgouropoulou, S.; Ender, J.; Kiefer, P.; Borger, M.A. Minimally invasive coronary artery bypass grafting via left anterior minithoracotomy: Setup, results, and evolution of a new surgical procedure. JTCVS Tech. 2025, 29, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Hlavicka, J.; Janda, D.; Budera, P.; Tousek, P.; Maly, M.; Fojt, R.; Linkova, H.; Holubec, T.; Kacer, P. Partial upper sternotomy for aortic valve replacement provides similar mid-term outcomes as the full sternotomy. J. Thorac. Dis. 2022, 14, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Cammertoni, F.; Bruno, P.; Rosenhek, R.; Pavone, N.; Farina, P.; Mazza, A.; Iafrancesco, M.; Nesta, M.; Chiariello, G.A.; Comerci, G.; et al. Minimally Invasive Aortic Valve Surgery in Octogenarians: Reliable Option or Fallback Solution? Innovations 2021, 16, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Masiello, P.; Coscioni, E.; Panza, A.; Triumbari, F.; Preziosi, G.; Di Benedetto, G. Surgical results of aortic valve replacement via partial upper sternotomy: Comparison with median sternotomy. Cardiovasc. Surg. 2002, 10, 333–338. [Google Scholar] [CrossRef]
- Xie, X.B.; Dai, X.F.; Qiu, Z.H.; Jiang, D.B.; Wu, Q.S.; Dong, Y.; Chen, L.W. Do obese patients benefit from isolated aortic valve replacement through a partial upper sternotomy? J. Cardiothorac. Surg. 2022, 17, 179. [Google Scholar] [CrossRef]
- Irace, F.G.; Chirichilli, I.; Russo, M.; Ranocchi, F.; Bergonzini, M.; Lio, A.; Nicolo, F.; Musumeci, F. Aortic Valve Replacement: Understanding Predictors for the Optimal Ministernotomy Approach. J. Clin. Med. 2023, 12, 6717. [Google Scholar] [CrossRef]
- Fischlein, T.; Folliguet, T.; Meuris, B.; Shrestha, M.L.; Roselli, E.E.; McGlothlin, A.; Kappert, U.; Pfeiffer, S.; Corbi, P.; Lorusso, R.; et al. Sutureless versus conventional bioprostheses for aortic valve replacement in severe symptomatic aortic valve stenosis. J. Thorac. Cardiovasc. Surg. 2021, 161, 920–932. [Google Scholar] [CrossRef]
- Okiljevic, B.; Raickovic, T.; Zivkovic, I.; Vukovic, P.; Milicic, M.; Stojanovic, I.; Milacic, P.; Micovic, S. Right anterior thoracotomy vs. upper hemisternotomy for aortic valve replacement with Perceval S: Is there a difference? Front. Cardiovasc. Med. 2024, 11, 1369204. [Google Scholar] [CrossRef]
- Bocianski, M.; Puslecki, M.; Ratajczak, M.; Stefaniak, S.; Buczkowski, P.; Perek, B.; Jemielity, M. Comparative study of quality of life after aortic valve replacement through partial upper ministernotomy versus full median sternotomy. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2025, 34, 895–900. [Google Scholar] [CrossRef]
- Sandoval, E.; Bhoera, R.A.; Tomsic, A.; Morales-Rey, I.; Garcia-Alvarez, A.; Palmen, M.; Pereda, D. Learning curve of robotic mitral repair: Prospective two-centre study of proficiency and clinical outcomes. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2024, 66, ezae426. [Google Scholar] [CrossRef]
- Chia, R.H.M.; Joshi, P. Sternal sparing aortic valve replacement via right anterior minithoracotomy: An early experience. Indian J. Thorac. Cardiovasc. Surg. 2024, 40, 191–197. [Google Scholar] [CrossRef]
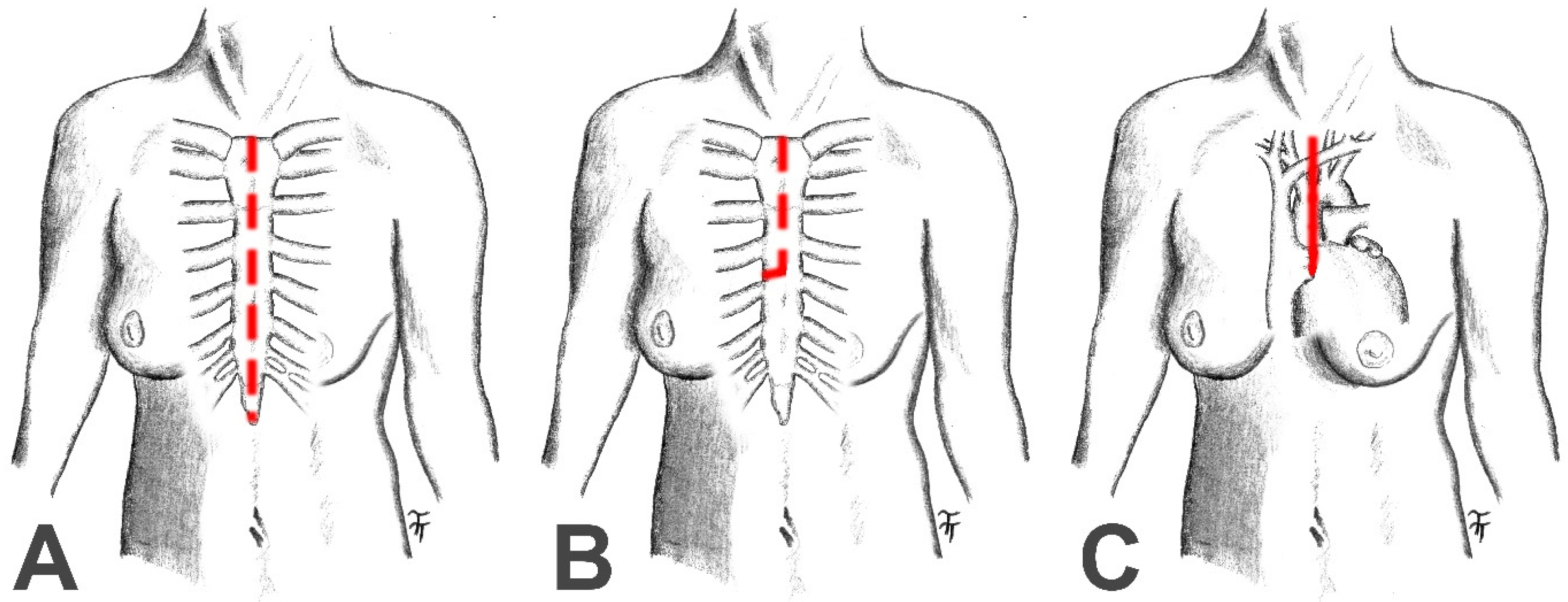
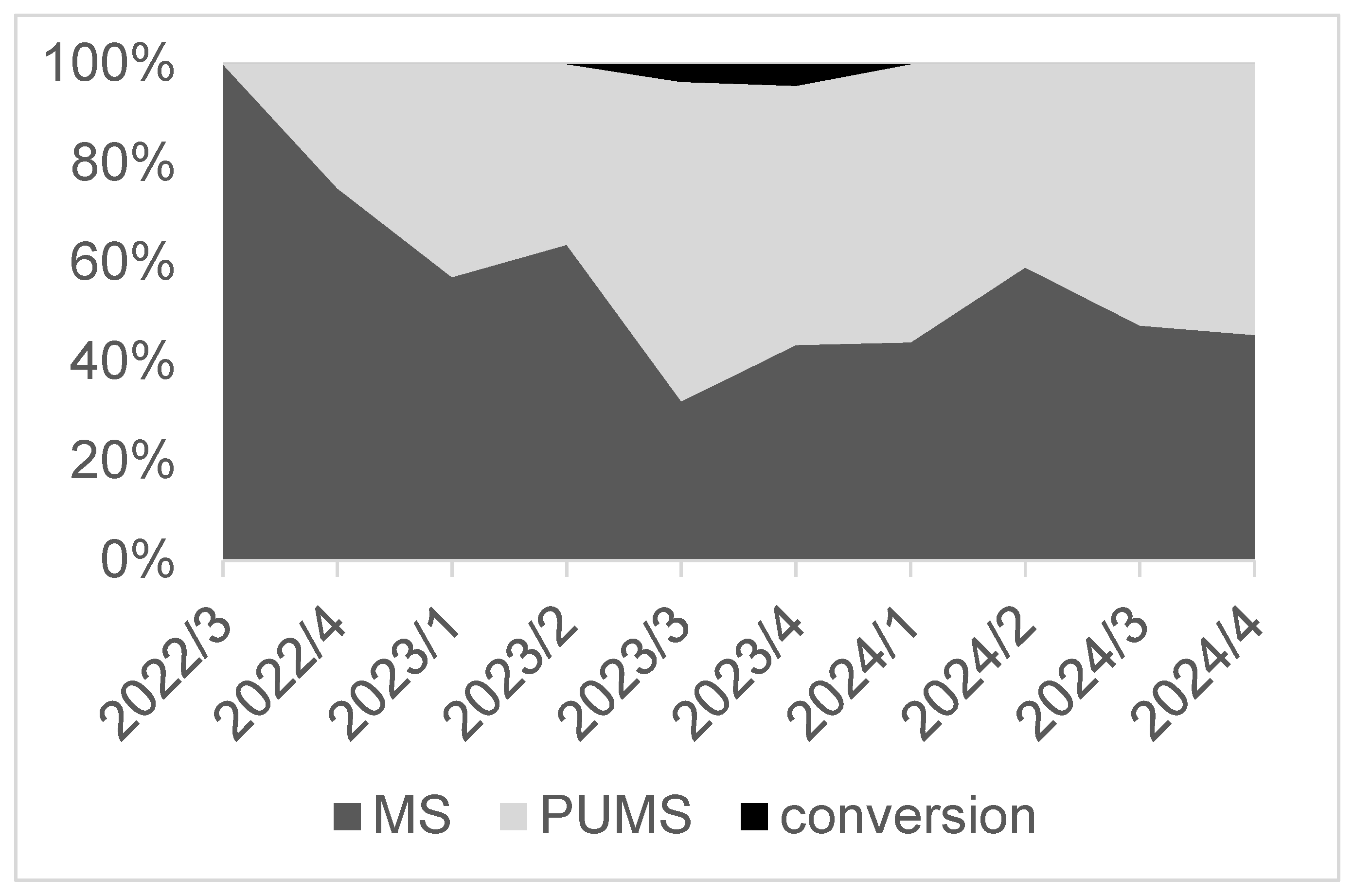
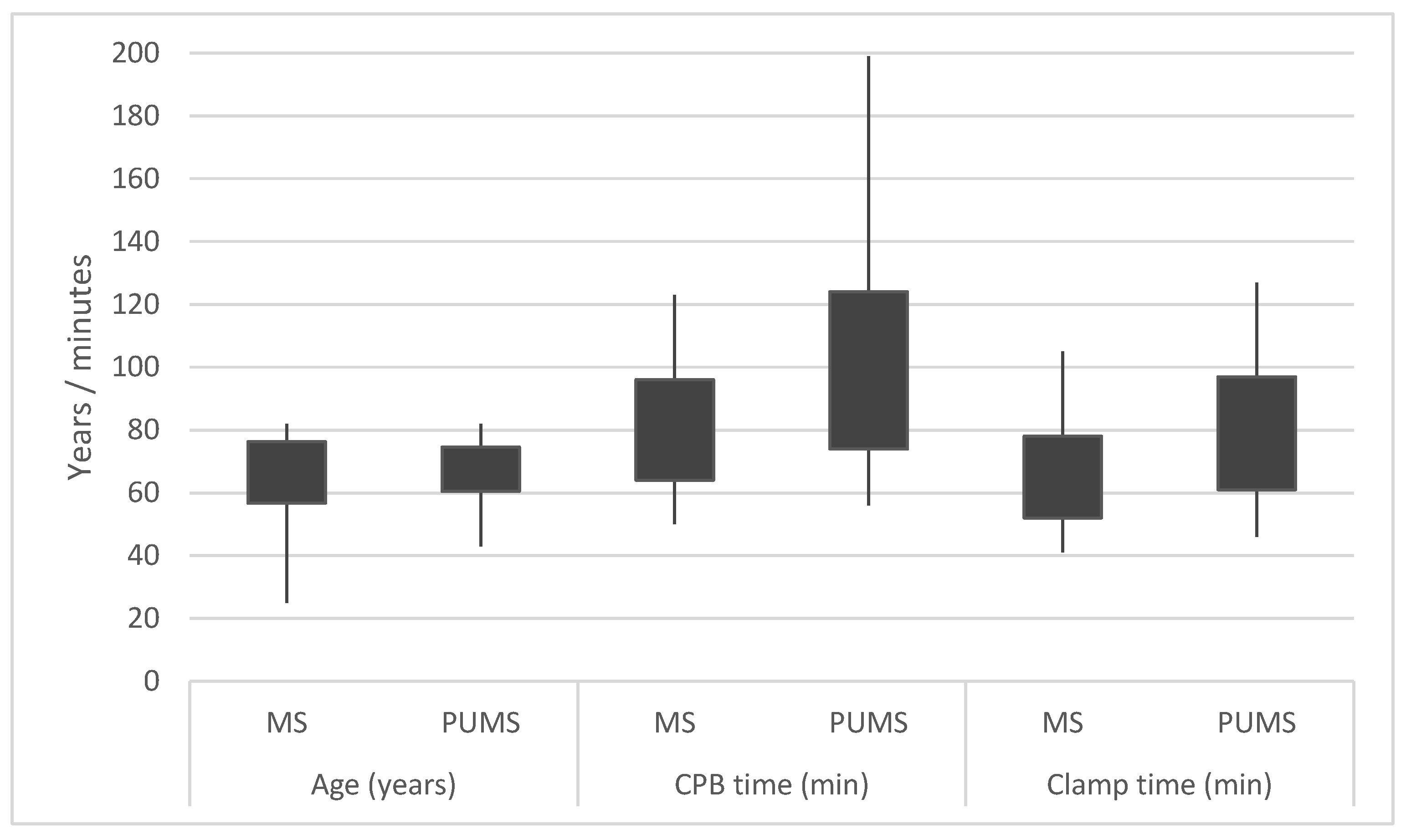
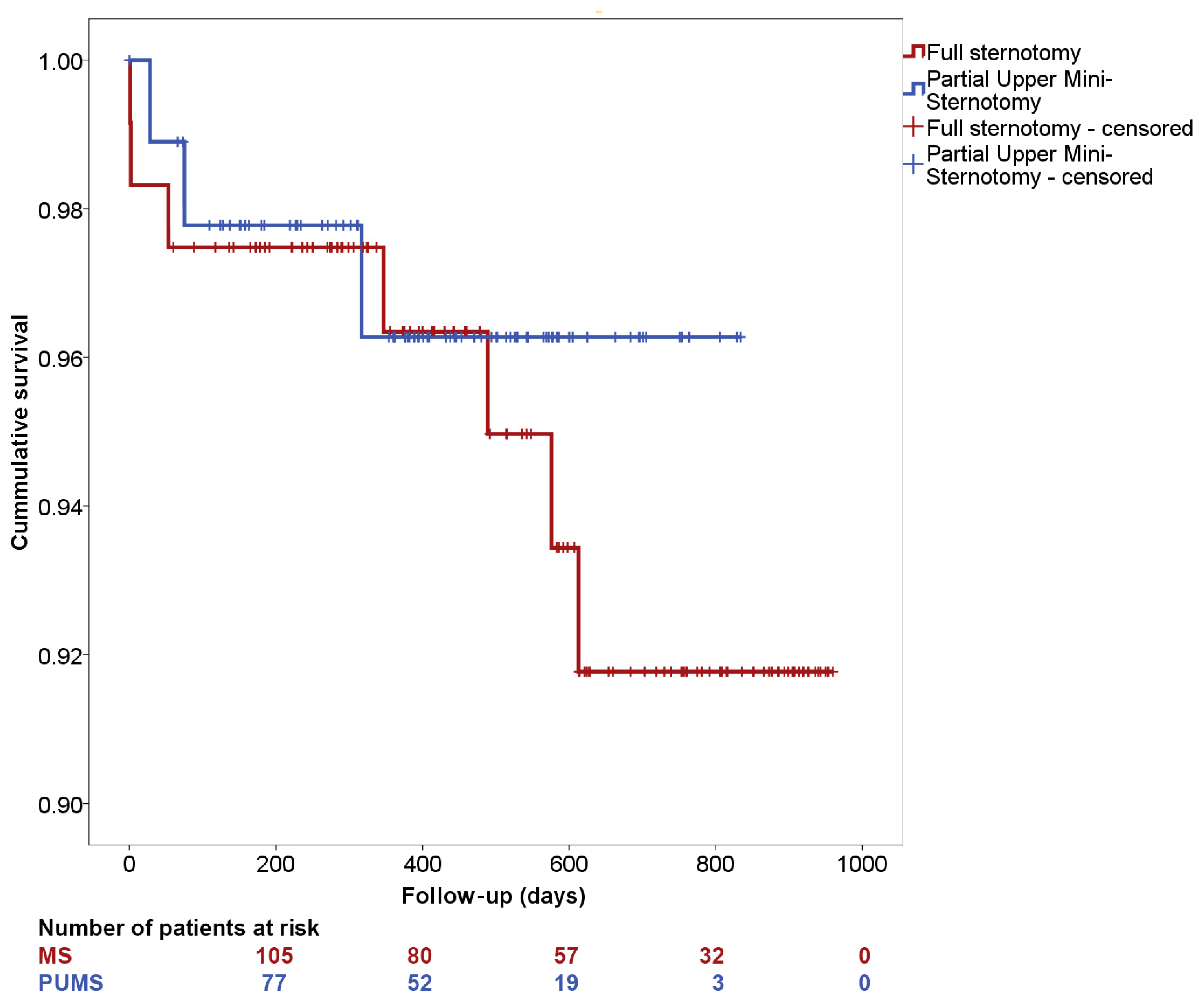
| Full Median Sternotomy (n = 119) | Partial Upper Mini-Sternotomy (n = 92) | p | |
|---|---|---|---|
| Age | 66.5 ± 9.8 | 67.5 ± 7.0 | 0.010 |
| Women | 37% | 36% | 0.492 |
| IHD history | 40% | 30% | 0.090 |
| Pacemaker preoperatively | 3% | 0% | 0.177 |
| AF | 3% | 1% | 0.275 |
| LVEF | 54 ± 10% | 58 ± 9% | 0.704 |
| iAVA | 0.4 ± 0.1 cm2/m2 | 0.4 ± 0.1 cm2/m2 | 0.825 |
| Velocity | 4.6 ± 0.7m/s | 4.7 ± 0.7 m/s | 0.868 |
| BSA | 2 ± 0.2 m2 | 2.0 ± 0.2 m2 | 0.386 |
| BMI | 30.7 ± 5.9 | 30.5 ± 5.5 | 0.539 |
| Diabetes mellitus | 28% | 26% | 0.457 |
| Liver disease | 13% | 12% | 0.530 |
| Stroke history | 7% | 4% | 0.335 |
| COPD | 13% | 17% | 0.218 |
| Full Median Sternotomy (n = 119) | Partial Upper Mini-Sternotomy (n = 92) | p | |
|---|---|---|---|
| Surgery: | |||
| CPB time | 80 ± 16 min | 99 ± 25 min | 0.002 |
| Clamp time | 65 ± 13 min | 79 ± 18 min | 0.024 |
| Conversion to MS | - | 2% | - |
| Early revisions | 1% | 2% | 0.404 |
| Late revisions | 8% | 3% | 0.103 |
| Deep sternal infections | 1% | 1% | 0.683 |
| Any revisions | 10% | 5% | 0.165 |
| Bioprosthesis | 96% | 98% | 0.341 |
| Prosthesis diameter | 22.7 ± 1.8 mm | 22.7 ± 1.5 mm | 0.060 |
| iEOA | 0.88 ± 0.12 cm2/m2 | 0.9 ± 0.1 cm2/m2 | 0.298 |
| Hospitalisation: | |||
| Pacemaker implantation (AVB) | 5% | 1% | 0.112 |
| PPOAF | 4% | 3% | 0.509 |
| POAF | 36% | 32% | 0.290 |
| ICU | 5.5 ± 5.2 days | 4.1 ± 3.3 days | 0.191 |
| Hospitalisation | 10.8 ± 6.2 days | 10.3 ± 4.5 days | 0.110 |
| Follow-up: | |||
| Medial gradient | 13 ± 5 mmHg | 14 ± 4 mmHg | 0.720 |
| Peak gradient | 24 ± 9 mmHg | 25 ± 8 mmHg | 0.557 |
| Velocity | 2.4 ± 0.4 m/s | 2.5 ± 0.4 m/s | 0.913 |
| Paravalvular leak | 7% | 11% | 0.546 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toporcer, T.; Homola, M.; Bereš, A.; Trebišovský, M.; Lopuchovský, T.; Mižáková, Š.; Vajda, L.; Lukačín, Š.; Kolesár, A. Short-Term Outcomes of Partial Upper Ministernotomy for Aortic Valve Replacement Within the Learning Curve Context. J. Cardiovasc. Dev. Dis. 2025, 12, 254. https://doi.org/10.3390/jcdd12070254
Toporcer T, Homola M, Bereš A, Trebišovský M, Lopuchovský T, Mižáková Š, Vajda L, Lukačín Š, Kolesár A. Short-Term Outcomes of Partial Upper Ministernotomy for Aortic Valve Replacement Within the Learning Curve Context. Journal of Cardiovascular Development and Disease. 2025; 12(7):254. https://doi.org/10.3390/jcdd12070254
Chicago/Turabian StyleToporcer, Tomáš, Marián Homola, Anton Bereš, Michal Trebišovský, Tomáš Lopuchovský, Štefánia Mižáková, Lukáš Vajda, Štefan Lukačín, and Adrián Kolesár. 2025. "Short-Term Outcomes of Partial Upper Ministernotomy for Aortic Valve Replacement Within the Learning Curve Context" Journal of Cardiovascular Development and Disease 12, no. 7: 254. https://doi.org/10.3390/jcdd12070254
APA StyleToporcer, T., Homola, M., Bereš, A., Trebišovský, M., Lopuchovský, T., Mižáková, Š., Vajda, L., Lukačín, Š., & Kolesár, A. (2025). Short-Term Outcomes of Partial Upper Ministernotomy for Aortic Valve Replacement Within the Learning Curve Context. Journal of Cardiovascular Development and Disease, 12(7), 254. https://doi.org/10.3390/jcdd12070254






