Psychosocial Risk and Recurrent Hospitalizations in Women and Men Following LVAD Implantation: A Multi-State Analysis of the INTERMACS Registry
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
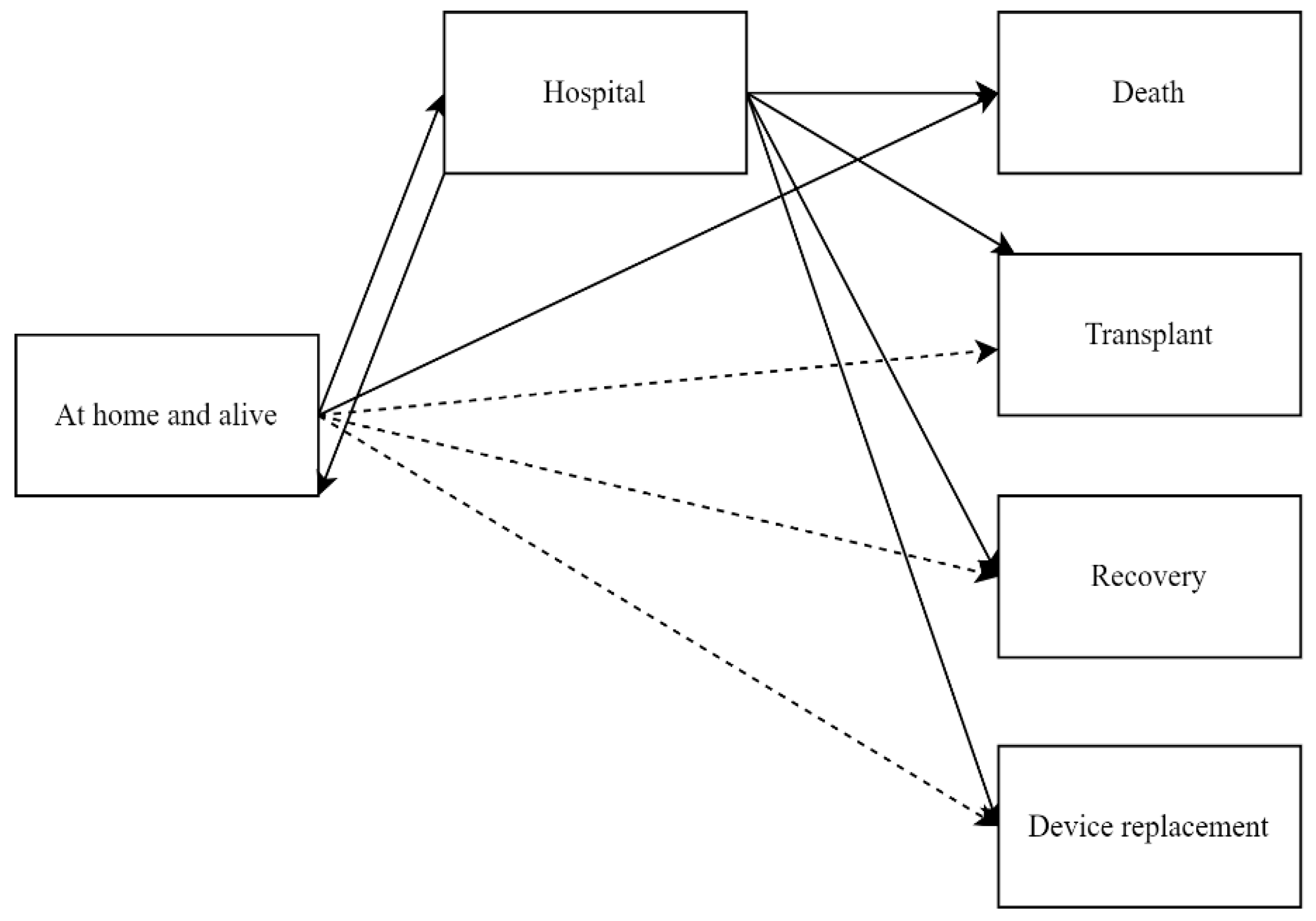
2.2. Outcome Measurements
2.3. Statistical Analysis
3. Results
3.1. Sex Differences Preimplant
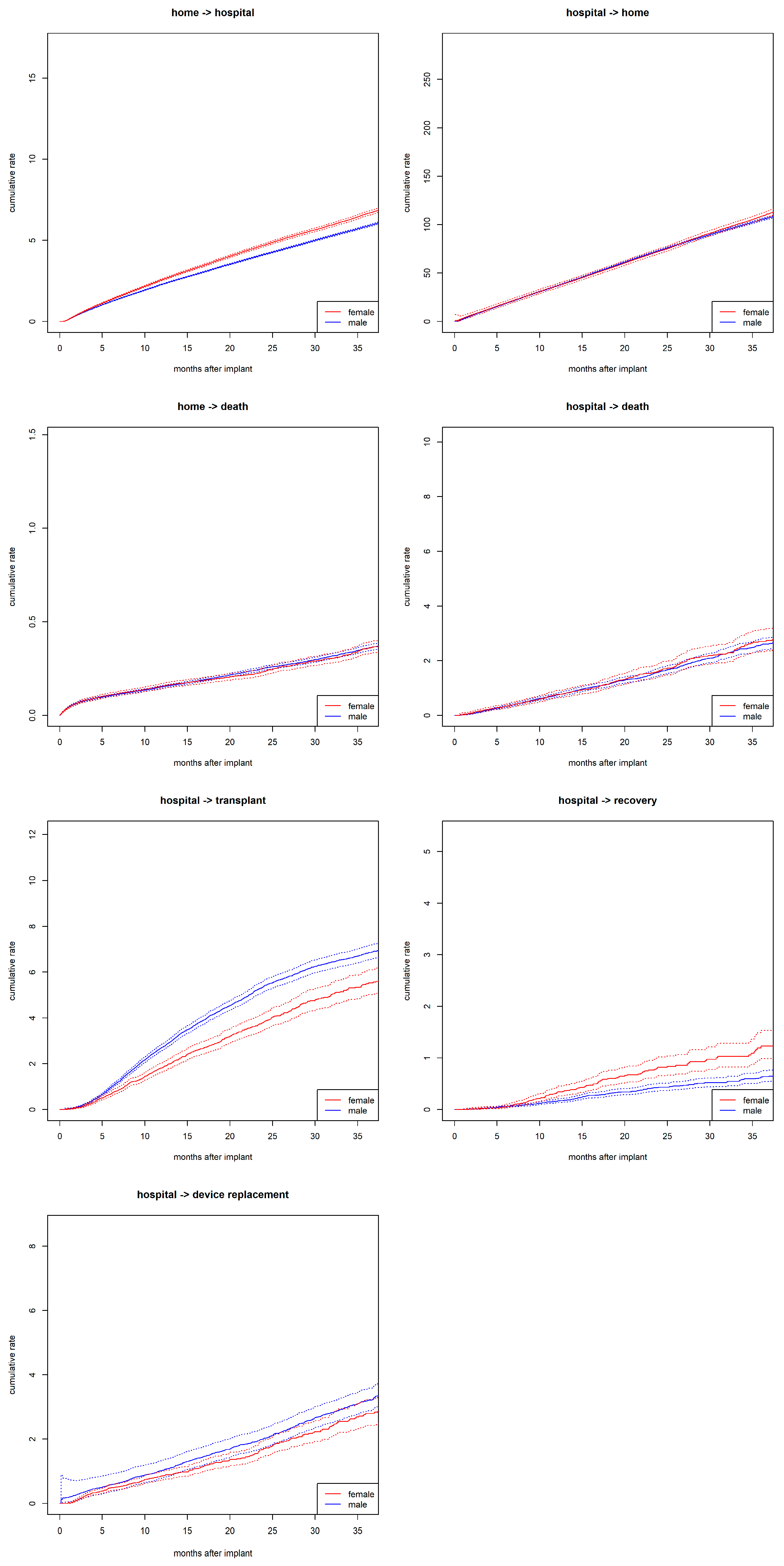
3.2. Sex Differences in State Transitions
3.3. Probability of Being Hospitalized
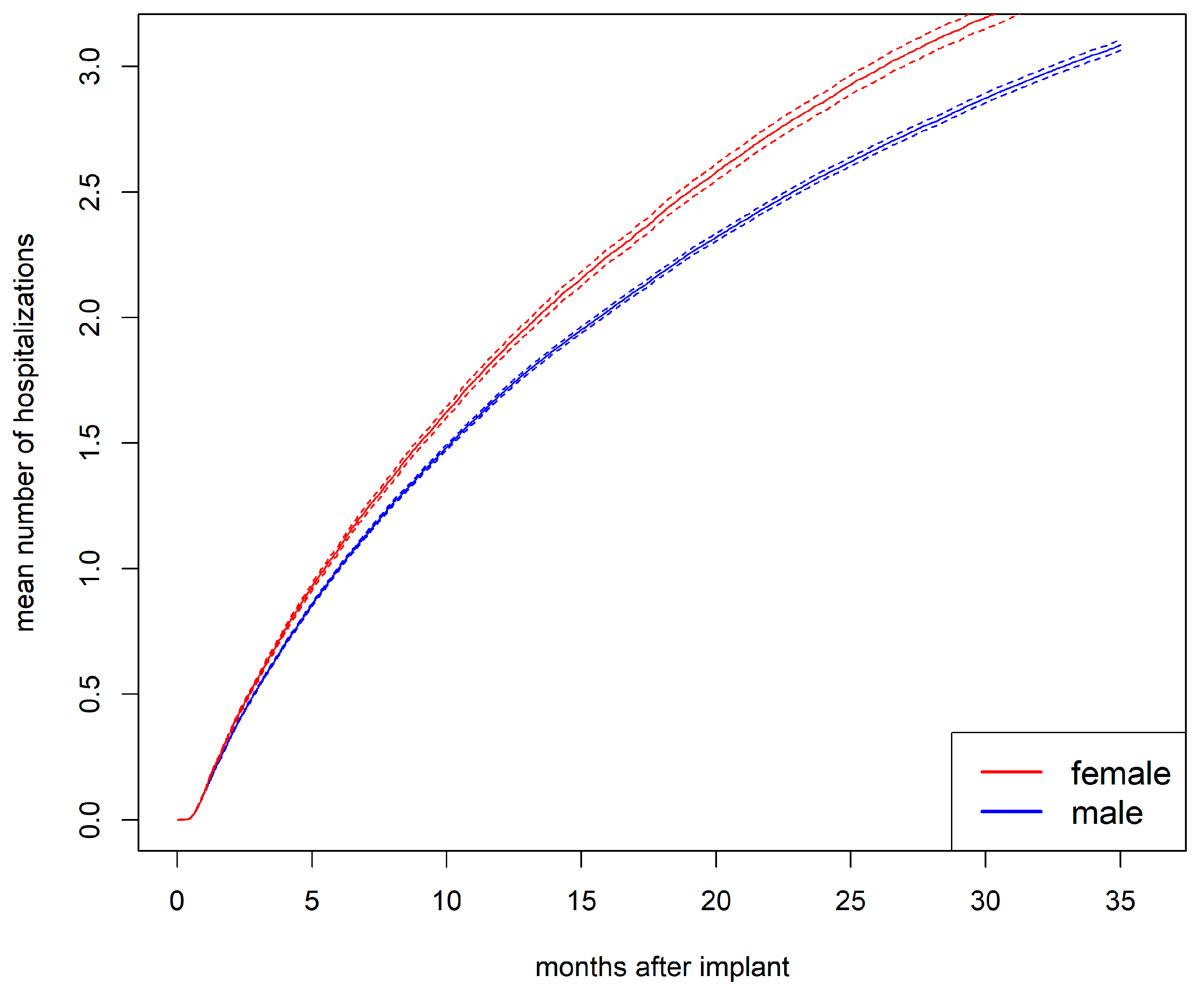
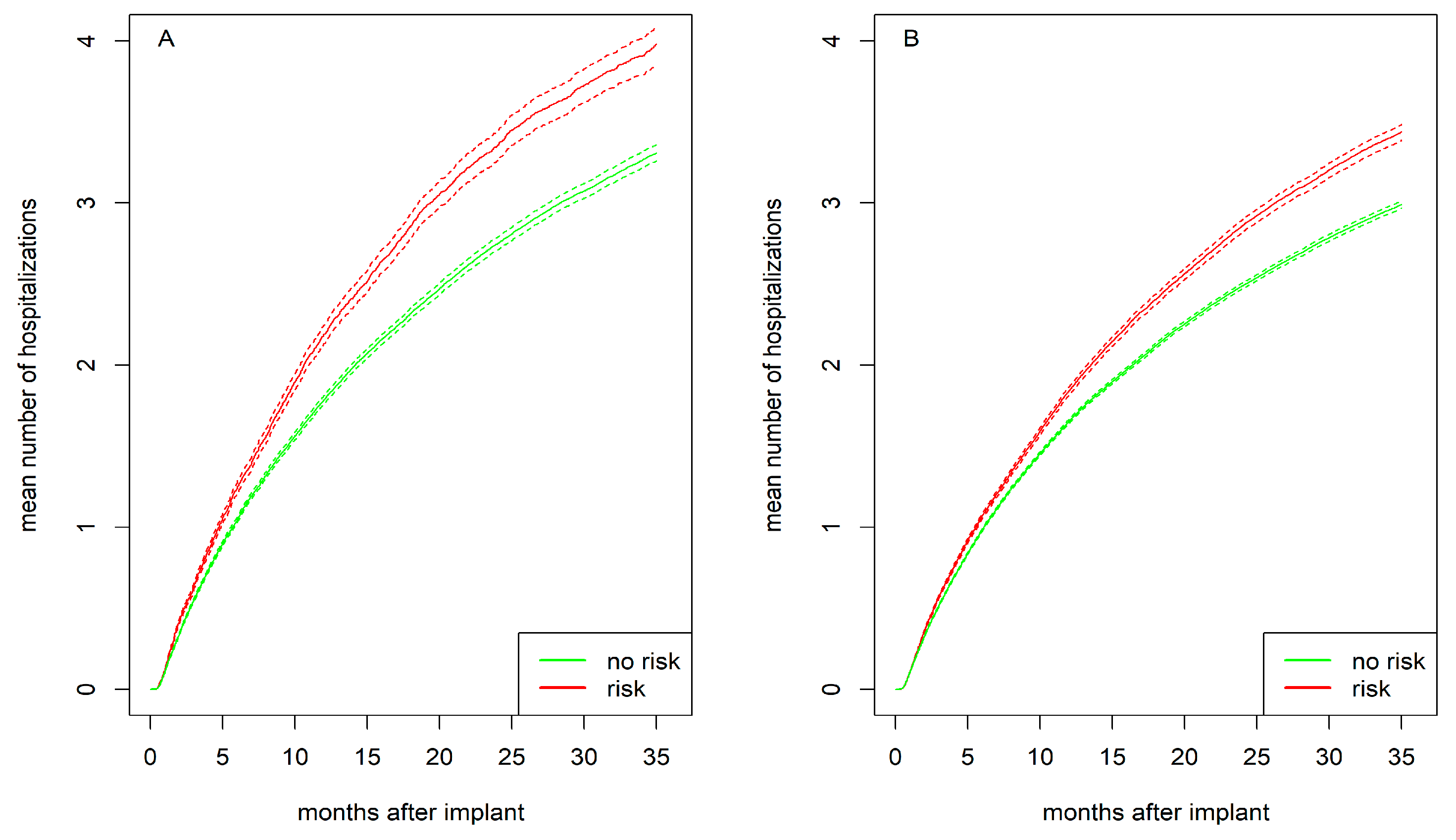
3.4. Mean Number of Hospitalizations
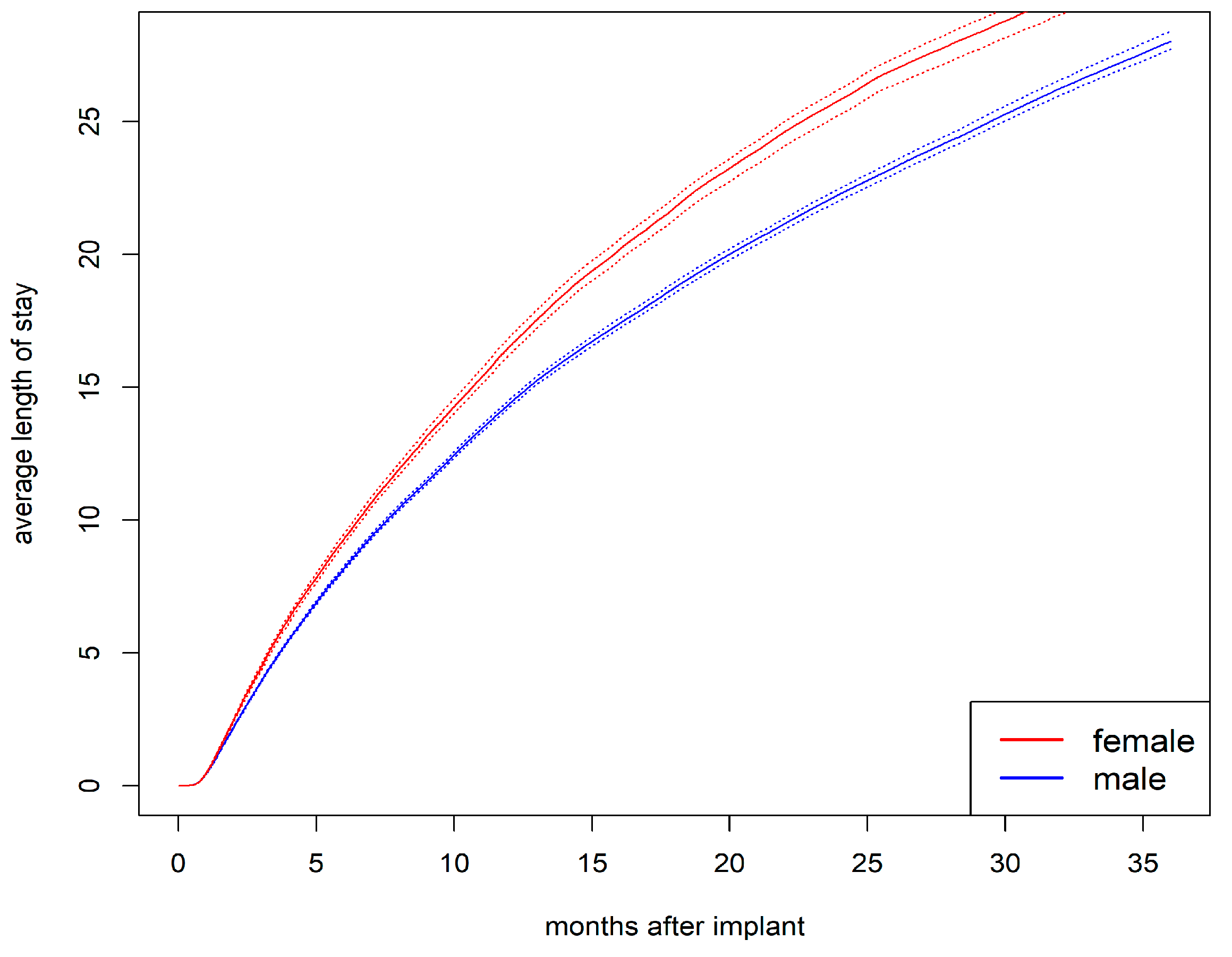
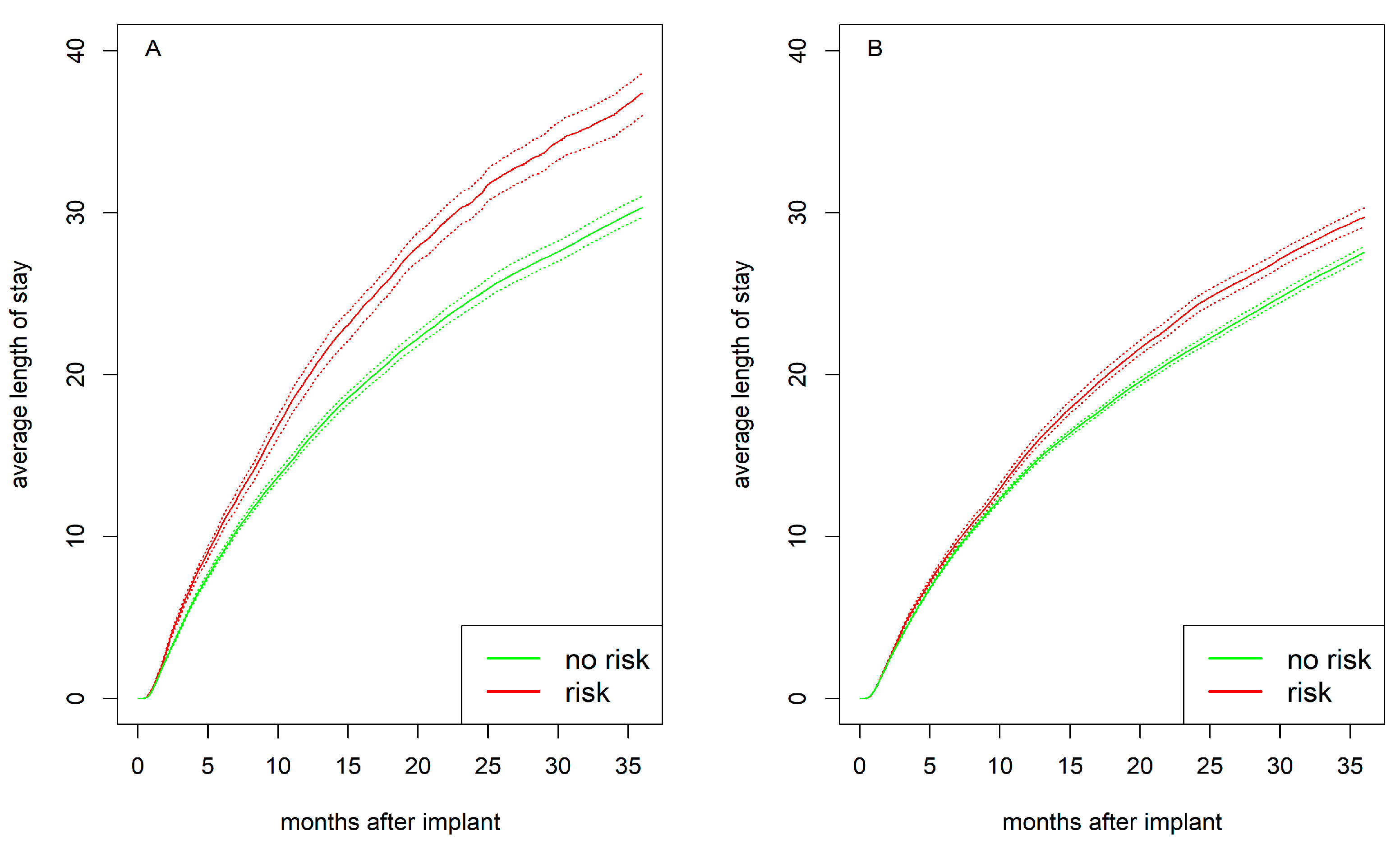
3.5. Cumulative Average Length of Stay in Hospital
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme inhibitor |
| ARB | Angiotensin II receptor blocker |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| CF-LVAD | Continuous-flow left ventricular assist device |
| HFrEF | Heart failure with reduced ejection fraction |
| ICD | Implantable cardioverter–defibrillator |
| INTERMACS | Interagency Registry for Mechanically Assisted Circulatory Support |
| LVAD | Left ventricular assist device |
| LVEDD | Left ventricular end-diastolic diameter |
References
- Shah, P.; Yuzefpolskaya, M.; Hickey, G.W.; Breathett, K.; Wever-Pinzon, O.; Ton, V.-K.; Hiesinger, W.; Koehl, D.; Kirklin, J.K.; Cantor, R.S.; et al. Twelfth Interagency Registry for Mechanically Assisted Circulatory Support report: Readmissions after left ventricular assist device. Ann. Thorac. Surg. 2022, 113, 722–737. [Google Scholar] [CrossRef]
- Molina, E.J.; Shah, P.; Kiernan, M.S.; Cornwell, W.K.; Copeland, H.; Takeda, K.; Fernandez, F.G.; Badhwar, V.; Habib, R.H.; Jacobs, J.P.; et al. The Society of Thoracic Surgeons Intermacs 2020 annual report. Ann. Thorac. Surg. 2021, 111, 778–792. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Allen, N.B.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Bansal, N.; Beaton, A.Z.; et al. 2025 Heart disease and stroke statistics: A report of US and global data from the American Heart Association. Circulation 2025, 151, 8. [Google Scholar] [CrossRef]
- Desai, R.J.; Mahesri, M.; Chin, K.; Levin, R.; Lahoz, R.; Studer, R.; Vaduganathan, M.; Patorno, E. Epidemiologic characterization of heart failure with reduced or preserved ejection fraction populations identified using medicare claims. Am. J. Med. 2021, 134, e241–e251. [Google Scholar] [CrossRef]
- Rose, S.W.; Strackman, B.W.; Gilbert, O.N.; Lasser, K.E.; Paasche-Orlow, M.K.; Lin, M.; Saylor, G.; Hanchate, A.D. Disparities by sex, race, and ethnicity in use of left ventricular assist devices and heart transplants among patients with heart failure with reduced ejection fraction. J. Am. Heart Assoc. 2024, 13, e031021. [Google Scholar] [CrossRef] [PubMed]
- Khazanie, P. REVIVAL of the sex disparities debate: Are women denied, never referred, or ineligible for heart replacement therapies? JACC Heart Fail. 2019, 7, 612–614. [Google Scholar] [CrossRef]
- Joshi, A.A.; Lerman, J.B.; Sajja, A.P.; Dahiya, G.; Gokhale, A.V.; Dey, A.K.; Kyvernitakis, A.; Halbreiner, M.S.; Bailey, S.; Alpert, C.M.; et al. Sex-based differences in left ventricular assist device utilization: Insights from the Nationwide Inpatient Sample 2004 to 2016. Circ. Heart Fail. 2019, 12, e006082. [Google Scholar] [CrossRef]
- Teuteberg, J.J.; Cleveland, J.C.; Cowger, J.; Higgins, R.S.; Goldstein, D.J.; Keebler, M.; Kirklin, J.K.; Myers, S.L.; Salerno, C.T.; Stehlik, J.; et al. The Society of Thoracic Surgeons Intermacs 2019 annual report: The changing landscape of devices and indications. Ann. Thorac. Surg. 2020, 109, 649–660. [Google Scholar] [CrossRef]
- Radhoe, S.P.; Jakus, N.; Veenis, J.F.; Timmermans, P.; Pouleur, A.; Rubís, P.; Van Craenenbroeck, E.M.; Gaizauskas, E.; Barge-Caballero, E.; Paolillo, S.; et al. Sex-related differences in left ventricular assist device utilization and outcomes: Results from the PCHF-VAD registry. ESC Heart Fail. 2023, 10, 1054–1065. [Google Scholar] [CrossRef]
- Gruen, J.; Caraballo, C.; Miller, P.E.; McCullough, M.; Mezzacappa, C.; Ravindra, N.; Mullan, C.W.; Reinhardt, S.W.; Mori, M.; Velazquez, E.; et al. Sex differences in patients receiving left ventricular assist devices for end-stage heart failure. JACC Heart Fail. 2020, 8, 770–779. [Google Scholar] [CrossRef]
- Maukel, L.-M.; Weidner, G.; Beyersmann, J.; Spaderna, H. Adverse events after left ventricular assist device implantation linked to psychosocial risk in women and men. J. Heart Lung Transplant. 2023, 42, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Bernhardt, A.M.; Ojeda, F.M.; Wagner, F.M.; Gummert, J.; de By, T.M.; Krabatsch, T.; Mohacsi, P.; Rybczynski, M.; Knappe, D.; et al. Gender differences and outcomes in left ventricular assist device support: The European Registry for Patients with Mechanical Circulatory Support. J. Heart Lung Transplant. 2018, 37, 61–70. [Google Scholar] [CrossRef]
- Maukel, L.-M.; Weidner, G.; Beyersmann, J.; Spaderna, H. Sex differences in recovery and device replacement after left ventricular assist device implantation as destination therapy. J. Am. Heart Assoc. 2022, 11, e023294. [Google Scholar] [CrossRef] [PubMed]
- DeFilippis, E.M.; Breathett, K.; Donald, E.M.; Nakagawa, S.; Takeda, K.; Takayama, H.; Truby, L.K.; Sayer, G.; Colombo, P.C.; Yuzefpolskaya, M.; et al. Psychosocial risk and its association with outcomes in continuous-flow left ventricular assist device patients. Circ. Heart Fail. 2020, 13, e006910. [Google Scholar] [CrossRef]
- Dew, M.A.; Hollenberger, J.C.M.; Obregon, L.L.B.; Hickey, G.W.; Sciortino, C.M.; Lockard, K.L.M.; Kunz, N.M.R.; Mathier, M.A.; Ramani, R.N.; Kilic, A.; et al. The preimplantation psychosocial evaluation and prediction of clinical outcomes during mechanical circulatory support: What information is most prognostic? Transplantation 2021, 105, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Okoh, A.K.; Chen, Y.; Steinberg, R.S.; Gangavelli, A.; Patel, K.J.; Ko, Y.-A.; Alexis, J.D.; Patel, S.A.; Vega, D.J.; et al. Association of psychosocial risk factors with quality of life and readmissions 1 year after LVAD implantation. J. Card. Fail. 2024, 31, 40–48. [Google Scholar] [CrossRef]
- Chew, D.S.; Manns, B.; Miller, R.J.H.; Sharma, N.; Exner, D.V. Economic evaluation of left ventricular assist devices for patients with end stage heart failure who are ineligible for cardiac transplantation. Can. J. Cardiol. 2017, 33, 1283–1291. [Google Scholar] [CrossRef]
- Baras Shreibati, J.; Goldhaber-Fiebert, J.D.; Banerjee, D.; Owens, D.K.; Hlatky, M.A. Cost-effectiveness of left ventricular assist devices in ambulatory patients with advanced heart failure. JACC Heart Fail. 2017, 5, 110–119. [Google Scholar] [CrossRef]
- Agrawal, S.; Garg, L.; Shah, M.; Agarwal, M.; Patel, B.; Singh, A.; Garg, A.; Jorde, U.P.; Kapur, N.K. Thirty-day readmissions after left ventricular assist device implantation in the United States: Insights from the Nationwide Readmissions Database. Circ. Heart Fail. 2018, 11, e004628. [Google Scholar] [CrossRef]
- Imburgio, S.; Dandu, S.; Pannu, V.; Udongwo, N.; Johal, A.; Hossain, M.; Patel, P.; Sealove, B.; Almendral, J.; Heaton, J. Sex-based differences in left ventricular assist device clinical outcomes. Catheter. Cardiovasc. Interv. 2024, 103, 376–381. [Google Scholar] [CrossRef]
- Noly, P.-E.; Wu, X.; Hou, H.; Grady, K.L.; Stewart, J.W.; Hawkins, R.B.; Yang, G.; Kim, K.D.; Zhang, M.; Cabrera, L.; et al. Association of days alive and out of the hospital after ventricular assist device implantation with adverse events and quality of life. JAMA Surg. 2023, 158, e228127. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Adegbala, O.; Akintoye, E.; Inampudi, C.; Ajam, M.; Yassin, A.S.; Olawusi, E.; Shokr, M.; Alvarez, P.; Briasoulis, A. Gender differences in outcomes after implantation of left ventricular assist devices. Ann. Thorac. Surg. 2020, 109, 780–786. [Google Scholar] [CrossRef]
- Kirklin, J.K.; Pagani, F.D.; Kormos, R.L.; Stevenson, L.W.; Blume, E.D.; Myers, S.L.; Miller, M.A.; Baldwin, J.T.; Young, J.B.; Naftel, D.C. Eighth annual INTERMACS report: Special focus on framing the impact of adverse events. J. Heart Lung Transplant. 2017, 36, 1080–1086. [Google Scholar] [CrossRef]
- Bui, Q.M.; Allen, L.A.; LeMond, L.; Brambatti, M.; Adler, E. Psychosocial evaluation of candidates for heart transplant and ventricular assist devices: Beyond the current consensus. Circ. Heart Fail. 2019, 12, e006058. [Google Scholar] [CrossRef]
- Dew, M.A.; DiMartini, A.F.; Dobbels, F.; Grady, K.L.; Jowsey-Gregoire, S.G.; Kaan, A.; Kendall, K.; Young, Q.-R.; Abbey, S.E.; Butt, Z.; et al. Consensus Document: The 2018 ISHLT/APM/AST/ICCAC/STSW recommendations for the psychosocial evaluation of adult cardiothoracic transplant candidates and candidates for long-term mechanical circulatory support. J. Heart Lung Transplant. 2018, 37, 803–823. [Google Scholar] [CrossRef] [PubMed]
- Ozga, A.-K.; Kieser, M.; Rauch, G. A systematic comparison of recurrent event models for application to composite endpoints. BMC Med. Res. Methodol. 2018, 18, 2. [Google Scholar] [CrossRef]
- Furberg, J.K.; Rasmussen, S.; Andersen, P.K.; Ravn, H. Methodological challenges in the analysis of recurrent events for randomised controlled trials with application to cardiovascular events in LEADER. Pharm. Stat. 2022, 21, 241–267. [Google Scholar] [CrossRef] [PubMed]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–68. [Google Scholar] [CrossRef]
- Allignol, A.; Beyersmann, J.; Schmoor, C. Statistical issues in the analysis of adverse events in time-to-event data. Pharm. Stat. 2016, 15, 297–305. [Google Scholar] [CrossRef]
- Nießl, A.; Allignol, A.; Beyersmann, J.; Mueller, C. Statistical inference for state occupation and transition probabilities in non-Markov multi-state models subject to both random left-truncation and right-censoring. Econom. Stat. 2023, 25, 110–124. [Google Scholar] [CrossRef]
- Erdmann, A.; Beyersmann, J.; Bluhmki, E. Comparison of nonparametric estimators of the expected number of recurrent events. Pharm. Stat. 2024, 23, 339–369. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bauer, G.R. Sex and gender multidimensionality in epidemiologic research. Am. J. Epidemiol. 2023, 192, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Schiebinger, L.; Leopold, S.S.; Miller, V.M. Editorial policies for sex and gender analysis. Lancet 2016, 388, 2841–2842. [Google Scholar] [CrossRef]
- Heise, L.; Greene, M.E.; Opper, N.; Stavropoulou, M.; Harper, C.; Nascimento, M.; Zewdie, D.; Darmstadt, G.L.; Greene, M.E.; Hawkes, S.; et al. Gender inequality and restrictive gender norms: Framing the challenges to health. Lancet 2019, 393, 2440–2454. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Gebhard, C. Gender medicine: Effects of sex and gender on cardiovascular disease manifestation and outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef]
- Pelletier, R.; Khan, N.A.; Cox, J.; Daskalopoulou, S.S.; Eisenberg, M.J.; Bacon, S.L.; Lavoie, K.L.; Daskupta, K.; Rabi, D.; Humphries, K.H.; et al. Sex versus gender-related characteristics: Which predicts outcome after acute coronary syndrome in the young? J. Am. Coll. Cardiol. 2016, 67, 127–135. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Petrov, G.; Lehmkuhl, E.; Smits, J.M.; Babitsch, B.; Brunhuber, C.; Jurmann, B.; Stein, J.; Schubert, C.; Merz, N.B.; et al. Heart transplantation in women with dilated cardiomyopathy. Transplantation 2010, 89, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, R.S.; Nayak, A.; Burke, M.A.; Aldridge, M.; Laskar, S.R.; Bhatt, K.; Sridharan, L.; Abdou, M.; Attia, T.; Smith, A.; et al. Association of race and gender with primary caregiver relationships and eligibility for advanced heart failure therapies. Clin. Transplant. 2022, 36, e14502. [Google Scholar] [CrossRef]
- Breathett, K.; Yee, E.; Pool, N.; Hebdon, M.; Crist, J.D.; Yee, R.H.; Knapp, S.M.; Solola, S.; Luy, L.; Herrera-Theut, K.; et al. Association of gender and race with allocation of advanced heart failure therapies. JAMA Netw. Open 2020, 3, e2011044. [Google Scholar] [CrossRef]
- Lee, C.S.; Sethares, K.A.; Thompson, J.H.; Faulkner, K.M.; Aarons, E.; Lyons, K.S. Patterns of heart failure dyadic illness management: The important role of gender. J. Cardiovasc. Nurs. 2020, 35, 416–422. [Google Scholar] [CrossRef]
- Connelly, P.J.; Azizi, Z.; Alipour, P.; Delles, C.; Pilote, L.; Raparelli, V. The importance of gender to understand sex differences in cardiovascular disease. Can. J. Cardiol. 2021, 37, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Mwansa, H.; Lewsey, S.; Mazimba, S.; Breathett, K. Racial/ethnic and gender disparities in heart failure with reduced ejection fraction. Curr. Heart Fail. Rep. 2021, 18, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; A Figtree, G.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef] [PubMed]
- Forest, S.J.; Xie, R.; Kirklin, J.K.; Cowger, J.; Xia, Y.; Dipchand, A.I.; Sivathasan, C.; Merry, C.; Lund, L.H.; Kormos, R.; et al. Impact of body mass index on adverse events after implantation of left ventricular assist devices: An IMACS registry analysis. J. Heart Lung Transplant. 2018, 37, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Youmans, Q.R.; Zhou, A.; Harap, R.; Eskender, M.H.; Anderson, A.S.; Ezema, A.U.; Ghafourian, K.; Ohiomoba, R.; Pham, D.T.; Rich, J.D.; et al. Association of cigarette smoking and adverse events in left ventricular assist device patients. Int. J. Artif. Organs 2021, 44, 181–187. [Google Scholar] [CrossRef]
- Havranek, E.P.; Mujahid, M.S.; Barr, D.A.; Blair, I.V.; Cohen, M.S.; Cruz-Flores, S.; Smith, G.D.; Himmelfarb, C.D.; Lauer, M.S.; Lockwood, D.W.; et al. Social determinants of risk and outcomes for cardiovascular disease: A scientific statement from the American Heart Association. Circulation 2015, 132, 873–898. [Google Scholar] [CrossRef]
- Wever-Pinzon, O.; Drakos, S.G.; McKellar, S.H.; Horne, B.D.; Caine, W.T.; Kfoury, A.G.; Li, D.Y.; Fang, J.C.; Stehlik, J.; Selzman, C.H. Cardiac recovery during long-term left ventricular assist device support. J. Am. Coll. Cardiol. 2016, 68, 1540–1553. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 ACC/AHA/HFSA guideline for the management of heart failure. Circulation 2022, 145, e876–e894. [Google Scholar] [CrossRef]
- Nahlén Bose, C. A meta-review of systematic reviews and meta-analyses on outcomes of psychosocial interventions in heart failure. Front. Psychiatry 2023, 14, 1095665. [Google Scholar] [CrossRef]
- Chernoff, R.A.P.; Messineo, G.M.; Kim, S.; Pizano, D.M.; Korouri, S.B.; Danovitch, I.M.; IsHak, W.W.M. Psychosocial interventions for patients with heart failure and their impact on depression, anxiety, quality of life, morbidity, and mortality: A systematic review and meta-analysis. Psychosom. Med. 2022, 84, 560–580. [Google Scholar] [CrossRef] [PubMed]
- Orth-Gomer, K.; Schneiderman, N.; Wang, H.X.; Walldin, C.; Blom, M.; Jernberg, T. Stress reduction prolongs life in women with coronary disease: The stockholm women’s intervention trial for coronary disease (SWITCHD). Circ. Cardiovasc. Qual. Outcomes 2009, 2, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Clancy, M.J.; Jessop, A.B.; Eisen, H. Assessment of pre-operative psychosocial function among people receiving left ventricular assist devices: A national survey of US LVAD programs. Heart Lung 2019, 48, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Savarese, G.; Eryd, S.A.; Bodegård, J.; Cleland, J.G.; Khordoc, C.; Kishi, T.; Thuresson, M.; Vardeny, O.; Zhang, R.; et al. Mortality, outcomes, costs, and use of medicines following a first heart failure hospitalization: EVOLUTION HF. JACC Heart Fail. 2023, 11, 1320–1332. [Google Scholar] [CrossRef]
- Vidula, H.; Takeda, K.; Estep, J.D.; Silvestry, S.C.; Milano, C.; Cleveland, J.C.; Goldstein, D.J.; Uriel, N.; Kormos, R.L.; Dirckx, N.; et al. Hospitalization patterns and impact of a magnetically-levitated left ventricular assist device in the MOMENTUM 3 Trial. JACC Heart Fail. 2022, 10, 470–481. [Google Scholar] [CrossRef]
- Shih, H.; Mondellini, G.M.; Kurlansky, P.A.; Sun, J.; Ning, Y.; Feldman, V.R.; Tiburcio, M.; Maguire, C.W.; Ladanyi, A.; Clerkin, K.; et al. Unplanned hospital readmissions following HeartMate 3 implantation: Readmission rates, causes, and impact on survival. Artif. Organs 2024, 48, 1049–1059. [Google Scholar] [CrossRef]
| Women (n = 4282) 21.3% | Men (n = 15,817) 78.7% | Total (N = 20,123) | p-Value | |
|---|---|---|---|---|
| Psychosocial risk, n (%) | 554 (17.5) | 2490 (21.4) | 3044 (15.1) | <0.001 |
| Demographic and behavioral characteristics | ||||
| Age in years | 54.08 (13.44) | 57.55 (12.69) | 56.81 (12.93) | <0.001 |
| Educational attainment, n (%) | 0.222 | |||
| Up to primary | 112 (3.5) | 434 (3.7) | 546 (3.7) | |
| Secondary | 1464 (45.5) | 5284 (45.2) | 6750 (45.2) | |
| Post-secondary | 901 (28.0) | 3111 (26.6) | 4013 (26.9) | |
| Tertiary | 743 (23.1) | 2868 (24.5) | 3611 (24.2) | |
| Marital status: married, n (%) | 2189 (52.4) | 10,704 (68.8) | 12,896 (65.3) | <0.001 |
| Race White, n (%) | 2430 (56.7) | 11042 (69.8) | 13474 (67.0) | <0.001 |
| Working for income, n (%) | 563 (14.5) | 2717 (18.9) | 3280 (18.0) | <0.001 |
| BMI, n (%) | <0.001 | |||
| Underweight | 208 (4.9) | 489 (3.1) | 697 (3.5) | |
| Non-obese | 2375 (55.9) | 9524 (60.7) | 11,909 (59.6) | |
| Obese | 1336 (31.4) | 4932 (31.4) | 6280 (31.4) | |
| Morbidly obese | 332 (7.8) | 752 (4.8) | 1086 (5.4) | |
| Smoking history, n (%) | <0.001 | |||
| Currently | 162 (5.1) | 611 (5.3) | 773 (5.2) | |
| Past | 648 (20.5) | 3287 (28.3) | 3939 (26.6) | |
| Never | 2347 (74.3) | 7716 (66.4) | 10,082 (68.1) | |
| Clinical variables | ||||
| LVEDD | 6.51 (1.08) | 6.90 (1.12) | 6.82 (1.12) | <0.001 |
| LVAD axial, n (%) | 3245 (75.8) | 12,704 (80.3) | 15,961 (79.3) | <0.001 |
| Device strategy, n (%) | 0.001 | |||
| Destination therapy | 1730 (40.4) | 6875 (43.5) | 8615 (42.8) | |
| Bridge to transplant | 2515 (58.8) | 8840 (55.9) | 11,369 (56.5) | |
| Bridge to recovery | 23 (0.5) | 55 (0.3) | 78 (0.4) | |
| Rescue therapy | 12 (0.3) | 39 (0.2) | 51 (0.3) | |
| INTERMACS profile, n (%) | 0.005 | |||
| 1 | 723 (16.9) | 2454 (15.6) | 3182 (15.9) | |
| 2 | 1541 (36.1) | 5683 (36.1) | 7230 (36.1) | |
| Primary ischemic diagnosis, n (%) | 1236 (29.1) | 7911 (50.4) | 9160 (45.8) | <0.001 |
| Time since diagnosis, n (%) | <0.001 | |||
| <1 month | 269 (6.5) | 778 (5.1) | 1049 (5.4) | |
| 1 month–1 year | 530 (12.8) | 1559 (10.2) | 2089 (10.8) | |
| 1–2 years | 379 (9.2) | 985 (6.5) | 1365 (7.0) | |
| >2 years | 2947 (71.4) | 11,921 (78.2) | 14,886 (76.8) | |
| Previous cardiac surgery, n (%) | 1088 (25.4) | 5689 (36.0) | 6784 (33.7) | <0.001 |
| Current ICD, n (%) | 3248 (76.4) | 12,717 (80.9) | 15,982 (79.9) | <0.001 |
| Severe diabetes, n (%) | 314 (9.9) | 1130 (9.7) | 1445 (9.8) | 0.742 |
| Pulmonary Hypertension, n (%) | 655 (20.7) | 2616 (22.5) | 3275 (22.1) | 0.035 |
| Preoperative blood values | ||||
| Albumin g/dL | 3.38 (0.66) | 3.40 (0.65) | 3.40 (0.65) | 0.071 |
| Bilirubin total mg/dL | 1.21 (1.61) | 1.42 (1.82) | 1.38 (1.79) | <0.001 |
| BUN mg/dL | 26.07 (16.86) | 30.14 (18.27) | 29.27 (18.05) | <0.001 |
| Creatinine mg/dL | 1.22 (0.65) | 1.45 (0.71) | 1.40 (0.70) | <0.001 |
| Platelets × 1000/µL | 209.32 (87.46) | 193.68 (79.19) | 197.00 (81.25) | <0.001 |
| Medication n (%) | ||||
| Beta-Blocker | 3140 (75.8) | 11,989 (78.5) | 15,141 (77.9) | <0.001 |
| ACE | 1911 (48.0) | 7317 (49.9) | 9233 (49.4) | 0.034 |
| ARB | 817 (20.9) | 2555 (18.0) | 3372 (18.6) | <0.001 |
| Aldosterone | 2457 (60.6) | 8307 (55.9) | 10,775 (56.9) | <0.001 |
| Loop Diuretics | 3605 (84.9) | 13,492 (86.2) | 17,117 (85.9) | 0.046 |
| HR (95% CI) | p-Value | |
|---|---|---|
| Female sex | 1.11 (1.06–1.16) | <0.001 |
| Psychosocial risk | 1.07 (1.02–1.12) | 0.003 |
| Female sex × psychosocial risk | 1.11 (1.01–1.22) | 0.036 |
| Age in 10 years | 0.97 (0.96–0.99) | 0.002 |
| Race White | 0.97 (0.94–1.01) | 0.124 |
| Marital status | ||
| Married/domestic partners | REF | |
| Single | 1.04 (0.99–1.09) | 0.115 |
| Divorced/widowed | 1.05 (1.00–1.10) | 0.050 |
| Widowed | 1.04 (0.95–1.14) | 0.363 |
| Educational attainment | 0.92 (0.87–0.97) | 0.004 |
| Not working for income | 1.05 (1.01–1.10) | 0.021 |
| BMI | ||
| Non-obese | REF | |
| Underweight | 1.01 (0.92–1.10) | 0.828 |
| Obese | 1.09 (1.05–1.13) | <0.001 |
| Morbidly obese | 1.08 (1.00–1.16) | 0.053 |
| Smoking history | ||
| Never | REF | |
| Past | 1.07 (1.03–1.11) | <0.001 |
| Currently | 1.10 (1.02–1.18) | 0.013 |
| Device strategy | ||
| Destination therapy | REF | |
| Bridge to transplant | 1.00 (0.97–1.04) | 0.916 |
| Bridge to recovery | 0.92 (0.74–1.14) | 0.447 |
| Rescue therapy | 0.76 (0.54–1.08) | 0.125 |
| LVEDD | 0.99 (0.98–1.00) | 0.181 |
| LVAD axial | 0.87 (0.84–0.91) | <0.001 |
| INTERMACS profile | 1.05 (0.98–1.12) | 0.169 |
| Primary diagnosis | ||
| Ischemic | REF | |
| Idiopathic | 0.96 (0.92–1.00) | 0.069 |
| Other | 0.91 (0.87–0.95) | <0.001 |
| Time since diagnosis | 1.10 (1.04–1.17) | 0.002 |
| Previous cardiac surgery | 1.10 (1.05–1.14) | <0.001 |
| Current ICD | 1.12 (1.07–1.18) | <0.001 |
| Severe diabetes | 1.09 (1.04–1.14) | <0.001 |
| Pulmonary hypertension | 0.99 (0.95–1.03) | 0.561 |
| Albumin g/dL | 1.01 (0.99–1.04) | 0.363 |
| Bilirubin total mg/dL | 0.97 (0.95–0.98) | <0.001 |
| BUN mg/dL | 1.00 (1.00–1.00) | 0.558 |
| Creatinine mg/dL | 1.06 (1.03–1.09) | <0.001 |
| Platelets × 1000/µL | 1.00 (1.00–1.00) | <0.001 |
| Beta blocker | 1.01 (0.97–1.05) | 0.671 |
| ACE | 0.98 (0.95–1.01) | 0.247 |
| ARB | 0.99 (0.95–1.03) | 0.573 |
| Aldosterone | 1.01 (0.98–1.04) | 0.575 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maukel, L.-M.; Schmeller, S.; Weidner, G.; Beyersmann, J.; Spaderna, H. Psychosocial Risk and Recurrent Hospitalizations in Women and Men Following LVAD Implantation: A Multi-State Analysis of the INTERMACS Registry. J. Cardiovasc. Dev. Dis. 2025, 12, 198. https://doi.org/10.3390/jcdd12060198
Maukel L-M, Schmeller S, Weidner G, Beyersmann J, Spaderna H. Psychosocial Risk and Recurrent Hospitalizations in Women and Men Following LVAD Implantation: A Multi-State Analysis of the INTERMACS Registry. Journal of Cardiovascular Development and Disease. 2025; 12(6):198. https://doi.org/10.3390/jcdd12060198
Chicago/Turabian StyleMaukel, Lisa-Marie, Sandra Schmeller, Gerdi Weidner, Jan Beyersmann, and Heike Spaderna. 2025. "Psychosocial Risk and Recurrent Hospitalizations in Women and Men Following LVAD Implantation: A Multi-State Analysis of the INTERMACS Registry" Journal of Cardiovascular Development and Disease 12, no. 6: 198. https://doi.org/10.3390/jcdd12060198
APA StyleMaukel, L.-M., Schmeller, S., Weidner, G., Beyersmann, J., & Spaderna, H. (2025). Psychosocial Risk and Recurrent Hospitalizations in Women and Men Following LVAD Implantation: A Multi-State Analysis of the INTERMACS Registry. Journal of Cardiovascular Development and Disease, 12(6), 198. https://doi.org/10.3390/jcdd12060198






