Spontaneous Intracranial Vertebral Artery Dissection: A Rare Cause of Ischemic Stroke
Abstract
1. Introduction
2. Epidemiology and Pathophysiology
3. Anatomical Remarks
4. Pathophysiology and Clinical Phenotypes
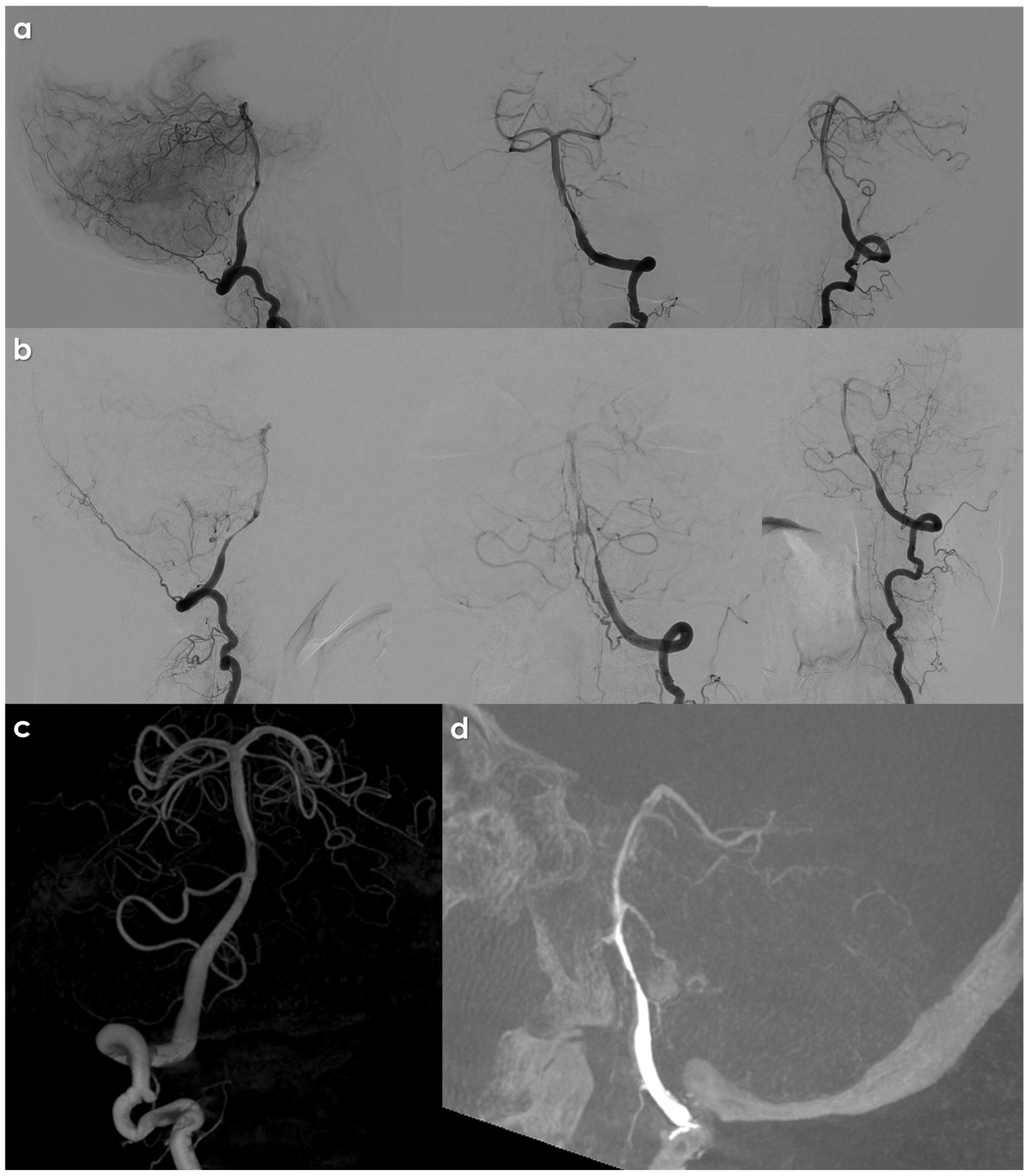
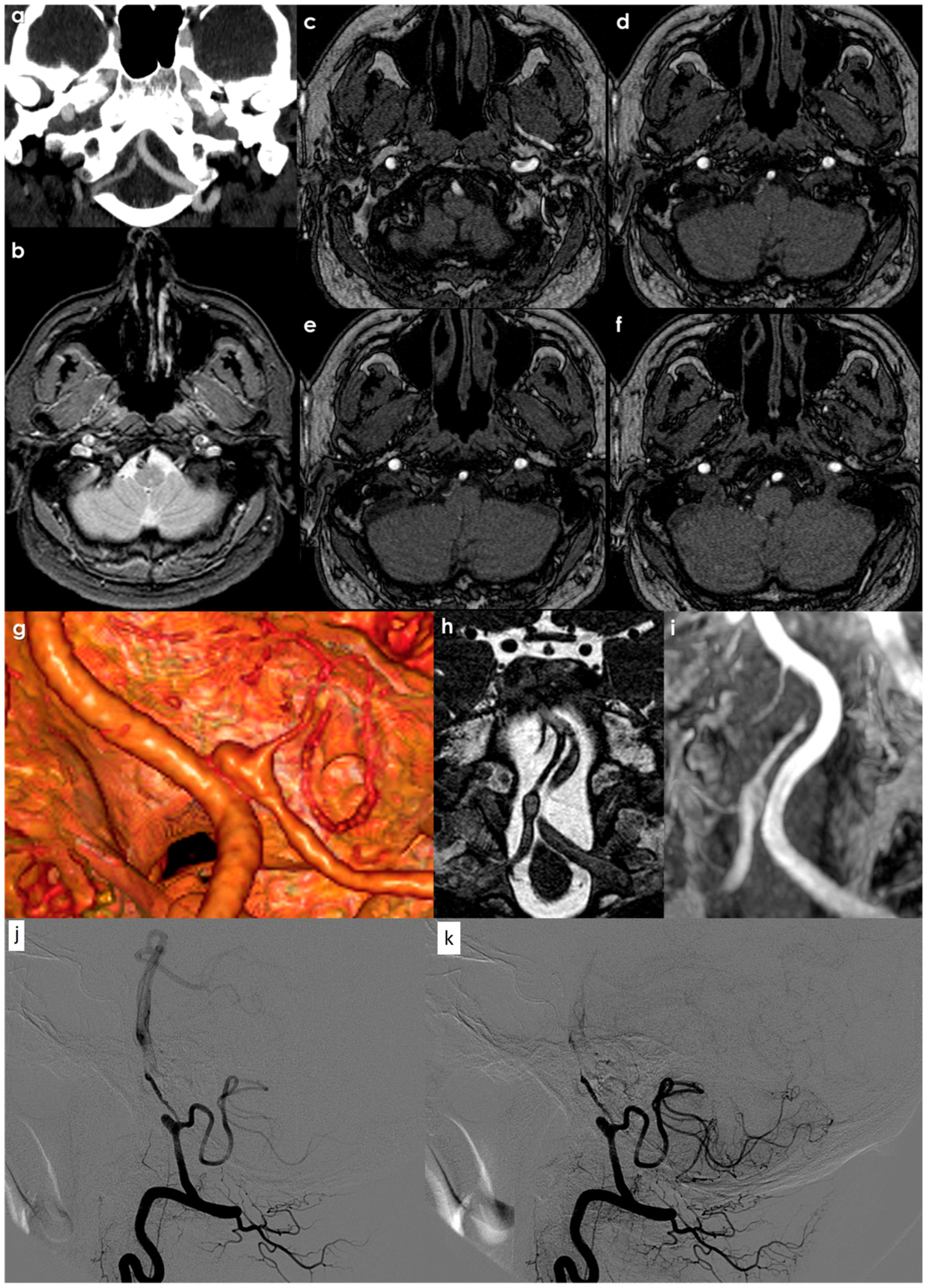
5. Neuroradiological Diagnosis
6. Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putaala, J.; Metso, A.J.; Metso, T.M.; Konkola, N.; Kraemer, Y.; Haapaniemi, E.; Kaste, M.; Tatlisumak, T. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: The Helsinki young stroke registry. Stroke 2009, 40, 1195–1203. [Google Scholar] [CrossRef] [PubMed]
- Leys, D.; Bandu, L.; Hénon, H.; Lucas, C.; Mounier-Vehier, F.; Rondepierre, P.; Godefroy, O. Clinical outcome in 287 consecutive young adults (15 to 45 years) with ischemic stroke. Neurology 2002, 59, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Amlie-Lefond, C.; Bernard, T.J.; Sébire, G.; Friedman, N.R.; Heyer, G.L.; Lerner, N.B.; DeVeber, G.; Fullerton, H.J.; International Pediatric Stroke Study Group. International Pediatric Stroke Study Group. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: Results of the International Pediatric Stroke Study. Circulation 2009, 119, 1417–1423. [Google Scholar] [CrossRef]
- Caplan, L.R. Dissections of brain-supplying arteries. Nat. Clin. Pract. Neurol. 2008, 4, 34–42. [Google Scholar] [CrossRef]
- Lyrer, P.; Engelter, S. Antithrombotic drugs for carotid artery dissection. Cochrane Database Syst. Rev. 2010, 4, CD000255. [Google Scholar] [CrossRef]
- Schievink, W.I. Spontaneous dissection of the carotid and vertebral arteries. N. Engl. J. Med. 2001, 344, 898–906. [Google Scholar] [CrossRef]
- Debette, S.; Leys, D. Cervical-artery dissections: Predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009, 8, 668–678. [Google Scholar] [CrossRef]
- Arnold, M.; Fischer, U.; Bousser, M.G. Treatment issues in spontaneous cervicocephalic artery dissections. Int. J. Stroke 2011, 6, 213–218. [Google Scholar] [CrossRef]
- Arnold, M.; Pannier, B.; Chabriat, H.; Nedeltchev, K.; Stapf, C.; Buffon, F.; Crassard, I.; Thomas, F.; Guize, L.; Baumgartner, R.W.; et al. Vascular risk factors and morphometric data in cervical artery dissection: A case-control study. J. Neurol. Neurosurg. Psychiatry 2009, 80, 232–234. [Google Scholar] [CrossRef]
- Arnold, M.; Kappeler, L.; Georgiadis, D.; Berthet, K.; Keserue, B.; Bousser, M.G.; Baumgartner, R.W. Gender differences in spontaneous cervical artery dissection. Neurology 2006, 67, 1050–1052. [Google Scholar] [CrossRef]
- Debette, S.; Grond-Ginsbach, C.; Bodenant, M.; Kloss, M.; Engelter, S.; Metso, T.; Pezzini, A.; Brandt, T.; Caso, V.; Touzé, E.; et al. Differential features of carotid and vertebral artery dissections: The CADISP study. Neurology 2011, 77, 1174–1181. [Google Scholar] [CrossRef]
- Debette, S.; Metso, T.; Pezzini, A.; Abboud, S.; Metso, A.; Leys, D.; Bersano, A.; Louillet, F.; Caso, V.; Lamy, C.; et al. Association of vascular risk factors with cervical artery dissection and ischemic stroke in young adults. Circulation 2011, 123, 1537–1544. [Google Scholar] [CrossRef]
- Yonas, H.; Agamanolis, D.; Takaoka, Y.; White, R.J. Dissecting intracranial aneurysms. Surg. Neurol. 1977, 8, 407–415. [Google Scholar] [PubMed]
- Adams, H.P., Jr.; Aschenbrener, C.A.; Kassell, N.F.; Ansbacher, L.; Cornell, S.H. Intracranial hemorrhage produced by spontaneous dissecting intracranial aneurysm. Arch. Neurol. 1982, 39, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, Y.; Wakai, S. Unruptured intracranial vertebral artery dissection. Clinical course and serial radiographic imagings. Stroke 1997, 28, 370–374. [Google Scholar] [CrossRef]
- Debette, S.; Compter, A.; Labeyrie, M.A.; Uyttenboogaart, M.; Metso, T.M.; Majersik, J.J.; Goeggel-Simonetti, B.; Engelter, S.T.; Pezzini, A.; Bijlenga, P.; et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015, 14, 640–654. [Google Scholar] [CrossRef] [PubMed]
- Bejot, Y.; Daubail, B.; Debette, S.; Durier, J.; Giroud, M. Incidence and outcome of cerebrovascular events related to cervical artery dissection: The Dijon Stroke Registry. Int. J. Stroke 2014, 9, 879–882. [Google Scholar] [CrossRef]
- Lee, V.H.; Brown, R.D., Jr.; Mandrekar, J.N.; Mokri, B. Incidence and outcome of cervical artery dissection: A population-based study. Neurology 2006, 67, 1809–1812. [Google Scholar] [CrossRef]
- Arnold, M.; Bousser, M.G.; Fahrni, G.; Fischer, U.; Georgiadis, D.; Gandjour, J.; Benninger, D.; Sturzenegger, M.; Mattle, H.P.; Baumgartner, R.W. Vertebral artery dissection: Presenting findings and predictors of outcome. Stroke 2006, 37, 2499–2503. [Google Scholar] [CrossRef]
- Kwak, J.H.; Choi, J.W.; Park, H.J.; Chae, E.Y.; Park, E.S.; Lee, D.H.; Suh, D.C. Cerebral artery dissection: Spectrum of clinical presentations related to angiographic findings. Neurointervention 2011, 6, 78–83. [Google Scholar] [CrossRef]
- Kim, B.M.; Kim, S.H.; Kim, D.I.; Shin, Y.S.; Suh, S.H.; Kim, D.J.; Park, S.I.; Park, K.Y.; Ahn, S.S. Outcomes and prognostic factors of intracranial unruptured vertebrobasilar artery dissection. Neurology 2011, 76, 1735–1741. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, S.; Tsukahara, T.; Minematsu, K. A study of vertebrobasilar artery dissection with subarachnoid hemorrhage. Acta Neurochir. Suppl. 2010, 107, 45–49. [Google Scholar]
- Kim, B.M.; Shin, Y.S.; Kim, S.H.; Suh, S.H.; Ihn, Y.K.; Kim, D.I.; Kim, D.J.; Park, S.I. Incidence and risk factors of recurrence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke 2011, 42, 2425–2430. [Google Scholar] [CrossRef] [PubMed]
- Songsaeng, D.; Srivatanakul, K.; Krings, T.; Geibprasert, S.; Ozanne, A.; Lasjaunias, P. Symptomatic spontaneous vertebrobasilar dissections in children: Review of 29 consecutive cases. J. Neurosurg. Pediatr. 2010, 6, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, J.G.; Canham, P.B. Orientation of nuclei as indicators of smooth muscle cell alignment in the cerebral artery. Blood Vessel. 1979, 16, 43–51. [Google Scholar] [CrossRef]
- Lee, R.M. Morphology of cerebral arteries. Pharmacol. Ther. 1995, 66, 149–173. [Google Scholar] [CrossRef]
- Wilkinson, I.M. The vertebral artery. Extracranial and intracranial structure. Arch. Neurol. 1972, 27, 392–396. [Google Scholar] [CrossRef]
- Masuoka, T.; Hayashi, N.; Hori, E.; Kuwayama, N.; Ohtani, O.; Endo, S. Distribution of internal elastic lamina and external elastic lamina in the internal carotid artery: Possible relationship with atherosclerosis. Neurol. Med. Chir. 2010, 50, 179–182. [Google Scholar] [CrossRef]
- Chen, M.; Caplan, L. Intracranial dissections. In Handbook on Cerebral Artery Dissection; S.Karger AG: Basel, Switzerland, 2005; pp. 160–173. [Google Scholar] [CrossRef]
- Krings, T.; Choi, I.S. The many faces of intracranial arterial dissections. Interv. Neuroradiol. 2010, 16, 151–160. [Google Scholar] [CrossRef]
- Halbach, V.V.; Higashida, R.T.; Dowd, C.F.; Fraser, K.W.; Smith, T.P.; Teitelbaum, G.P.; Wilson, C.B.; Hieshima, G.B. Endovascular treatment of vertebral artery dissections and pseudoaneurysms. J. Neurosurg. 1993, 79, 183–191. [Google Scholar] [CrossRef]
- Sasaki, O.; Ogawa, H.; Koike, T.; Koizumi, T.; Tanaka, R. A clinicopathological study of dissecting aneurysms of the intracranial vertebral artery. J. Neurosurg. 1991, 75, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Yamaura, I.; Tani, E.; Yokota, M.; Nakano, A.; Fukami, M.; Kaba, K.; Matsumoto, T. Endovascular treatment of ruptured dissecting aneurysms aimed at occlusion of the dissected site by using Guglielmi detachable coils. J. Neurosurg. 1999, 90, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Komiyama, M.; Nishikawa, M.; Nakajima, H.; Kobayashi, Y.; Inoue, T. Fusiform vertebral artery aneurysms as a cause of dissecting aneurysms. Report of two autopsy cases and a review of the literature. J. Neurosurg. 1999, 91, 139–144. [Google Scholar] [CrossRef]
- Friedman, A.H.; Drake, C.G. Subarachnoid hemorrhage from intracranial dissecting aneurysm. J. Neurosurg. 1984, 60, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Aruga, T.; Kirino, T.; Miki, Y.; Saito, I.; Tsuchida, T. Recurrent subarachnoid hemorrhage from untreated ruptured vertebrobasilar dissecting aneurysms. Neurosurgery 1995, 36, 905–911, discussion 912–903. [Google Scholar] [CrossRef]
- Tsukahara, T.; Wada, H.; Satake, K.; Yaoita, H.; Takahashi, A. Proximal balloon occlusion for dissecting vertebral aneurysms accompanied by subarachnoid hemorrhage. Neurosurgery 1995, 36, 914–919, discussion 919–920. [Google Scholar] [CrossRef]
- Yamaura, A.; Watanabe, Y.; Saeki, N. Dissecting aneurysms of the intracranial vertebral artery. J. Neurosurg. 1990, 72, 183–188. [Google Scholar] [CrossRef]
- Sano, H.; Kato, Y.; Okuma, I.; Yamaguchi, S.; Ninomiya, T.; Arunkumar, R.; Kanno, T. Classification and treatment of vertebral dissecting aneurysm. Surg. Neurol. 1997, 48, 598–605. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, Y.F.; Wang, Y.H.; Tu, Y.K.; Jeng, J.S.; Liu, H.M. Cervicocranial arterial dissection: Experience of 73 patients in a single center. Surg. Neurol. 2009, 72 (Suppl. S2), S20–S27. [Google Scholar] [CrossRef]
- Yamada, M.; Kitahara, T.; Kurata, A.; Fujii, K.; Miyasaka, Y. Intracranial vertebral artery dissection with subarachnoid hemorrhage: Clinical characteristics and outcomes in conservatively treated patients. J. Neurosurg. 2004, 101, 25–30. [Google Scholar] [CrossRef]
- Ali, M.S.; Amenta, P.S.; Starke, R.M.; Jabbour, P.M.; Gonzalez, L.F.; Tjoumakaris, S.I.; Flanders, A.E.; Rosenwasser, R.H.; Dumont, A.S. Intracranial vertebral artery dissections: Evolving perspectives. Interv. Neuroradiol. 2012, 18, 469–483. [Google Scholar] [CrossRef]
- Ducrocq, X.; Lacour, J.C.; Debouverie, M.; Bracard, S.; Girard, F.; Weber, M. Cerebral ischemic accidents in young subjects. A prospective study of 296 patients aged 16 to 45 years. Rev. Neurol. 1999, 155, 575–582. [Google Scholar] [PubMed]
- Arnold, M.; Kurmann, R.; Galimanis, A.; Sarikaya, H.; Stapf, C.; Gralla, J.; Georgiadis, D.; Fischer, U.; Mattle, H.P.; Bousser, M.G.; et al. Differences in demographic characteristics and risk factors in patients with spontaneous vertebral artery dissections with and without ischemic events. Stroke 2010, 41, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, T.; Adachi, M.; Yamaguchi, K.; Haku, T.; Kayama, T.; Kato, T. Clinical and neuroradiological features of intracranial vertebrobasilar artery dissection. Stroke 1999, 30, 1083–1090. [Google Scholar] [CrossRef]
- Chaalala, C.; El Hage, G.; Gilbert, V.; Martin, T.; Iancu, D.; Labidi, M.; Bojanowski, M.W. Spontaneous intracranial vertebral artery dissections presenting with subarachnoid hemorrhage. Neurochirurgie 2024, 70, 101526. [Google Scholar] [CrossRef] [PubMed]
- Tudose, R.C.; Rusu, M.C.; Hostiuc, S. The vertebral artery: A systematic review and a meta-analysis of the current literature. Diagnostics 2023, 13, 2036. [Google Scholar] [CrossRef]
- Magklara, E.P.; Pantelia, E.T.; Solia, E.; Panagouli, E.; Piagkou, M.; Mazarakis, A.; Skandalakis, P.; Troupis, T.; Filippou, D. Vertebral artery variations revised: Origin, course, branches and embryonic development. Folia Morphol. 2021, 80, 1–12. [Google Scholar] [CrossRef]
- Woraputtaporn, W.; Ananteerakul, T.; Iamsaard, S.; Namking, M. Incidence of vertebral artery of aortic arch origin, its level of entry into transverse foramen, length, diameter and clinical significance. Anat. Sci. Int. 2019, 94, 275–279. [Google Scholar] [CrossRef]
- Komiyama, M.; Morikawa, T.; Nakajima, H.; Nishikawa, M.; Yasui, T. High incidence of arterial dissection associated with left vertebral artery of aortic origin. Neurol. Med. Chir. 2001, 41, 8–11. [Google Scholar] [CrossRef]
- Yamaura, A. Nontraumatic Intracranial arterial dissection: Natural history, diagnosis, and treatment. Contemp. Neurosurg. 1994, 16, 1. [Google Scholar] [CrossRef]
- Jeng, J.S.; Yip, P.K. Evaluation of vertebral artery hypoplasia and asymmetry by color-coded duplex ultrasonography. Ultrasound Med. Biol. 2004, 30, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.; El Hage, G.; Peeters, J.B.; Chaalala, C.; Bojanowski, M.W. Hemodynamic factors of spontaneous vertebral artery dissecting aneurysms assessed with numerical and deep learning algorithms: Role of blood pressure and asymmetry. Neurochirurgie 2024, 70, 101519. [Google Scholar] [CrossRef]
- Dzierżanowski, J.; Szarmach, A.; Baścik, B.; Czapiewski, P.; Muc, A.; Piskunowicz, M.; Krakowiak, M.; Szmuda, T.; Słoniewski, P.; Szurowska, E.; et al. Intracranial region of the vertebral artery: Morphometric study in the context of clinical usefulness. Folia Morphol. 2017, 76, 379–387. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hori, S.; Hori, E.; Umemura, K.; Shibata, T.; Okamoto, S.; Kubo, M.; Horie, Y.; Kuroda, S. Anatomical variations of vertebrobasilar artery are closely related to the occurrence of vertebral artery dissection—An MR angiography study. J. Stroke Cerebrovasc. Dis. 2020, 29, 104636. [Google Scholar] [CrossRef]
- Zhou, M.; Zheng, H.; Gong, S.; Guo, J.; Chen, N.; Zhou, D.; Yang, R.; Zhu, C.; He, L. Vertebral artery hypoplasia and vertebral artery dissection: A hospital-based cohort study. Neurology 2015, 84, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Park, G.J.; Cho, J.H.; Kim, K.H. Angiographic characteristics of ruptured versus unruptured vertebral artery dissecting aneurysm. J. Cerebrovasc. Endovasc. Neurosurg. 2022, 24, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ariyada, K.; Shibahashi, K.; Fujika, N.; Sakakura, Y.; Hanakawa, K.; Murao, M. Posterior communicating artery hypoplasia: A risk factor for vertebral artery dissection causing subarachnoid hemorrhage. J. Stroke Cerebrovasc. Dis. 2022, 31, 106224. [Google Scholar] [CrossRef]
- Vogels, V.; Dammers, R.; van Bilsen, M.; Volovici, V. Deep cerebral perforators: Anatomical distribution and clinical symptoms: An overview. Stroke 2021, 52, e660–e674. [Google Scholar] [CrossRef]
- Er, U.; Fraser, K.; Lanzino, G. The anterior spinal artery origin: A microanatomical study. Spinal Cord. 2008, 46, 45–49. [Google Scholar] [CrossRef]
- Rojas, S.; Ortega, M.; RodríGuez-Baeza, A. Variable anatomic configuration of the posterior spinal arteries in humans. Clin. Anat. 2018, 31, 1137–1143. [Google Scholar] [CrossRef]
- Seçkin, H.; Ateş, Ö.; Bauer, A.M.; Başkaya, M.K. Microsurgical anatomy of the posterior spinal artery via a far-lateral transcondylar approach. J. Neurosurg. Spine 2009, 10, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Cironi, K.; Mathkour, M.; Lockwood, J.; Aysenne, A.; Iwanaga, J.; Loukas, M.; Bui, C.J.; Dumont, A.S.; Tubbs, R.S. Anatomical study of the posterior spinal artery branches to the medulla oblongata. World Neurosurg. 2021, 149, e1098–e1104. [Google Scholar] [CrossRef] [PubMed]
- Marinković, S.; Milisavljević, M.; Gibo, H.; Maliković, A.; Djulejić, V. Microsurgical anatomy of the perforating branches of the vertebral artery. Surg. Neurol. 2004, 61, 190–197. [Google Scholar] [CrossRef]
- Mercier, P.H.; Brassier, G.; Fournier, H.D.; Picquet, J.; Papon, X.; Lasjaunias, P. Vascular microanatomy of the pontomedullary junction, posterior inferior cerebellar arteries, and the lateral spinal arteries. Interv. Neuroradiol. 2008, 14, 49–58. [Google Scholar] [CrossRef]
- Ro, A.; Kageyama, N. Pathomorphometry of ruptured intracranial vertebral arterial dissection: Adventitial rupture, dilated lesion, intimal tear, and medial defect. J. Neurosurg. 2013, 119, 221–227. [Google Scholar] [CrossRef]
- Sato, T.; Sasaki, T.; Suzuki, K.; Matsumoto, M.; Kodama, N.; Hiraiwa, K. Histological study of the normal vertebral artery—Etiology of dissecting aneurysms. Neurol. Med. Chir. 2004, 44, 629–635. [Google Scholar] [CrossRef]
- Mizutani, T.; Miki, Y.; Kojima, H.; Suzuki, H. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery 1999, 45, 253–259. [Google Scholar] [CrossRef]
- De Bray, J.M.; Penisson-Besnier, I.; Dubas, F.; Emile, J. Extracranial and intracranial vertebrobasilar dissections: Diagnosis and prognosis. J. Neurol. Neurosurg. Psychiatry 1997, 63, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lylyk, P.; Cohen, J.E.; Ceratto, R.; Ferrario, A.; Miranda, C. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J. Neurosurg. 2001, 94, 427–432. [Google Scholar] [CrossRef]
- Mehta, B.; Burke, T.; Kole, M.; Bydon, A.; Seyfried, D.; Malik, G. Stent-within-a-stent technique for the treatment of dissecting vertebral artery aneurysms. Am. J. Neuroradiol. 2003, 24, 1814–1818. [Google Scholar]
- Wakhloo, A.K.; Lanzino, G.; Lieber, B.B.; Hopkins, L.N. Stents for intracranial aneurysms: The beginning of a new endovascular era? Neurosurgery 1998, 43, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Santos-Franco, J.A.; Zenteno, M.; Lee, A. Dissecting aneurysms of the vertebrobasilar system. A comprehensive review on natural history and treatment options. Neurosurg. Rev. 2008, 31, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, F.C.; Fiorella, D.J.; Han, P.P.; Deshmukh, V.R.; Kim, L.J.; McDougall, C.G. Endovascular management of intracranial vertebral artery dissecting aneurysms. Neurosurg. Focus 2005, 18, E3. [Google Scholar] [CrossRef] [PubMed]
- Schievink, W.I.; Michels, V.V.; Piepgras, D.G. Neurovascular manifestations of heritable connective tissue disorders. A review. Stroke 1994, 25, 889–903. [Google Scholar] [CrossRef]
- Engelter, S.T.; Brandt, T.; Debette, S.; Caso, V.; Lichy, C.; Pezzini, A.; Abboud, S.; Bersano, A.; Dittrich, R.; Grond-Ginsbach, C.; et al. Antiplatelets verus anticoagulation in cervical artery dissection. Stroke 2007, 38, 2605–2611. [Google Scholar] [CrossRef]
- Anson, J.; Crowell, R.M. Cervicocranial arterial dissection. Neurosurgery 1991, 29, 89–96. [Google Scholar] [CrossRef]
- Germain, D.P. Ehlers-Danlos syndrome type IV. Orphanet. J. Rare Dis. 2007, 2, 32. [Google Scholar] [CrossRef]
- Schievink, W.I.; Mokri, B.; Whisnant, J.P. Internal carotid artery dissection in a community. Rochester, Minnesota, 1987–1992. Stroke 1993, 24, 1678–1680. [Google Scholar] [CrossRef]
- Debette, S. Pathophysiology and risk factors of cervical artery dissection: What have we learnt from large hospital-based cohorts? Curr. Opin. Neurol. 2014, 27, 20–28. [Google Scholar] [CrossRef]
- Matouk, C.C.; Hanbidge, A.; Mandell, D.M.; Terbrugge, K.G.; Agid, R. Osteogenesis imperfecta, multiple intra-abdominal arterial dissections and a ruptured dissecting-type intracranial aneurysm. Interv. Neuroradiol. 2011, 17, 371–375. [Google Scholar] [CrossRef]
- Touzé, E.; Oppenheim, C.; Trystram, D.; Nokam, G.; Pasquini, M.; Alamowitch, S.; Hervé, D.; Garnier, P.; Mousseaux, E.; Plouin, P.F. Fibromuscular dysplasia of cervical and intracranial arteries. Int. J. Stroke 2010, 5, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, C.; Yan, M. Clinical outcomes of atlantoaxial dislocation combined with High-riding vertebral artery using C2 translaminar screws. World Neurosurg. 2019, 122, e1511–e1518. [Google Scholar] [CrossRef] [PubMed]
- Bojanowski, M.W.; Stefanovic, K.; Bergeron, D.; Farzin, B.; Letourneau-Guillon, L.; Chaalala, C. Pregnancy as a subgroup in the pathophysiologic classification of spinal aneurysms. World Neurosurg. 2022, 157, e264–e270. [Google Scholar] [CrossRef] [PubMed]
- Azad, H.A.; Prasad, N.; Shlobin, N.A.; Mitra, A.; Cloney, M.B.; Hopkins, B.S.; Jahromi, B.S.; Potts, M.B.; Dahdaleh, N.S. Clinical characteristics, course, and outcomes of vertebral artery dissections in the postpartum period. Neurosurgery 2021, 89, 792–799. [Google Scholar] [CrossRef]
- Lee, J.S.; Yong, S.W.; Bang, O.Y.; Shin, Y.S.; Kim, B.M.; Kim, S.Y. Comparison of spontaneous intracranial vertebral artery dissection with large artery disease. Arch. Neurol. 2006, 63, 1738–1744. [Google Scholar] [CrossRef]
- Nakazawa, T.; Takeichi, Y.; Yokoi, T.; Fukami, T.; Jito, J.; Nitta, N.; Takagi, K.; Nozaki, K. Treatment of spontaneous intradural vertebral artery dissections. Neuroradiol. J. 2011, 24, 699–711. [Google Scholar] [CrossRef]
- Kocaeli, H.; Chaalala, C.; Andaluz, N.; Zuccarello, M. Spontaneous intradural vertebral artery dissection: A single-center experience and review of the literature. Skull Base 2009, 19, 209–218. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Sharma, P.; Robinson, K.A.; Arnan, M.; Tsui, M.; Saber-Tehrani, A.; Newman-Toker, D.E. Imaging characteristics of symptomatic vertebral artery dissection: A systematic review. Neurologist 2012, 18, 255–260. [Google Scholar] [CrossRef]
- Aoki, N.; Sakai, T. Rebleeding from intracranial dissecting aneurysm in the vertebral artery. Stroke 1990, 21, 1628–1631. [Google Scholar] [CrossRef]
- Gomez-Rojas, O.; Hafeez, A.; Gandhi, N.; Berghea, R.; Halalau, A. Bilateral vertebral artery dissection: A case report with literature review. Case Rep. Med. 2020, 25, 8180926. [Google Scholar] [CrossRef]
- Meng, Y.Y.; Dou, L.; Wang, C.M.; Kong, D.Z.; Wei, Y.; Wu, L.S.; Yang, Y.; Zhou, H.W. Spinal cord infarction presenting as Brown-Séquard syndrome from spontaneous vertebral artery dissection: A case report and literature review. BMC Neurol. 2019, 19, 321. [Google Scholar] [CrossRef] [PubMed]
- Bond, K.M.; Krings, T.; Lanzino, G.; Brinjikji, W. Intracranial dissections: A pictorial review of pathophysiology, imaging features, and natural history. J. Neuroradiol. 2021, 48, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Mokri, B.; Houser, O.W.; Sandok, B.A.; Piepgras, D.G. Spontaneous dissections of the vertebral arteries. Neurology 1988, 38, 880–885. [Google Scholar] [CrossRef]
- Shimoji, T.; Bando, K.; Nakajima, K.; Ito, K. Dissecting aneurysm of the vertebral artery. Report of seven cases and angiographic findings. J. Neurosurg. 1984, 61, 1038–1046. [Google Scholar] [CrossRef]
- Sturzenegger, M. Vertebral artery dissection. Clinical aspects, non-invasive diagnosis, therapy-observations in 14 patients. Der Nervenarzt 1994, 65, 402–410. [Google Scholar]
- Ben Hassen, W.; Machet, A.; Edjlali-Goujon, M.; Legrand, L.; Ladoux, A.; Mellerio, C.; Bodiguel, E.; Gobin-Metteil, M.P.; Trystram, D.; Rodriguez-Regent, C.; et al. Imaging of cervical artery dissection. Diagn. Interv. Imaging 2014, 95, 1151–1161. [Google Scholar] [CrossRef]
- Takemoto, K.; Takano, K.; Abe, H.; Okawa, M.; Iwaasa, M.; Higashi, T.; Inoue, T. The new MRI modalities “BPAS and VISTA” for the diagnosis of VAD. Acta Neurochir. Suppl. 2011, 112, 59–65. [Google Scholar]
- Fujiwara, S.; Yokoyama, N.; Fujii, K.; Matsushima, T.; Matsubara, T.; Fukui, M. Repeat angiography and magnetic resonance imaging (MRI) of dissecting aneurysms of the intracranial vertebral artery. Report of four cases. Acta Neurochir. 1993, 121, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.S.; Kim, B.M.; Suh, S.H.; Kim, D.J.; Kim, D.I.; Shin, Y.S.; Ha, S.Y.; Kwon, Y.S. Spontaneous symptomatic intracranial vertebrobasilar dissection: Initial and follow-up imaging findings. Radiology 2012, 264, 196–202. [Google Scholar] [CrossRef]
- Tsukahara, T.; Minematsu, K. Overview of spontaneous cervicocephalic arterial dissection in Japan. Acta Neurochir. Suppl. 2010, 107, 35–40. [Google Scholar]
- Hosoya, T.; Nagahata, M.; Yamaguchi, K. Prevalence of vertebral artery dissection in Wallenberg syndrome: Neuroradiological analysis of 93 patients in the Tohoku District, Japan. Radiat. Med. 1996, 14, 241–246. [Google Scholar] [PubMed]
- Hosoya, T.; Watanabe, N.; Yamaguchi, K.; Kubota, H.; Onodera, Y. Intracranial vertebral artery dissection in Wallenberg syndrome. Am. J. Neuroradiol. 1994, 15, 1161–1165. [Google Scholar]
- Kanoto, M.; Hosoya, T. Diagnosis of Intracranial Artery Dissection. Neurol. Med. Chir. 2016, 56, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Yoon, W.; Seo, J.J.; Kim, T.S.; Do, H.M.; Jayaraman, M.V.; Marks, M.P. Dissection of the V4 segment of the vertebral artery: Clinicoradiologic manifestations and endovascular treatment. Eur. Radiol. 2007, 17, 983–993. [Google Scholar] [CrossRef]
- Koyama, S.; Kotani, A.; Sasaki, J. Spontaneous dissecting aneurysm of the anterior cerebral artery: Report of two cases. Surg. Neurol. 1996, 46, 55–61. [Google Scholar] [CrossRef]
- Kanoto, M.; Oda, A.; Hosoya, T.; Kokubo, Y.; Kayama, T. Megadolichobasilar artery with arterial dissection; a case report. Jpn. J. Clin. Radiol. 2007, 52, 1699–1702. [Google Scholar]
- Zenteno, M.A.; Santos-Franco, J.A.; Freitas-Modenesi, J.M.; Gómez, C.; Murillo-Bonilla, L.; Aburto-Murrieta, Y.; Díaz-Romero, R.; Nathal, E.; Gómez-Llata, S.; Lee, A. Use of the sole stenting technique for the management of aneurysms in the posterior circulation in a prospective series of 20 patients. J. Neurosurg. 2008, 108, 1104–1118. [Google Scholar] [CrossRef]
- Soper, J.R.; Parker, G.D.; Hallinan, J.M. Vertebral artery dissection diagnosed with CT. AJNR Am. J. Neuroradiol. 1995, 16, 952–954. [Google Scholar] [PubMed]
- Vertinsky, A.T.; Schwartz, N.E.; Fischbein, N.J.; Rosenberg, J.; Albers, G.W.; Zaharchuk, G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. Am. J. Neuroradiol. 2008, 29, 1753–1760. [Google Scholar] [CrossRef]
- Lum, C.; Chakraborty, S.; Schlossmacher, M.; Santos, M.; Mohan, R.; Sinclair, J.; Sharma, M. Vertebral artery dissection with a normal-appearing lumen at multisection CT angiography: The importance of identifying wall hematoma. AJNR Am. J. Neuroradiol. 2009, 30, 787–792. [Google Scholar] [CrossRef]
- Provenzale, J.M.; Barboriak, D.P.; Taveras, J.M. Exercise-related dissection of craniocervical arteries: CT, M R, and angiographic findings. J. Comput. Assist. Tomogr. 1995, 19, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Kraus, R.R.; Bergstein, J.M.; DeBord, J.R. Diagnosis, treatment, and outcome of blunt carotid arterial injuries. Am. J. Surg. 1999, 178, 190–193. [Google Scholar] [CrossRef]
- Provenzale, J.M.; Sarikaya, B. Comparison of test performance characteristics of M RI, M R angiography, and CT angiography in the diagnosis of carotid and vertebral artery dissection: A review of the medical literature. Am. J. Roentgenol. 2009, 193, 1167–1174. [Google Scholar] [CrossRef]
- Provenzale, J.M. MRI and MRA for evaluation of dissection of craniocerebral arteries: Lessons from the medical literature. Emerg. Radiol. 2009, 16, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Choi, H.S.; Koo, J.; Jung, S.L.; Ahn, K.J.; Kim, B.S.; Shin, Y.S.; Lee, K.S. Intramural hematoma detection by susceptibility-weighted imaging in intracranial vertebral artery dissection. Cerebrovasc. Dis. 2013, 36, 292–298. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, L.; Yao, L.; Li, X.; Li, T.; Wang, H.; Wang, X.; Jian, Y.; Sun, M.; Li, Y.; et al. Basi-parallel anatomic scanning (BPAS-MRI) compared with high-resolution MRI for the diagnosis of vertebrobasilar artery abnormalities. Eur. J. Radiol. 2020, 123, 108791. [Google Scholar] [CrossRef]
- Nagahata, M.; Abe, Y.; Ono, S.; Hosoya, T.; Uno, S. Surface appearance of the vertebrobasilar artery revealed on basiparallel anatomic scanning (BPAS)-MR imaging: Its role for brain MR examination. Am. J. Neuroradiol. 2005, 26, 2508–2513. [Google Scholar] [PubMed]
- Fatima, Z.; Motosugi, U.; Okumura, A.; Ishigame, K.; Araki, T. Basi-parallel anatomical scanning (BPAS)-MRI can improve discrimination of vertebral artery dissection from atherosclerosis and hypoplasia. Acad. Radiol. 2012, 19, 1362–1367. [Google Scholar] [CrossRef]
- Dieleman, N.; van der Kolk, A.G.; Zwanenburg, J.J.; Harteveld, A.A.; Biessels, G.J.; Luijten, P.R.; Hendrikse, J. Imaging intracranial vessel wall pathology with magnetic resonance imaging: Current prospects and future directions. Circulation 2014, 130, 192–201. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jung, S.C.; Lee, D.H. Vessel wall imaging of the intracranial and cervical carotid arteries. J. Stroke 2015, 17, 238–255. [Google Scholar] [CrossRef]
- Hu, X.Y.; Li, Z.X.; Liu, H.Q.; Zhang, M.; Wei, M.L.; Fang, S.; Chen, W.; Pan, H.; Huang, J.X.; Zhu, Y.M.; et al. Relationship between vertebral artery hypoplasia and posterior circulation stroke in Chinese patients. Neuroradiology 2013, 55, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lou, X.; Li, Y.; Sui, B.; Sun, S.; Li, C.; Jiang, P.; Siddiqui, A.; Yang, X. Imaging investigation of intracranial arterial dissecting aneurysms by using 3 T high-resolution MRI and DSA: From the interventional neuroradiologists’ view. Acta Neurochir. 2014, 156, 515–525. [Google Scholar] [CrossRef]
- Obara, M.; Kuroda, K.; Wang, J.; Honda, M.; Yoneyama, M.; Imai, Y.; Cauteren, M.V. Comparison between two types of improved motion-sensitized driven-equilibrium (iMSDE) for intracranial black-blood imaging at 3.0 Tesla. J. Magn. Reson. Imaging 2014, 40, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Nagao, E.; Yoshiura, T.; Hiwatashi, A.; Obara, M.; Yamashita, K.; Kamano, H.; Takayama, Y.; Kobayashi, K.; Honda, H. 3D turbo spin-echo sequence with motion-sensitized driven-equilibrium preparation for detection of brain metastases on 3T MR imaging. Am. J. Neuroradiol. 2011, 32, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Pelkonen, O.; Tikkakoski, T.; Pyhtinen, J.; Sotaniemi, K. Cerebral CT and MRI findings in cervicocephalic artery dissection. Acta Radiol. 2004, 45, 259–265. [Google Scholar] [CrossRef]
- Shi, Z.; Tian, X.; Tian, B.; Meddings, Z.; Zhang, X.; Li, J.; Saloner, D.; Liu, Q.; Teng, Z.; Lu, J. Identification of high risk clinical and imaging features for intracranial artery dissection using high-resolution cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2021, 23, 74. [Google Scholar] [CrossRef]
- Saliou, G.; Sacho, R.H.; Power, S.; Kostynskyy, A.; Willinsky, R.A.; Tymianski, M.; terBrugge, K.G.; Rawal, S.; Krings, T. Natural history and management of basilar trunk artery aneurysms. Stroke 2015, 46, 948–953. [Google Scholar] [CrossRef]
- Pfefferkorn, T.; Saam, T.; Rominger, A.; Habs, M.; Gerdes, L.A.; Schmidt, C.; Cyran, C.; Straube, A.; Linn, J.; Nikolaou, K.; et al. Vessel wall inflammation in spontaneous cervical artery dissection: A prospective, observational positron emission tomography, computed tomography, and magnetic resonance imaging study. Stroke 2011, 42, 1563–1568. [Google Scholar] [CrossRef]
- Sakurai, K.; Miura, T.; Sagisaka, T.; Hattori, M.; Matsukawa, N.; Mase, M.; Kasai, H.; Arai, N.; Kawai, T.; Shimohira, M.; et al. Evaluation of luminal and vessel wall abnormalities in subacute and other stages of intracranial vertebrobasilar artery dissections using the volume isotropic turbo-spin-echo acquisition (VISTA) sequence: A preliminary study. J. Neuroradiol. 2013, 40, 19–28. [Google Scholar] [CrossRef]
- Coppenrath, E.; Lenz, O.; Sommer, N.; Lummel, N.; Linn, J.; Treitl, K.; Bamberg, F.; Reiser, M.; Pfefferkorn, T.; Saam, T. Clinical significance of intraluminal contrast enhancement in patients with spontaneous cervical artery dissection: A black-blood MRI study. RoFo 2017, 189, 624–631. [Google Scholar] [CrossRef]
- Park, K.J.; Jung, S.C.; Kim, H.S.; Choi, C.G.; Kim, S.J.; Lee, D.H.; Suh, D.C.; Kwon, S.U.; Kang, D.W.; Kim, J.S. Multi-contrast high-resolution magnetic resonance findings of spontaneous and unruptured intracranial vertebral artery dissection: Qualitative and quantitative analysis according to stages. Cerebrovasc. Dis. 2016, 42, 23–31. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamaguchi, Y.; Makita, N.; Morita, Y.; Ide, T.; Wada, S.; Mizoguchi, T.; Ikenouchi, H.; Miwa, K.; Yi, K.; et al. Clinical and radiological characteristics of intracranial artery dissection using recently proposed diagnostic criteria. J. Stroke Cerebrovasc. Dis. 2019, 28, 1691–1702. [Google Scholar] [CrossRef]
- Amenta, P.; Jabbour, P.; Rosenwasser, R. Approaches to extracranial and intracranial dissections. In Hemorrhagic and Ischemic Stroke: Surgical, Interventional, Imaging, and Medical Approaches; Bendok, B.R., Batjer, H.H., Naidech, W.M.T., Eds.; Thieme: New York, NY, USA, 2011. [Google Scholar]
- Katsuno, M.; Kobayashi, S. Diagnosis of vertebral artery dissection with basiparallel anatomical scanning magnetic resonance imaging. J. Nippon Med. Sch. 2011, 78, 367–373. [Google Scholar] [CrossRef]
- Kitanaka, C.; Tanaka, J.; Kuwahara, M.; Teraoka, A. Magnetic resonance imaging study of intracranial vertebrobasilar artery dissections. Stroke 1994, 25, 571–575. [Google Scholar] [CrossRef]
- Bousson, V.; Lévy, C.; Brunereau, L.; Djouhri, H.; Tubiana, J.M. Dissections of the internal carotid artery: Three-dimensional time-of flight MR angiography and M R imaging features. Am. J. Roentgenol. 1999, 173, 139–143. [Google Scholar] [CrossRef]
- Boet, R.; Wong, H.T.; Yu, S.C.; Poon, W.S. Vertebrobasilar artery dissection: Current practice. HKMJ 2002, 8, 33–38. [Google Scholar]
- Mascalchi, M.; Bianchi, M.C.; Mangiafico, S.; Ferrito, G.; Puglioli, M.; Marin, E.; Mugnai, S.; Canapicchi, R.; Quilici, N.; Inzitari, D. MRI and MR angiography of vertebral artery dissection. Neuroradiology 1997, 39, 329–340. [Google Scholar] [CrossRef]
- Lévy, C.; Laissy, J.P.; Raveau, V.; Amarenco, P.; Servois, V.; Bousser, M.G.; Tubiana, J.M. Carotid and vertebral artery dissections: Three-dimensional time-of flight MR angiography and MR imaging versus conventional angiography. Radiology 1994, 190, 97–103. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwak, H.S.; Hwang, S.B.; Chung, G.H. Differential diagnosis of intraplaque hemorrhage and dissection on high-resolution MR imaging in patients with focal high signal of the vertebrobasilar artery on TOF imaging. Diagnostics 2021, 11, 1024. [Google Scholar] [CrossRef]
- Tanoue, S.; Endo, H.; Hiramatsu, M.; Matsumaru, Y.; Matsumoto, Y.; Sato, K.; Tsuruta, W.; Sato, M.; Hirohata, M.; Abe, T.; et al. Delineability and anatomical variations of perforating arteries from normal vertebral artery on 3D DSA: Implications for endovascular treatment of dissecting aneurysms. Neuroradiology 2021, 63, 609–617. [Google Scholar] [CrossRef]
- Dobrocky, T.; Piechowiak, E.I.; Goldberg, J.; Barvulsky Aleman, E.; Nicholson, P.; Lynch, J.; Bervini, D.; Kaesmacher, J.; Agid, R.; Krings, T.; et al. Absence of pontine perforators in vertebrobasilar dolichoectasia on ultra-high resolution cone-beam computed tomography. J. NeuroIntervent. Surg. 2021, 13, 580–584. [Google Scholar] [CrossRef]
- Fukumoto, H.; Morishita, T.; Takemoto, K.; Kobayashi, H.; Kawano, D.; Horio, Y. Clinical characteristics and management of vertebral artery dissection without definitive imaging features: A single center cohort study. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2024, 37, 101985. [Google Scholar] [CrossRef]
- Takano, K.; Yamashita, S.; Takemoto, K.; Inoue, T.; Kuwabara, Y.; Yoshimitsu, K. MRI of intracranial vertebral artery dissection: Evaluation of intramural haematoma using a black blood, variable-flip-angle 3D turbo spin-echo sequence. Neuroradiology 2013, 55, 845–851. [Google Scholar] [CrossRef]
- Horio, Y.; Ogata, T.; Abe, H.; Fukuda, K.; Morishita, T.; Higashi, T.; Inoue, T. Factors predictive of enlargement of dissecting aneurysms in the vertebral artery. World Neurosurg. 2021, 151, e935–e942. [Google Scholar] [CrossRef]
- Sato, S.; Toyoda, K.; Matsuoka, H.; Okatsu, H.; Kasuya, J.; Takada, T.; Shimode, A.; Uehara, T.; Naritomi, H.; Minematsu, K. Isolated anterior cerebral artery territory infarction: Dissection as an etiological mechanism. Cerebrovasc. Dis. 2010, 29, 170–177. [Google Scholar] [CrossRef]
- Kobayashi, N.; Murayama, Y.; Yuki, I.; Ishibashi, T.; Ebara, M.; Arakawa, H.; Irie, K.; Takao, H.; Kajiwara, I.; Nishimura, K.; et al. Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am. J. Neuroradiol. 2014, 35, 1371–1375. [Google Scholar] [CrossRef]
- Sakata, N.; Takebayashi, S.; Kojima, M.; Masawa, N.; Suzuki, K.; Takatama, M. Pathologyof a dissecting intracranial aneurysm. Neuropathology 2000, 20, 104–108. [Google Scholar] [CrossRef]
- Kai, Y.; Nishi, T.; Watanabe, M.; Morioka, M.; Hirano, T.; Yano, S.; Ohmori, Y.; Kawano, T.; Hamada, J.; Kuratsu, J. Strategy for treating unruptured vertebralartery dissecting aneurysms. Neurosurgery 2011, 69, 1085–1091. [Google Scholar] [CrossRef]
- Mizutani, T. Natural course of intracranial arterial dissections. J. Neuro-Surg. 2011, 114, 1037–1044. [Google Scholar] [CrossRef]
- Schievink, W.I.; Mokri, B.; O’Fallon, W.M. Recurrent spontaneous cervical-artery dissection. N. Engl. J. Med. 1994, 330, 393–397. [Google Scholar] [CrossRef]
- Kasner, S.E. CADISS: A feasibility trial that answered its question. Lancet Neurol. 2015, 14, 342–343. [Google Scholar] [CrossRef]
- Kennedy, F.; Lanfranconi, S.; Hicks, C.; Reid, J.; Gompertz, P.; Price, C.; Kerry, S.; Norris, J.; Markus, H.S.; CADISS Investigators. Antiplatelets vs anticoagulationfor dissection: CADISS nonrandomized arm and meta-analysis. Neurology 2012, 79, 686–689. [Google Scholar] [CrossRef]
- Daou, B.; Hammer, C.; Mouchtouris, N.; Starke, R.M.; Koduri, S.; Yang, S.; Jabbour, P.; Rosenwasser, R.; Tjoumakaris, S. Anticoagulation vs Antiplatelet Treatment in Patients with Carotid and Vertebral Artery Dissection: A Study of 370 Patients and Literature Review. Neurosurgery 2017, 80, 368–379. [Google Scholar] [CrossRef]
- Chowdhury, M.M.; Sabbagh, C.N.; Jackson, D.; Coughlin, P.A.; Ghosh, J. Antithrombotic treatment for acute extracranial carotid artery dissections: A meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 148–156. [Google Scholar] [CrossRef]
- Zinkstok, S.M.; Vergouwen, M.D.; Engelter, S.T.; Lyrer, P.A.; Bonati, L.H.; Arnold, M.; Mattle, H.P.; Fischer, U.; Sarikaya, H.; Baumgartner, R.W.; et al. Safety and functional out-come of thrombolysis in dissection-related ischemic stroke: A meta-analysis of individual patient data. Stroke 2011, 42, 2515–2520. [Google Scholar] [CrossRef]
- Kitanaka, C.; Tanaka, J.; Kuwahara, M.; Teraoka, A.; Sasaki, T.; Takakura, K.; Tanaka, J. Nonsurgical treatment of unruptured intracranial vertebral artery dissection with serial follow-up angiography. J. Neurosurg. 1994, 80, 667–674. [Google Scholar] [CrossRef]
- Doijiri, R.; Yokota, C.; Suzuki, R.; Toyoda, K.; Minematsu, K. Intravenous Recombinant Tissue Plasminogen Activator Thrombolysis in Acute Ischemic Stroke due to Middle Cerebral Artery Dissection. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Association 2012, 21, 915.e7–915.e9. [Google Scholar] [CrossRef]
- Chen, M.; Caplan, L. Intracranial dissections. Front. Neurol. Neurosci. 2005, 20, 160–173. [Google Scholar]
- Guillon, B.; Levy, C.; Bousser, M.G. Internal carotid artery dissection: An update. J. Neurol. Sci. 1998, 153, 146–158. [Google Scholar] [CrossRef]
- Srinivasan, J.; Newell, D.W.; Sturzenegger, M.; Mayberg, M.R.; Winn, H.R. Prognosis and safety of anticoagulation in intracranial artery dissections in adults. Stroke 2007, 38, 1837–1842. [Google Scholar]


| Subtype | Features |
|---|---|
| Transmural Dissection | If the hematoma disrupts the entire vessel wall, a transmural dissection is present. The clinical symptoms will depend on the surrounding structures:
|
| Subintimal Dissection | If the dissection remains subintimal, a subadventitial hematoma forms within the vessel wall. The clinical consequences depend on the subsequent behavior of this hematoma:
|
| Clinical Symptoms | |
Consequently, the symptoms of arterial dissection can be attributed to
| |
| Issues | Features |
|---|---|
| Etiologies | Multiple factors implicated; associated with inconsistent terminology. |
| Initial Mechanism | Begins as a tear in the intimal lining of the vessel, creating a “false lumen” for alternative blood flow [32,74,75,76]. |
| True Dissection | Result of subintimal extravasation of blood or blood between the intima and media; most common form of extracranial dissection [51]. |
| Intimal Damage | Exposes pro-thrombotic subendothelial vessel wall, which may lead to thrombus formation and potential embolization [76,77]. |
| Dissection Mechanism | High-pressure arterial blood enters the false lumen, dissecting through the tunica media or between layers. |
| Hematoma Formation | An expanding hematoma accumulates within the vessel wall due to pro-thrombotic subendothelial exposure. |
| Possible Complications | - Thrombotic phenomena. - Parent vessel occlusion (stenosis or hematoma expansion). - SAH due to adventitial extension. |
| Pseudoaneurysm Formation | Occurs if dissection involves all three vessel layers, leading to the encapsulation of the extravascular hematoma. |
| Characteristics of Pseudoaneurysms | - Lack of external elastic lamina. - Fewer elastic fibers in tunica media. - May rupture into subarachnoid space. |
| Incidence of Pseudoaneurysms | - 28% of posterior circulation aneurysms. - 3.3% of all intracranial aneurysms. - Causative in ~10% of non-traumatic SAH cases. |
| Rupture Propensity | High; up to 73% of pseudoaneurysms present with SAH; only 27% show bulbar signs or cerebellar ischemia. |
| Major Criteria | “Double lumen” or “intimal flap” demonstrated on DSA, MRI, MRA, or CTA. “Pearl and string sign” or “string sign” demonstrated on DSA. Pathological confirmation of arterial dissection. |
| Minor Criteria | “Pearl sign” or “tapered occlusion” demonstrated on DSA. “Pearl and string sign”, “string sign”, or “tapered occlusion” demonstrated on MRA. “Hyperintense intramural signal” (indicative of intramural hematoma) demonstrated on T1-weighted MRI. |
| Additional Criteria | Change in arterial shape demonstrated on DSA, MRI, MRA, CTA, or duplex ultrasonography. Absence of other causes of arterial abnormalities. |
| Definite Dissection | The presence of one or more major criteria, or the presence of one or more minor criteria, along with both of the additional criteria. |
| Probable Dissection | The presence of one or more minor criteria. |
| Diagnostic Criteria |
|---|
| At least one of the three following features must be present: |
| 1. Fusiform or irregular aneurysmal dilation at a non-branching site of an intracranial artery, with at least one of the following: - Intramural hematoma (hyperintense rim on T1-weighted MRI), intimal flap, or double lumen. - Rapid change in morphology on repeated imaging (increase/reduction in size, new stenosis). - Association with focal stenosis (pearl-and-string sign). |
| 2. Long filiform or irregular stenosis of an intracranial artery, with at least one of the following: - Intramural hematoma (hyperintense rim on T1-weighted MRI), intimal flap, or double lumen. - Rapid change in morphology on repeated imaging (increase/reduction in size, new aneurysmal dilation). - Association with fusiform or irregular aneurysmal dilation (pearl-and-string sign). |
| 3. The occlusion of an intracranial artery that recanalizes into either a fusiform or irregular aneurysmal dilation at a non-branching site, or a long filiform or irregular stenosis. |
| Grading | Criteria |
|---|---|
| Definite Intracranial Artery Dissection | - Stenosis or occlusion developing into fusiform/irregular aneurysmal dilation at a non-branching site. - Intramural hematoma, intimal flap, or double lumen. - Pathological confirmation of dissection. |
| Probable Intracranial Artery Dissection | - Fusiform/irregular aneurysmal dilation and focal, long filiform, or irregular stenosis (pearl-and-string sign) without SAH, or persisting > 1 month after SAH. - Fusiform/irregular aneurysmal dilation at a non-branching site with rapid morphological changes (increase/reduction in size, new stenosis). |
| Possible Intracranial Artery Dissection | - Fusiform/irregular aneurysmal dilation at a non-branching site without morphological changes on imaging within 6–12 months. - Long filiform or irregular stenosis with reduction in size or disappearance over time. |
| Finding | Description | Incidence |
|---|---|---|
| Pearl-and-String Sign | Represents a dilatation adjacent to the narrowed dissected segment. | Observed in up to 91% of VAD cases [108]. |
| Double Lumen or Rosette | Indicates the presence of two lumens within the dissected vessel. | Reliable angiographic finding for VAD diagnosis [13,38,45]. |
| Fusiform Dilatation | Simple fusiform dilation of the dissected segment. | Common finding in VAD cases. |
| Delayed Contrast Clearance | The delayed clearance of the contrast from the false lumen of the dissected vessel. | Indicative of dissection. |
| Abrupt or Tapered “Cut Off” | Observed in cases of vessel occlusion secondary to dissection, where the flow cannot be visualized beyond a certain point. | Characteristic of severe dissection [45,73]. |
| Issue | Features |
|---|---|
| Technical Evolution | MDCT has facilitated the development of CTA. |
| Imaging Efficiency | Efficient scanners can image large body segments within seconds of intravascular contrast injection. |
| Time for Evaluation | CTA evaluation of cranio-cervical circulation can be obtained in less than thirty seconds. |
| Image Quality | Reconstructed images are of angiographic quality. |
| Data Acquisition | Unlimited projections can be obtained from one data acquisition, allowing offline interrogation of vasculature. |
| Emergent Screening | Particularly beneficial for suspected VAD, aiding in the diagnosis of hemorrhage and ischemia. |
| Time to Treatment | Significant reductions in time to treatment, morbidity, and cost due to combined non-contrast CT and CTA findings. |
| Findings on CTA | Reliable demonstration of vessel caliber changes, false lumens, fusiform dilatations, and pseudoaneurysms [112,113]. |
| Reliable Diagnostic Criteria | Narrowed, eccentric lumen associated with increased overall diameter of the dissected artery [84]. |
| Key Findings [73] | (1) Focal dilatation. (2) Intimal flap. (3) Increase in outer diameter. (4) Narrowing or occlusion of vessel lumen. |
| Comparison of Sensitivity | CTA excels in identifying intimal flaps, pseudoaneurysms, and high-grade stenoses compared to MRA [110]. |
| Wall Thickness Sensitivity | An alteration in wall thickness on CTA is a more sensitive criterion than a change in the luminal diameter in VAD [111]. |
| Meta-analysis Findings | Reported sensitivity and specificity for CTA and MRA are fairly similar in detecting carotid and vertebral artery injuries [114]. |
| MRI for Intramural Hematoma Detection |
|
| MRA for Arterial Dissection |
|
| 3D-T1WI for Intramural Hematoma and Intimal Flap |
|
| Susceptibility-Weighted Imaging (SWI) in Dissection |
|
| Atherosclerosis | Vertebrobasilar Dysplasia | Dissection | |
|---|---|---|---|
| TOF MRA | Irregularly stenosis or unclear or invisible. | Smoothly narrowed, diameter < 2 mm, or Invisible. | Eccentric stenosis with local vascular dilation or segmental stenosis with double-lumen signs. |
| HR-MRI | The lumen was narrow and the wall had annular or eccentric atherosclerotic plaques. | Without any thickening of the vascular wall, the lumen diameter was <1/2 of the contralateral side, or <2 mm, or absent. | Vascular dilation with the double-lumen sign, intimal flap, or intramural hematoma. |
| BPAS-MRI | Diffuse dilatation with or without a rough wall, or almost normal. | Smoothly narrowed or invisible. | Segmental dilatation or aneurysmal dilatation. |
| Issue | Features |
|---|---|
| Detection Methods | MRI and MRA offer sensitive, non-invasive means to detect cervical and intracranial dissection [134]. |
| Advantages of MRA | - Reproducibility of technique. - Uniformity between vendors. - Minimal interoperator variation. |
| Accuracy of Imaging Techniques | Higher accuracy for predicting intracranial VAD with T2-weighted MRI and basi-parallel anatomical scanning (BPAS-MRI) compared to T2-weighted MRI and conventional angiography [135]. |
| Diagnosis of VAD | Depends on the demonstration of intramural hematoma and alteration in the caliber of the patent lumen. |
| Shapes of Intramural Hematoma | - Curvilinear. - Crescentic (circumferential). - Bamboo-cut. - Band-line. - Spotty [136] |
| Signal Intensity Changes (T1-weighted) | Changes from isointense/slightly hyperintense in acute settings to hyperintense in subacute and back to isointense in chronic settings. |
| Fat Suppression | Aids in distinguishing periarterial atheroma from intramural hematoma [137]. |
| Complementary MRI Findings | - Identifiable intimal flap on proton density or T2-weighted images. - Increased vessel diameter compared to normal side. - Double lumen. - Wall and septum enhancement on contrast-enhanced images (3D SPGR) [45]. |
| MRA Findings | Vascular dissection may appear as a tapered or narrowed vessel lumen; medium-to-large pseudoaneurysms or sacculations can be reliably identified with non-contrast MRA [138]. |
| Diagnosis of Exclusion | VAD may sometimes be diagnosed by exclusion when thrombosis occurs. |
| Sensitivity and Specificity | - MRA: 20% sensitivity, 100% specificity. - MRI: 60% sensitivity, 98% specificity [139,140]. |
| Dynamic Contrast Enhanced MRA (DCEMRA) | Improves the visualization of complex cervical vasculature anatomy; gadolinium-DTPA provides the rapid imaging of the cerebrovascular circulation. |
| Disadvantages of MRI/MRA | - Lower spatial resolution compared to CTA or DSA. - Larger slice partitions may obscure subtle or short segment dissection. - Complex anatomy of V3 and V4 segments may cause artifactual loss of flow. - Fat-suppression techniques may fail at cervico-thoracic and cranio-cervical junctions, lowering sensitivity. |
| Modality | Limitation |
|---|---|
| MRA | The potential misinterpretation of a double lumen as a flow artifact. |
| T1WI | The risk of mistaking an intramural hematoma for an in-flow effect. |
| 3D-GdT1WI | The possibility of misidentifying a double lumen as a flow artifact. |
| MSDE | The data may be insufficient to differentiate intramural hematoma from atherosclerotic plaque. |
| CTA | The potential for misinterpreting a double lumen as a flow artifact. |
| Angiography | The data may be inadequate for depicting an intramural hematoma. |
| Issue | Features |
|---|---|
| Management Approach | A large number of patients with ischemic symptoms from VAD may be managed medically. |
| Primary Etiology of Ischemia | Thromboembolism is the primary cause of ischemia following VAD, rather than hypo-perfusion [159]. |
| Non-Invasive Imaging | CT and MRI exhibit infarct patterns consistent with thromboembolism [76]. |
| Angiographic Findings | Demonstrates the branch occlusion of intracranial vasculature distal to the dissected vessel, indicative of thromboembolic phenomena. |
| Anticoagulant Treatment | Some authors recommend avoiding anticoagulants in all intracranial dissections or a performing lumbar puncture to rule out SAH before treatment [76,160]. |
| Aspirin Treatment | Majority of intracranial VAD patients treated with Aspirin (300 mg/day for 3–6 months); stopped upon evidence of recanalization. |
| Outcomes by Treatment | - Aspirin: 82% favorable outcomes. - Aspirin/Warfarin: 77%. - Heparin/Warfarin: 8%. |
| Heparin to Warfarin Bridge | Reported favorable outcomes in non-aneurysmal intracranial VAD; minimal cases of hemorrhage [151,161]. |
| Monitoring Without Intervention | Limited studies; in Mizutani’s study, 155 of 190 patients with intracranial dissection involved vertebral artery [151]. |
| Unruptured Cases | 54 unruptured cases with infarction treated with antiplatelet or anticoagulant; others followed with blood pressure control [162]. |
| Complications in Follow-Up | One patient died from the rupture of a dilated IAD and another from brainstem infarction; 18 patients had recurrent dissection [151]. |
| Inconclusive Data | Existing data are inconclusive due to lack of randomized trials, variable treatment algorithms, and differing rates of treatment-associated hemorrhages. |
| Recommendations | - Treat patients without pseudoaneurysms or significant stenosis with antiplatelet therapy. - Endovascular or surgical intervention for continued thromboembolic symptoms despite antiplatelet therapy. |
| Need for Further Research | A randomized controlled trial is needed to clarify the best medical therapy, requiring a large sample size. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedde, M.; Pascarella, R. Spontaneous Intracranial Vertebral Artery Dissection: A Rare Cause of Ischemic Stroke. J. Cardiovasc. Dev. Dis. 2025, 12, 187. https://doi.org/10.3390/jcdd12050187
Zedde M, Pascarella R. Spontaneous Intracranial Vertebral Artery Dissection: A Rare Cause of Ischemic Stroke. Journal of Cardiovascular Development and Disease. 2025; 12(5):187. https://doi.org/10.3390/jcdd12050187
Chicago/Turabian StyleZedde, Marialuisa, and Rosario Pascarella. 2025. "Spontaneous Intracranial Vertebral Artery Dissection: A Rare Cause of Ischemic Stroke" Journal of Cardiovascular Development and Disease 12, no. 5: 187. https://doi.org/10.3390/jcdd12050187
APA StyleZedde, M., & Pascarella, R. (2025). Spontaneous Intracranial Vertebral Artery Dissection: A Rare Cause of Ischemic Stroke. Journal of Cardiovascular Development and Disease, 12(5), 187. https://doi.org/10.3390/jcdd12050187







