Patterning Defects in Mice with Defective Ventricular Wall Maturation and Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Lines and Genotyping
2.2. Tissue Processing
2.3. Bright Field Imaging
2.4. RNA-Seq Data Analysis
2.5. RNA Probe Synthesis and In Situ Hybridization on Sections
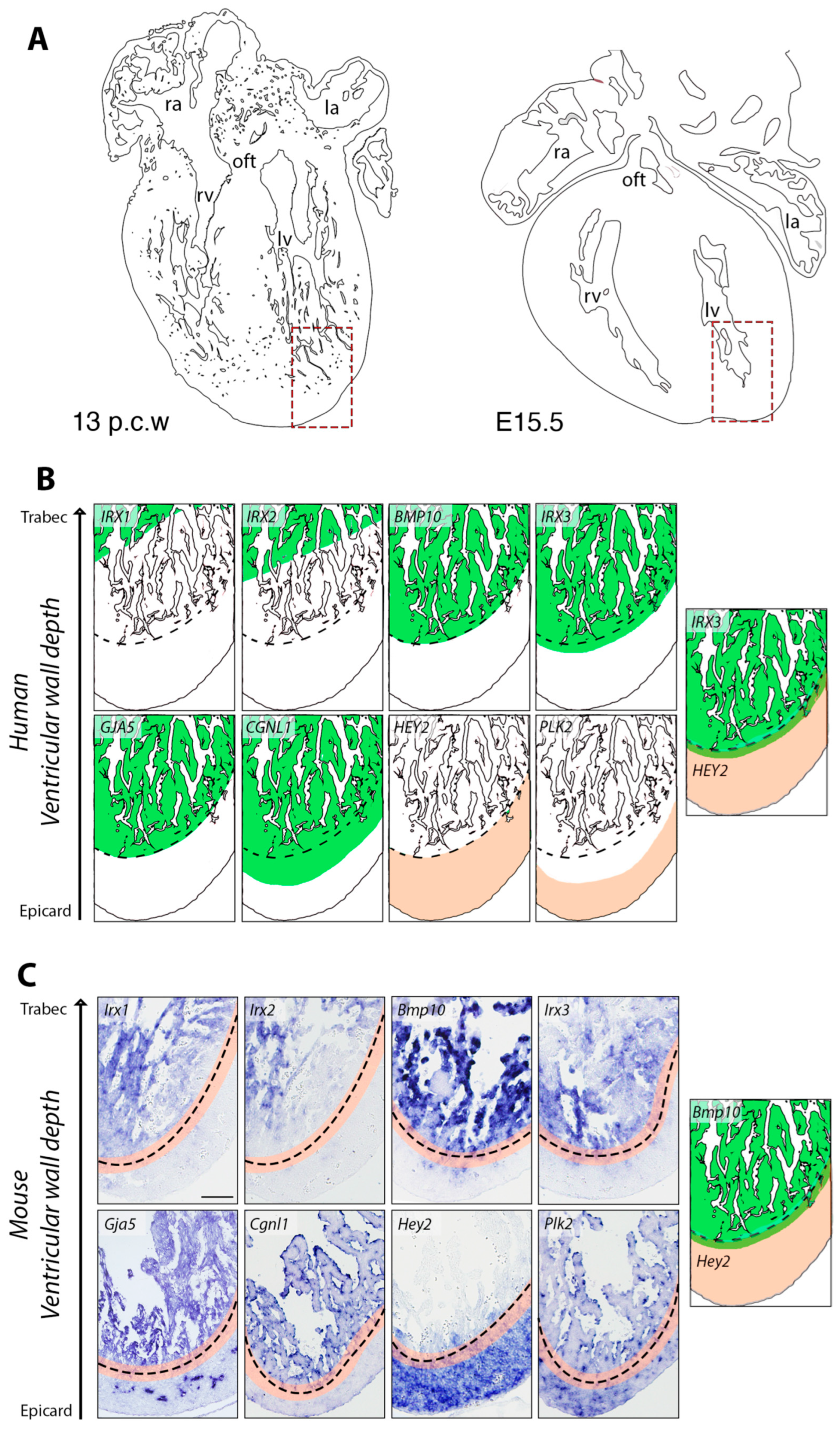
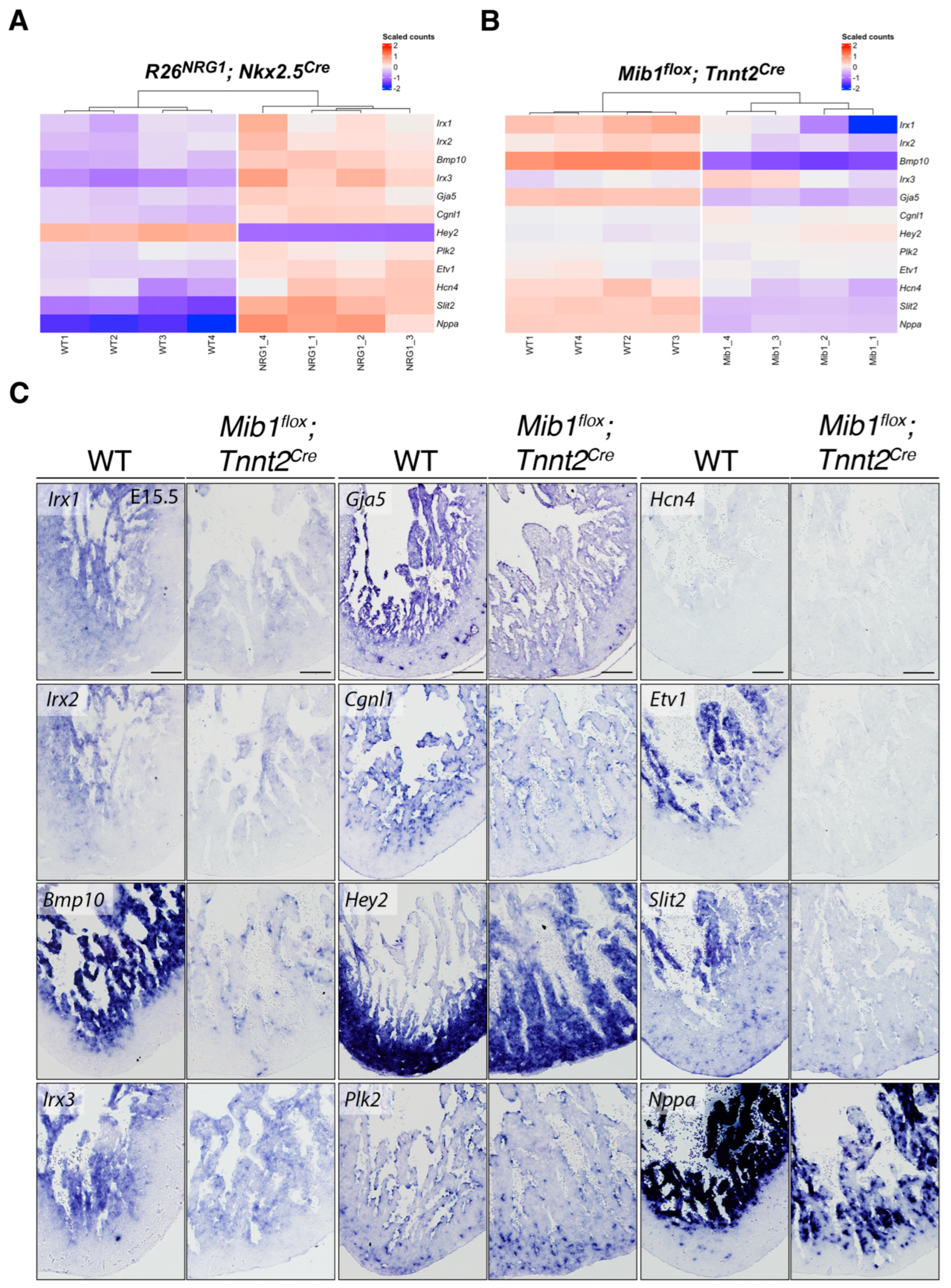
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelly, R.G.; Buckingham, M.E.; Moorman, A.F. Heart fields and cardiac morphogenesis. Cold Spring Harb. Perspect. Med. 2014, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.; Sedmera, D. Developmental anatomy of the heart: A tale of mice and man. Physiol. Genom. 2003, 15, 165–176. [Google Scholar] [CrossRef]
- Christoffels, V.M.; Habets, P.E.; Franco, D.; Campione, M.; de Jong, F.; Lamers, W.H.; Bao, Z.Z.; Palmer, S.; Biben, C.; Harvey, R.P.; et al. Chamber formation and morphogenesis in the developing mammalian heart. Dev. Biol. 2000, 223, 266–278. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; Christoffels, V.M. Cardiac Chamber Formation: Development, Genes, and Evolution. Am. Physiol. Soc. 2003, 223, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Sedmera, D.; Pexieder, T.; Vuillemin, M.; Thompson, R.P.; Anderson, R.H. Developmental patterning of the myocardium. Anat. Rec. 2000, 258, 319–337. [Google Scholar] [CrossRef]
- Samsa, L.A.; Yang, B.; Liu, J. Embryonic cardiac chamber maturation: Trabeculation, conduction, and cardiomyocyte proliferation. Am. J. Med. Genet. Part C Semin. Med. Genet. 2013, 163C, 157–168. [Google Scholar] [CrossRef]
- Miquerol, L.; Moreno-Rascon, N.; Beyer, S.; Dupays, L.; Meilhac, S.M.; Buckingham, M.E.; Franco, D.; Kelly, R.G. Biphasic development of the mammalian ventricular conduction system. Circ. Res. 2010, 107, 153–161. [Google Scholar] [CrossRef]
- D’Amato, G.; Phansalkar, R.; Naftaly, J.A.; Fan, X.; Amir, Z.A.; Rios Coronado, P.E.; Cowley, D.O.; Quinn, K.E.; Sharma, B.; Caron, K.M.; et al. Endocardium-to-coronary artery differentiation during heart development and regeneration involves sequential roles of Bmp2 and Cxcl12/Cxcr4. Dev. Cell 2022, 57, 2517–2532.e6. [Google Scholar] [CrossRef] [PubMed]
- Luxán, G.; Casanova, J.C.; Martínez-Poveda, B.; Prados, B.; D’Amato, G.; MacGrogan, D.; Gonzalez-Rajal, A.; Dobarro, D.; Torroja, C.; Martinez, F. Mutations in the NOTCH pathway regulator MIB1 cause left ventricular noncompaction cardiomyopathy. Nat. Med. 2013, 19, 193–201. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; He, L.; Zhang, H.; Huang, X.; Liu, Q.; Pu, W.; Zhang, L.; Li, Y.; Zhao, H. Identification of a hybrid myocardial zone in the mammalian heart after birth. Nat. Commun. 2017, 8, 87. [Google Scholar] [CrossRef]
- Chiang, I.K.; Humphrey, D.; Mills, R.J.; Kaltzis, P.; Pachauri, S.; Graus, M.; Saha, D.; Wu, Z.; Young, P.; Sim, C.B.; et al. Sox7-positive endothelial progenitors establish coronary arteries and govern ventricular compaction. EMBO Rep. 2023, 24, e55043. [Google Scholar] [CrossRef]
- Asp, M.; Giacomello, S.; Larsson, L.; Wu, C.; Fürth, D.; Qian, X.; Wärdell, E.; Custodio, J.; Reimegård, J.; Salmén, F. A spatiotemporal organ-wide gene expression and cell atlas of the developing human heart. Cell 2019, 179, 1647–1660.e19. [Google Scholar] [CrossRef] [PubMed]
- Holman, A.R.; Tran, S.; Destici, E.; Farah, E.N.; Li, T.; Nelson, A.C.; Engler, A.J.; Chi, N.C. Single-cell multi-modal integrative analyses highlight functional dynamic gene regulatory networks directing human cardiac development. Cell Genom. 2024, 4, 100680. [Google Scholar] [CrossRef]
- Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Farah, E.N.; Hu, R.K.; Kern, C.; Zhang, Q.; Lu, T.-Y.; Ma, Q.; Tran, S.; Zhang, B.; Carlin, D.; Monell, A. Spatially organized cellular communities form the developing human heart. Nature 2024, 627, 854–864. [Google Scholar] [CrossRef]
- De Bono, C.; Xu, Y.; Kausar, S.; Herbane, M.; Humbert, C.; Rafatov, S.; Missirian, C.; Moreno, M.; Shi, W.; Gitton, Y.; et al. Multi-modal refinement of the human heart atlas during the first gestational trimester. Development 2025, 152, DEV204555. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.; Boulgakoff, L.; Kelly, R.G.; Miquerol, L. New Insights into the Development and Morphogenesis of the Cardiac Purkinje Fiber Network: Linking Architecture and Function. J. Cardiovasc. Dev. Dis. 2021, 8, 95. [Google Scholar] [CrossRef]
- D’Amato, G.; Luxán, G.; de la Pompa, J.L. Notch signalling in ventricular chamber development and cardiomyopathy. FEBS J. 2016, 283, 4223–4237. [Google Scholar] [CrossRef]
- Grego-Bessa, J.; Gómez-Apiñaniz, P.; Prados, B.; Gómez, M.J.; MacGrogan, D.; de la Pompa, J.L. Nrg1 Regulates cardiomyocyte migration and cell cycle in ventricular development. Circ. Res. 2023, 133, 927–943. [Google Scholar] [CrossRef]
- Siguero-Álvarez, M.; Salguero-Jiménez, A.; Grego-Bessa, J.; de la Barrera, J.; MacGrogan, D.; Prados, B.; Sánchez-Sáez, F.; Piñeiro-Sabarís, R.; Felipe-Medina, N.; Torroja, C. A human hereditary cardiomyopathy shares a genetic substrate with bicuspid aortic valve. Circulation 2023, 147, 47–65. [Google Scholar] [CrossRef]
- Choquet, C.; Nguyen, T.H.M.; Sicard, P.; Buttigieg, E.; Tran, T.T.; Kober, F.; Varlet, I.; Sturny, R.; Costa, M.W.; Harvey, R.P.; et al. Deletion of Nkx2-5 in trabecular myocardium reveals the developmental origins of pathological heterogeneity associated with ventricular non-compaction cardiomyopathy. PLoS Genet. 2018, 14, e1007502. [Google Scholar] [CrossRef]
- Jiao, K.; Kulessa, H.; Tompkins, K.; Zhou, Y.; Batts, L.; Baldwin, H.S.; Hogan, B.L.M. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003, 17, 2362–2367. [Google Scholar] [CrossRef]
- Christoffels, V.M.; Keijser, A.G.M.; Houweling, A.C.; Clout, D.E.W.; Moorman, A.F.M. Patterning the embryonic heart: Identification of five mouse Iroquois homeobox genes in the developing heart. Dev. Biol. 2000, 224, 263–274. [Google Scholar] [CrossRef]
- Kanzler, B.; Kuschert, S.J.; Liu, Y.H.; Mallo, M. Hoxa-2 restricts the chondrogenic domain and inhibits bone formation during development of the branchial area. Development 1998, 125, 2587–2597. [Google Scholar] [CrossRef]
- Krishnan, A.; Samtani, R.; Dhanantwari, P.; Lee, E.; Yamada, S.; Shiota, K.; Donofrio, M.T.; Leatherbury, L.; Lo, C.W. A detailed comparison of mouse and human cardiac development. Pediatr. Res. 2014, 76, 500–507. [Google Scholar] [CrossRef]
- Gomez-Del Arco, P.; Isern, J.; Jimenez-Carretero, D.; Lopez-Maderuelo, D.; Pineiro-Sabaris, R.; El Abdellaoui-Soussi, F.; Torroja, C.; Vera-Pedrosa, M.L.; Grima-Terren, M.; Benguria, A.; et al. The G4 resolvase Dhx36 modulates cardiomyocyte differentiation and ventricular conduction system development. Nat. Commun. 2024, 15, 8602. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Bezprozvannaya, S.; Hao, T.; Elnwasany, A.; Szweda, L.I.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Transcription factor NFYa controls cardiomyocyte metabolism and proliferation during mouse fetal heart development. Dev. Cell 2023, 58, 2867–2880.e7. [Google Scholar] [CrossRef]
- Feng, W.; Bais, A.; He, H.; Rios, C.; Jiang, S.; Xu, J.; Chang, C.; Kostka, D.; Li, G. Single-cell transcriptomic analysis identifies murine heart molecular features at embryonic and neonatal stages. Nat. Commun. 2022, 13, 7960. [Google Scholar] [CrossRef] [PubMed]
- Boulgakoff, L.; D’Amato, G.; Miquerol, L. Molecular Regulation of Cardiac Conduction System Development. Curr. Cardiol. Rep. 2024, 26, 943–952. [Google Scholar] [CrossRef]
- Hu, W.; Xin, Y.; Zhang, L.; Hu, J.; Sun, Y.; Zhao, Y. Iroquois Homeodomain transcription factors in ventricular conduction system and arrhythmia. Int. J. Med. Sci. 2018, 15, 808. [Google Scholar] [CrossRef]
- Kim, K.H.; Rosen, A.; Bruneau, B.G.; Hui, C.C.; Backx, P.H. Iroquois homeodomain transcription factors in heart development and function. Circ. Res. 2012, 110, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-S.; Kim, K.-H.; Rosen, A.; Smyth, J.W.; Sakuma, R.; Delgado-Olguín, P.; Davis, M.; Chi, N.C.; Puviindran, V.; Gaborit, N. Iroquois homeobox gene 3 establishes fast conduction in the cardiac His–Purkinje network. Proc. Natl. Acad. Sci. USA 2011, 108, 13576–13581. [Google Scholar] [CrossRef]
- Shekhar, A.; Lin, X.; Liu, F.-Y.; Zhang, J.; Mo, H.; Bastarache, L.; Denny, J.C.; Cox, N.J.; Delmar, M.; Roden, D.M. Transcription factor ETV1 is essential for rapid conduction in the heart. J. Clin. Investig. 2016, 126, 4444–4459. [Google Scholar] [CrossRef] [PubMed]
- Chrifi, I.; Hermkens, D.; Brandt, M.M.; Van Dijk, C.G.M.; Bürgisser, P.E.; Haasdijk, R.; Pei, J.; van de Kamp, E.H.M.; Zhu, C.; Blonden, L. Cgnl1, an endothelial junction complex protein, regulates GTPase mediated angiogenesis. Cardiovasc. Res. 2017, 113, 1776–1788. [Google Scholar] [CrossRef] [PubMed]
- Lotto, J.; Cullum, R.; Drissler, S.; Arostegui, M.; Garside, V.C.; Fuglerud, B.M.; Clement-Ranney, M.; Thakur, A.; Underhill, T.M.; Hoodless, P.A. Cell diversity and plasticity during atrioventricular heart valve EMTs. Nat. Commun. 2023, 14, 5567. [Google Scholar] [CrossRef]
- Künzel, S.R.; Hoffmann, M.; Weber, S.; Künzel, K.; Kämmerer, S.; Günscht, M.; Klapproth, E.; Rausch, J.S.E.; Sadek, M.S.; Kolanowski, T. Diminished PLK2 induces cardiac fibrosis and promotes atrial fibrillation. Circ. Res. 2021, 129, 804–820. [Google Scholar] [CrossRef]
- Mochizuki, M.; Lorenz, V.; Ivanek, R.; Della Verde, G.; Gaudiello, E.; Marsano, A.; Pfister, O.; Kuster, G.M. Polo-Like Kinase 2 is Dynamically Regulated to Coordinate Proliferation and Early Lineage Specification Downstream of Yes-Associated Protein 1 in Cardiac Progenitor Cells. J. Am. Heart Assoc. 2017, 6, e005920. [Google Scholar] [CrossRef]
| Gene | Primers (5′ → 3′) | Fragment Length (bp) |
|---|---|---|
| Irx1 | F: 5′ CAG CAA GGA CCA GGAAGA CG 3′ R: 5′ ATC CGG TCT GGT GAC TAG GT 3′ | 751 |
| Irx2 | F: 5′ GGG CCT TCT GCG GTA CAA TA 3′ R: 5′ TTT CTT GTA GGC GCG TCT GT 3′ | 850 |
| Cgnl1 | F: 5′ ACC TCA AAG AAG GAG GCA CA 3′ R: 5′ CAT TTC CGA CAG CTG GTC CT 3′ | 446 |
| Plk2 | F: 5′ CGG CAT GAT TTG AAG AAA GTG TCG AT 3′ R: 5′ CGT CAG TCT GAA AGT TGT AGA GAT CC 3′ | 811 |
| Etv1 | F: 5′ ACT TAG TCG TTC TCT CGG CT 3′ R: 5′ TCA TCC GCC ATC TTC CTG TT 3′ | 520 |
| Slit2 | F: 5′ ACA GAA TCA GCA CCA TCG AG 3′ R: 5′ TGT GTT GGT GTC GGA TGT GT 3′ | 1065 |
| Hcn4 | F: 5′ GCA TGA TGC TTC TGC TGT GT 3′ R: 5′ CCA GCT TTC GGC AGT TAA AG 3′ | 501 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Cantador, J.; Siguero-Álvarez, M.; de la Pompa, J.L. Patterning Defects in Mice with Defective Ventricular Wall Maturation and Cardiomyopathy. J. Cardiovasc. Dev. Dis. 2025, 12, 224. https://doi.org/10.3390/jcdd12060224
Santos-Cantador J, Siguero-Álvarez M, de la Pompa JL. Patterning Defects in Mice with Defective Ventricular Wall Maturation and Cardiomyopathy. Journal of Cardiovascular Development and Disease. 2025; 12(6):224. https://doi.org/10.3390/jcdd12060224
Chicago/Turabian StyleSantos-Cantador, Javier, Marcos Siguero-Álvarez, and José Luis de la Pompa. 2025. "Patterning Defects in Mice with Defective Ventricular Wall Maturation and Cardiomyopathy" Journal of Cardiovascular Development and Disease 12, no. 6: 224. https://doi.org/10.3390/jcdd12060224
APA StyleSantos-Cantador, J., Siguero-Álvarez, M., & de la Pompa, J. L. (2025). Patterning Defects in Mice with Defective Ventricular Wall Maturation and Cardiomyopathy. Journal of Cardiovascular Development and Disease, 12(6), 224. https://doi.org/10.3390/jcdd12060224








