Abstract
(1) Background: Antiphospholipid syndrome (APS) is associated with thrombotic events and the laboratory identification of antiphospholipid antibodies (aPL), in which lupus anticoagulant (LA), anticardiolipin (aCL), and anti-β2 glycoprotein I antibodies are included. The aim of the current retrospective study is to examine clinical characteristics and risk factors of ischemic stroke as a clinical manifestation of APS. (2) Methods: Adult patients diagnosed with APS between 1 January 2009 and 1 June 2024 were retrospectively enrolled in this study. Sydney-revised Sapporo criteria were used for the diagnosis of APS, while ischemic stroke was diagnosed based on the acute onset of focal neurologic deficits and confirmed with radiological findings. (3) Results: We studied 115 patients with APS. Specifically, 28 (24.35%) patients, with a mean age (standard deviation) of 54 (±12.5), had ischemic stroke as a clinical manifestation of APS. In univariate analysis, stroke development was associated with the following factors: age (p < 0.001), livedo reticularis (p = 0.046), avascular necrosis (AVN) (p = 0.046), hypertension (p < 0.001), dyslipidemia (p = 0.013), aCL IgG (U/L) antibodies title (p = 0.035), and adjusted global APS score (aGAPSS) (p = 0.047), while in multivariate analysis, it was associated with age (p = 0.006), hypertension (p < 0.001), AVN (p = 0.006), livedo reticularis (p = 0.035), aCL IgG title (p = 0.004), and aGAPSS (p = 0.002). (4) Conclusions: Stroke is a common initial manifestation of APS, with cardiovascular risk factors, particularly hypertension, being highly prevalent.
1. Introduction
Antiphospholipid syndrome (APS) is a systemic autoimmune disorder that is characterized by the persistent presence of antiphospholipid antibodies (aPL) combined with thrombotic events, obstetric complications, and other non-thrombotic manifestations [1]. APS can be categorized as primary or secondary, with secondary APS occurring in the context of another autoimmune disorder, most commonly systemic lupus erythematosus (SLE) [2]. The pathogenesis of this clinical entity includes multiple interactions between aPL, endothelium, coagulation cascade, and complement system [3]. Thrombosis can be either arterial, venous, or microvascular, leading to the development of thrombotic microangiopathy [4]. The most common sites of thrombosis include the deep veins of the lower extremities and cerebral arteries, while cases of thrombosis in unusual sites can also be observed. Other manifestations of APS are considered as thrombocytopenia, pulmonary hemorrhage, aPL nephropathy, and heart valve disease [5,6].
The up-to-date diagnosis of APS is based on the revised Sydney–Sapporo Criteria [7]. Recently, the 2023 ACR/EULAR antiphospholipid syndrome criteria have been suggested for the classification of APS patients [8]. Vitamin K antagonists remain the cornerstone of management for most patients with thrombotic APS [9]. Other agents used in the treatment of APS include low-dose aspirin, hydroxychloroquine (HCQ), and prednisone [9]. Catastrophic APS is characterized by micro- and macro-thrombosis in multiple sites, organ failure, and high mortality and morbidity. The differential diagnosis of this syndrome from other clinical entities, such as thrombotic microangiopathies, is challenging [10]. Moreover, novel agents such as complement inhibitors have been reported as beneficial for some patients [11].
The most common clinical manifestation of APS-associated arterial thrombosis is considered acute ischemic stroke [12]. At the same time, APS is an important etiology of stroke in individuals below the age of 45 years [13]. In addition to cerebrovascular disease, including acute ischemic stroke, transient ischemic attacks, and cerebral venous thrombosis, neurological manifestations of this condition include cognitive deficits, migraines, transverse myelitis, chorea, psychosis, and Sneddon syndrome [14]. Based on the common involvement of the central nervous system in APS patients and the burden of disease that stroke survivors experience, the aim of this study is to investigate the prevalence and risk factors associated with acute ischemic stroke in a real-world cohort of APS patients.
2. Materials and Methods
2.1. Study Design and Patient Population
In this study, adult patients (both inpatients and outpatients) with definite diagnoses of APS who visited the thrombosis–hemostasis referral clinic of the 2nd Propedeutic Department of Internal Medicine between 1st January 2009 and 1st June 2024 were enrolled. APS diagnosis was based on the revised Sydney–Sapporo Criteria [7]. Specifically, a diagnosis of APS was made when at least one of the clinical criteria and one of the laboratory criteria were present. Clinical criteria included vascular thrombosis and pregnancy morbidity, while the laboratory criteria were based on the presence of lupus anticoagulant (LA) in plasma, anticardiolipin antibody (aCL) of IgG, and/or the IgM isotype in serum or plasma, in medium or high titer, and the anti-b2 glycoprotein-I antibody (aβ2GPI) of IgG and/or IgM isotype in serum or plasma on two or more occasions at least 12 weeks apart. The study was conducted according to the Declaration of Helsinki and was approved by the Institutional Review Board of Hippocration General Hospital, with informed consent collected from all participants.
The following data were retrospectively collected from the patient’s medical records: gender, age at APS diagnosis, previous history of thrombosis, thrombocytopenia (platelet counts < 100 × 109/L), neuropsychiatric manifestations, other APS manifestations (such as history of avascular necrosis [AVN] or livedo reticularis), cardiovascular disease risk factors (arterial hypertension, dyslipidemia, diabetes mellitus), underlying autoimmune disease, homocysteine levels, thrombophilia genetic testing (factor V [FV] Leiden, factor II [FII] (G20210A) mutation, methylenetetrahydrofolate reductase [MTHFR] mutation), LA activity and positivity, aCL title and positivity, aβ2GPI title and positivity, and antinuclear antibody (ANA) positivity.
Acute ischemic stroke was diagnosed by experienced neurologists based on the patient’s history, clinical condition, and neuroimaging (magnetic resonance imaging [MRI] or computed tomography [CT]) findings. Data regarding follow-up visits were obtained from the patient’s records, while venous thromboembolism was diagnosed based on clinical manifestations and radiological imaging.
2.2. Detection of aPLs and aGAPSS Calculation
aCL and aβ2GPI were detected with the use of ELISA kits (EUROIMMUN, Luebeck, Germany). The aCL and aβ2GPI threshold for positivity was ≥40 units based on standardized ELISA results [8]. LA was measured according to the recommendations of ISTH, using diluted Russel’s viper venom time [15]. All patients diagnosed with APS had positive aPLs during at least 2 time points within an interval of a minimum of 12 weeks. Additionally, the adjusted global antiphospholipid syndrome score (aGAPSS) was retrospectively calculated based on the presence of dyslipidemia (3 points), arterial hypertension (1 point), aCL IgG/IgM antibodies (5 points), aβ2GPI IgG/IgM (4 points), and LA (4 Points) [16].
2.3. Statistical Analysis
The sample size calculation was based on previously published studies on stroke in APS patients [17,18]. Statistical analysis was performed with SPSS 28.0 (IBM SPSS Statistics for Windows, Version 28.0. Armonk, NY, USA: IBM Corp). Frequencies were used for the presentation of categorical variables. Shapiro–Wilk and Kolmogorov–Smirnov tests (based on the sample size for each time) were employed for the determination of the sample’s normality distribution. Normally distributed variables were presented as means with standard deviation (±SD), while those with non-normal distribution were presented as medians with an interquartile ratio (IQR: Q1–Q3). For categorical variables, chi-squared or Fisher’s exact tests, when required, were used to analyze the differences between the groups. For continuous variables, Student’s t-test (for normally distributed variables) and the Mann–Whitney U test (for non-normally distributed) were employed to identify differences. Univariate logistic regression analysis was used for the identification of potential factors associated with stroke development. Variables with a significance level of p < 0.05 were included in multivariate logistic regression models, along with gender. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated. The Hosmer–Lemeshow test was used to examine the multivariate logistic regression model’s goodness of fit and calibration. The fit was considered poor if the significance value was less than 0.05. The statistical significance level was set to p < 0.05.
3. Results
3.1. Clinical and Laboratory Characteristics of APS Patients with Stroke
Among 115 patients with APS, 28 (24.35%) presented with acute ischemic stroke. In Figure 1, the cases of APS diagnosed each year from 2009 to 2024 are presented. The mean age at diagnosis was 54 (±12.5) years, significantly higher compared to patients without acute ischemic stroke (41.5 ± 14.3) (p < 0.001), while 15 (53.6%) APS patients were also female. Four (14.28%) patients had a previous history of thrombosis, three (10.7%) of AVN of the femoral head, and three (10.7%) of livedo reticularis. Moreover, AVN (p = 0.043) and livedo reticularis (p = 0.043) were more frequent in the stroke group in comparison to other patients. Thrombocytopenia at diagnosis was observed in one (3.6%) patient with stroke and neuropsychiatric manifestations were shown in two patients (7.2%, migraine and cognitive dysfunction in both).
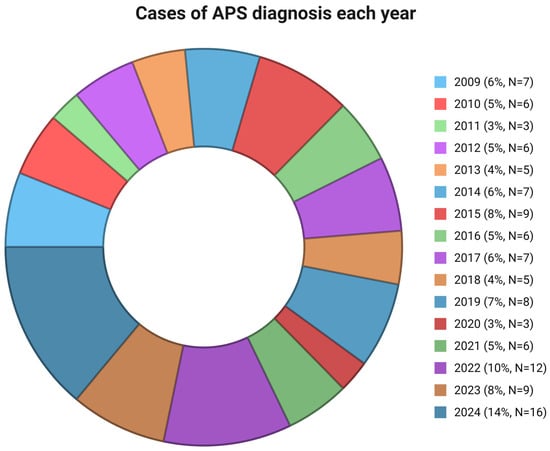
Figure 1.
Cases of APS diagnosis each year. APS: antiphospholipid syndrome.
Regarding cardiovascular disease risk factors, 15 (53.6%) stroke patients had a previous medical history of arterial hypertension, seven (25%) of dyslipidemia, and one (3.6%) of diabetes mellitus. Arterial hypertension (p < 0.001) and dyslipidemia (p = 0.008) were more common in the stroke group compared to patients without stroke. Furthermore, three individuals (10.7%) were diagnosed with secondary APS due to underlying SLE. The three patients with SLE in the stroke group were treated with HCQ, while among the eight other SLE patients without stroke, five received HCQ and three HCQ in combination with other immunosuppressant agents.
Three (10.7%) patients were heterozygous for the FV Leiden mutation, while one (3.6%) had the FII (G20210A) mutation. Furthermore, all patients with ischemic stroke were positive for LA, 12 (42.9%) for aCLs, and 13 (46.4%) for aβ2GPI, respectively. Triple positivity was observed in eight (28.6%) individuals, while ANA positivity was detected in eleven (39.3%). The median aGAPSS score was nine (4–13.75), which was higher in stroke patients compared to others (p = 0.048). The clinical and laboratory characteristics of APS patients with ischemic stroke in comparison to those without stroke are summarized in Table 1. All APS patients with stroke were treated with vitamin K antagonists. Follow-up data were available for seventeen stroke patients, and the recurrence of thrombosis (venous thromboembolism in all cases) was observed in three (17.65%) of them.

Table 1.
Clinical and laboratory characteristics of APS patients with ischemic stroke in comparison with those without.
3.2. Univariate Analysis
In univariate analysis, ischemic stroke in patients with APS was associated with the following factors: age at diagnosis (OR: 0.938, 95% CI: −0.520, −0.191, p < 0.001), livedo reticularis (OR: 10.44, 95% CI: 0.044, 0.391, p = 0.046), AVN of the femoral head (OR: 10.44, 95% CI: 0.044, 0.391, p = 0.046), arterial hypertension (OR: 15.769, 95% CI: 0.373, 0.641, p < 0.001), dyslipidemia (OR: 4.556, 95% CI: 0.067, 0.410, p = 0.013), title of aCL IgG (OR: 0.975, 95% CI: −0.031, −0.033, p = 0.035), and aGAPSS (OR: 0.916, 95% CI: −0.360, −0.003, p = 0.047). The findings of the univariate analysis are presented in Table 2.

Table 2.
Univariate analysis of factors associated with ischemic stroke in APS patients.
3.3. Multivariate Analysis
In the multivariate analysis, ischemic stroke was associated with age at diagnosis (OR: 0.925, 95% CI: 0.876, 0.977, p = 0.006), livedo reticularis (OR: 0.017, 95% CI: 0.000, 0.755, p = 0.035), AVN (OR: 228.573, 95% CI: 4.684, 11,153.959, p = 0.006), arterial hypertension (OR: 608.982, 95% CI: 20.519, 18,073.990, p < 0.001), aCL IgG title (OR: 0.916, 95% CI: 0.863, 0.973, p = 0.004) and aGAPSS (OR: 1.899, 95% CI: 1.263, 2.854, p = 0.002). In Table 3, the findings of the multivariate regression analysis are summarized.

Table 3.
Multivariate analysis of factors associated with ischemic stroke in APS patients.
4. Discussion
In this retrospective real-world study, 115 patients with APS were examined. Acute ischemic stroke (28 patients, 24.35%) was found as a common manifestation of APS. Moreover, age, livedo reticularis, AVN, aGAPSS, and cardiovascular disease risk factors, including arterial hypertension and dyslipidemia, were associated with stroke development in these patients. Furthermore, cases of APS diagnosis each year during the study period were presented. It is well known that the COVID-19 pandemic had a significant impact on risk factors associated with acute ischemic stroke [19,20]. However, in our real-world study, the number of patients referred to our center with APS was relatively small. This can be attributed to barriers in healthcare access during the pandemic that limited patients’ access to specialized centers such as ours [21].
The findings of our study were in line with previously published data in this field. García-Grimshaw et al. reported, in their retrospective study of 120 cases of APS with cerebrovascular events, that acute ischemic stroke was the most common type of these events [22]. In the study by Liu et al., 342 APS patients were enrolled, and neurological manifestations were recorded in 119 (34.8%) individuals [18]. In their analysis, older age (p = 0.047), livedo reticularis (p = 0.038), and dyslipidemia (p = 0.030) were correlated with central nervous system manifestations. Similarly, in our study, age at diagnosis, livedo reticularis, AVN, arterial hypertension, and dyslipidemia were associated with ischemic stroke [18]. Additionally, in the cohort of Fan et al., stroke was reported in 25.8% (93/361) of the participants, while in the multivariate regression analysis, arterial hypertension, diabetes mellitus, livedo reticularis, and other neurological manifestations were significantly related to stroke development [17]. In the cross-sectional study by Djokovic et al., it was shown that male gender and the presence of aβ2GPIs IgG were related significantly to stroke [23]. We showed that stroke development in APS patients was associated with the title of aCL IgG. Urbanski et al. compared the characteristics of APS patients with isolated IgM positivity (aCL or aβ2GPIs) with the non-isolated IgM populations, and they reported that stroke was more frequent in APS patients with isolated APS after adjustment for cardiovascular risk factors (odds ratio, 3.8; 95% CI, 1.3–11.5) [24].
aGAPSS, which can be easily calculated by commonly obtained clinical and laboratory factors, has been developed and validated for the prediction of thrombosis recurrence in APS patients [16]. Additionally, aGAPSS has been associated with coronary artery disease, stroke, and peripheral artery disease in this clinical entity [25]. In the study by Radin et al., the mean aGAPSS was significantly higher in APS patients with stroke compared to those without (p < 0.05) [26]. In our patient population, this score was correlated with stroke development in both univariate and multivariate analyses. Song et al. developed aGAPSS–ischemic stroke (aGAPSS-IS), which predicted the development of stroke in APS patients with higher accuracy compared to aGAPSS in both training and validating cohorts. This score is calculated based on the following factors: age (years), presence of diabetes mellitus, platelet count (×109/L), hyperuricemia, and aGAPSS > 10. Future work should focus on the predictive roles of these models in real-world patients.
Gašperšič et al. studied 89 patients with cerebrovascular events, and 22% of them fulfilled the criteria for APS diagnosis [27]. It was found that the persistent positivity of aPLs was an independent risk factor for stroke development (OR: 4.62), while a statistically significant difference in the incidence of thrombosis recurrence was not identified between APS and non-APS patients [27]. Saidi et al. compared aPL levels between patients with stroke and healthy controls [28]. Interestingly, positivity for LA resulted in an increased risk for stroke, while elevated levels of aCLs were identified in patients with lacunar, atherosclerotic, and cardioembolic stroke subtypes [28]. Moreover, Wang and colleagues evaluated aPLs in stroke patients and assessed post-stroke depression development 3 months after stroke onset by using the 24-item Hamilton Depression Rating Scale [29]. It was shown that increased aPL levels at baseline (acute phase of ischemic stroke) were associated with a higher risk of 3-month post-stroke depression [29]. APS has been shown as an independent risk factor for a hemorrhagic transformation of ischemic stroke [30]. Future studies should evaluate the possible associations between aPL type and title ischemic infract and radiological neuroimaging findings.
Ricarte et al., in their observational study, evaluated cerebral hemodynamic factors with transcranial Doppler in 46 patients with APS (22 with primary and 24 with secondary APS) in comparison to healthy controls [31]. They reported that right-to-left circulation shunts were more common in the APS group compared to controls (63.6% versus 38.1%, p = 0.05), underlying the importance of cardiac investigation in this patient population [31]. More data regarding hemodynamic alterations and brain microcirculation impairment in APS patients are essential. Interestingly, complement activation is implicated in the pathogenesis of thrombosis in APS, while in catastrophic APS, genetic predisposition to complement dysregulation has been recognized [32,33,34,35]. Markers of complement activation, such as soluble C5b-9 levels and the modified Ham test, as well as their associations with acute ischemic stroke development and severity in APS, should be further evaluated [36,37].
In our real-world cohort, all APS patients with ischemic stroke were treated with vitamin K antagonists. Current guidelines suggest that direct oral anticoagulants (DOACs) should not be used for the prevention of thrombosis in APS patients [9]. Sikorska et al., in their real-world study, studied 152 patients with APS, of whom 66 patients were treated with a DOAC (apixaban) and 86 with warfarin [38]. It was found that APS patients receiving DOAC exhibited a similar risk of thrombosis recurrency in comparison to those on warfarin [38]. In the clinical trial of Ordi-Ros et al., rivaroxaban was compared to dose-adjusted vitamin K antagonists in 190 adult patients with thrombotic APS. In a 3-year follow-up period, recurrent thrombosis was recorded in 11.6% of patients in the rivaroxaban group and six in the vitamin K antagonist arm without a statistically significant difference [39]. In a systematic review and meta-analysis of three clinical trials comparing DOACs with vitamin K antagonists as secondary APS prophylaxis, it was shown that DOACs might increase the risk of stroke in these patients [40]. Nevertheless, more data regarding the specific groups of APS patients who might experience benefits from DOAC use are essential, based both on clinical trials comparing DOACs with vitamin K antagonists but also on real-world data [41].
In a survey conducted by Cohen et al. investigating the attitudes of 107 physicians regarding the diagnosis and treatment of APS, variations were identified in aPL testing practices for brain ischemic injury beyond ischemic stroke or transient ischemic attack in cases where an alternative cause for stroke was present and in the age cutoff for aPL testing in stroke patients [38]. Our study mainly focused on ischemic stroke as a clinical manifestation of APS. However, beyond the thrombotic manifestations of this clinical entity, several other neuropsychiatric manifestations have been described in these patients, including migraines, cognitive dysfunction, seizures, transverse myelitis, multiple sclerosis-like symptoms, and psychiatric symptoms [39]. Notably, cognitive impairment in these patients has been described as a result of complex interactions between the coagulation cascade, immune dysregulation, and endothelial dysfunction [40]. Rosa et al. highlighted that diminished levels of brain-derived neurotrophic factor were correlated with cognitive dysfunction in patients with primary APS (p = 0.032) [42]. Other rare cerebrovascular manifestations of APS include the dissection of the cerebral artery, mainly in young adults [43].
Some limitations should be recognized in our research design. Firstly, it was a retrospective study, while the follow-up data were available only for some APS patients. A prospective design would have elucidated the potential biases of a retrospective study. Moreover, it was a monocenter study from a thrombosis and hemostasis referral center in Northern Greece. The study population was relatively small, and control and stroke groups were not age- and gender-matched, thus influencing statistical analysis findings. Multicenter collaboration and studies with a larger number of participants are essential in the field. Another limitation of our study includes the lack of significant cardiovascular disease and venous thromboembolism characteristics of the patients, given its retrospective design, which are incorporated in the new APS criteria [8]. Finally, our study included patients with secondary APS (SLE), given that the underlying autoimmune disease itself might contribute independently to ischemic stroke development.
5. Conclusions
In this retrospective monocenter study, acute ischemic stroke was identified as a common clinical manifestation of APS. Factors including age, livedo reticularis, AVN, arterial hypertension, aGAPSS, and dyslipidemia were associated with stroke development. Multicenter collaborative studies can be helpful towards a better understanding of APS-related stroke epidemiology and risk factors. Moreover, the role of endothelial dysfunction, microvascular inflammation, and complement activation and association of these factors with not only thrombotic risk in APS but also with other complications, such as cognitive impairment, should be further evaluated.
Author Contributions
Conceptualization, P.E. and E.G. (Eleni Gavriilaki); methodology, P.K., Z.N., T.P., S.C., A.S., S.K. and E.G. (Elisavet Grouzi); formal analysis, P.E.; investigation, N.K.; data curation, P.E. and N.K.; writing—original draft preparation, P.E.; writing—review and editing, N.K., M.D., S.V. and E.G. (Eleni Gavriilaki); visualization, E.G. (Eleni Gavriilaki); supervision, E.G. (Eleni Gavriilaki); project administration, E.G. (Eleni Gavriilaki). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board of Hippocration General Hospital (Greece 585/25.7.2023).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| aGAPSS | Adjusted global antiphospholipid syndrome |
| aGAPSS-IS | aGAPSS–ischemic stroke |
| aβ2GPI | Anti-b2 glycoprotein-I antibody |
| aCL | Anticardiolipin antibody |
| ANA | Antinuclear antibodies |
| aPL | Antiphospholipid antibodies |
| APS | Antiphospholipid syndrome |
| AVN | Avascular necrosis |
| CT | Computed tomography |
| CI | Confidence interval |
| DOAC | Direct oral anticoagulant |
| FII | Factor II |
| FV | Factor V |
| HCQ | Hydroxychloroquine |
| IQR | Interquartile ratio |
| LA | Lupus anticoagulant |
| MRI | Magnetic resonance imaging |
| MTHFR | Methylenetetrahydrofolate reductase |
| OR | Odds ratio |
| SD | Standard deviation |
References
- Garcia, D.; Erkan, D. Diagnosis and Management of the Antiphospholipid Syndrome. N. Engl. J. Med. 2018, 378, 2010–2021. [Google Scholar] [CrossRef] [PubMed]
- Arachchillage, D.J.; Platton, S.; Hickey, K.; Chu, J.; Pickering, M.; Sommerville, P.; MacCallum, P.; Breen, K. Guidelines on the Investigation and Management of Antiphospholipid Syndrome. Br. J. Haematol. 2024, 205, 855–880. [Google Scholar] [CrossRef] [PubMed]
- Patriarcheas, V.; Tsamos, G.; Vasdeki, D.; Kotteas, E.; Kollias, A.; Nikas, D.; Kaiafa, G.; Dimakakos, E. Antiphospholipid Syndrome: A Comprehensive Clinical Review. J. Clin. Med. 2025, 14, 733. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Branch, D.W.; Ortel, T.L. Antiphospholipid Syndrome: Advances in Diagnosis, Pathogenesis, and Management. BMJ 2023, 380, e069717. [Google Scholar] [CrossRef]
- Celia, A.I.; Galli, M.; Mancuso, S.; Alessandri, C.; Frati, G.; Sciarretta, S.; Conti, F. Antiphospholipid Syndrome: Insights into Molecular Mechanisms and Clinical Manifestations. J. Clin. Med. 2024, 13, 4191. [Google Scholar] [CrossRef]
- Evangelidis, P.; Gavriilaki, E.; Kotsiou, N.; Ntova, Z.; Kalmoukos, P.; Papadopoulou, T.; Chissan, S.; Vakalopoulou, S. Avascular Necrosis of the Femoral Head in Patients with Antiphospholipid Syndrome: A Case Series. Hematol. Rep. 2025, 17, 15. [Google Scholar] [CrossRef]
- Miyakis, S.; Lockshin, M.D.; Atsumi, T.; Branch, D.W.; Brey, R.L.; Cervera, R.; Derksen, R.H.W.M.; De Groot, P.G.; Koike, T.; Meroni, P.L.; et al. International Consensus Statement on an Update of the Classification Criteria for Definite Antiphospholipid Syndrome (APS). J. Thromb. Haemost. 2006, 4, 295–306. [Google Scholar] [CrossRef]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.-C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. 2023 ACR/EULAR Antiphospholipid Syndrome Classification Criteria. Ann. Rheum. Dis. 2023, 82, 1258–1270. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z.; Cervera, R.; Costedoat-Chalumeau, N.; Cuadrado, M.J.; Dörner, T.; Ferrer-Oliveras, R.; Hambly, K.; et al. EULAR Recommendations for the Management of Antiphospholipid Syndrome in Adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Anagnostopoulos, A.; Mastellos, D.C. Complement in Thrombotic Microangiopathies: Unraveling Ariadne’s Thread Into the Labyrinth of Complement Therapeutics. Front. Immunol. 2019, 10, 337. [Google Scholar] [CrossRef]
- Venturelli, V.; Maranini, B.; Tohidi-Esfahani, I.; Isenberg, D.A.; Cohen, H.; Efthymiou, M. Can Complement Activation Be the Missing Link in Antiphospholipid Syndrome? Rheumatology 2024, 63, 3243–3254. [Google Scholar] [CrossRef] [PubMed]
- Mezhov, V.; Segan, J.D.; Tran, H.; Cicuttini, F.M. Antiphospholipid Syndrome: A Clinical Review. Med. J. Aust. 2019, 211, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Berkman, S.A.; Song, S.S. Ischemic Stroke in the Young. Clin. Appl. Thromb./Hemost. 2021, 27, 107602962110022. [Google Scholar] [CrossRef]
- Mayer, M.; Cerovec, M.; Radoš, M.; Čikeš, N. Antiphospholipid Syndrome and Central Nervous System. Clin. Neurol. Neurosurg. 2010, 112, 602–608. [Google Scholar] [CrossRef]
- Wisløff, F.; Jacobsen, E.M.; Liestøl, S. Laboratory Diagnosis of the Antiphospholipid Syndrome. Thromb. Res. 2002, 108, 263–271. [Google Scholar] [CrossRef]
- Radin, M.; Sciascia, S.; Erkan, D.; Pengo, V.; Tektonidou, M.G.; Ugarte, A.; Meroni, P.; Ji, L.; Belmont, H.M.; Cohen, H.; et al. The Adjusted Global Antiphospholipid Syndrome Score (AGAPSS) and the Risk of Recurrent Thrombosis: Results from the APS ACTION Cohort. Semin. Arthritis Rheum. 2019, 49, 464–468. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Y.; Zhang, S.; Song, X.; Liu, Z.; Tu, W.; Li, C. Stroke and Risk Factors in Antiphospholipid Syndrome. J. Pers. Med. 2023, 14, 24. [Google Scholar] [CrossRef]
- Liu, M.; Li, G.; Song, X.; Fan, Y.; Li, C. Prevalence, Risk Factors, and Prognosis of Central Nervous System Manifestations in Antiphospholipid Syndrome. Sci. Rep. 2023, 13, 8915. [Google Scholar] [CrossRef] [PubMed]
- Belani, P.; Schefflein, J.; Kihira, S.; Rigney, B.; Delman, B.N.; Mahmoudi, K.; Mocco, J.; Majidi, S.; Yeckley, J.; Aggarwal, A.; et al. COVID-19 Is an Independent Risk Factor for Acute Ischemic Stroke. AJNR Am. J. Neuroradiol. 2020, 41, 1361–1364. [Google Scholar] [CrossRef]
- Mahroum, N.; Habra, M.; Alrifaai, M.A.; Shoenfeld, Y. Antiphospholipid Syndrome in the Era of COVID-19—Two Sides of a Coin. Autoimmun. Rev. 2024, 23, 103543. [Google Scholar] [CrossRef]
- Pujolar, G.; Oliver-Anglès, A.; Vargas, I.; Vázquez, M.-L. Changes in Access to Health Services during the COVID-19 Pandemic: A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 1749. [Google Scholar] [CrossRef] [PubMed]
- García-Grimshaw, M.; Posadas-Pinto, D.R.; Jiménez-Ruiz, A.; Valdés-Ferrer, S.I.; Cadena-Fernández, A.; Torres-Ruiz, J.J.; Barrientos-Guerra, J.D.; Amancha-Gabela, M.; Chiquete, E.; Flores-Silva, F.D.; et al. Antiphospholipid Syndrome–Mediated Acute Cerebrovascular Diseases and Long-Term Outcomes. Lupus 2022, 31, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Djokovic, A.; Stojanovich, L.; Stanisavljevic, N.; Banicevic, S.; Smiljanic, D.; Milovanovic, B. Relationship between Cerebrovascular and Valvular Manifestations in a Serbian Cohort of Patients with Antiphospholipid Syndrome. Clin. Exp. Rheumatol. 2018, 36, 850–855. [Google Scholar] [PubMed]
- Urbanski, G.; Yelnik, C.M.; Maillard, H.; Launay, D.; Dubucquoi, S.; Hachulla, E.; Hatron, P.-Y.; Lambert, M. Antiphospholipid Syndrome with Isolated Isotype M Anticardiolipin and/or Anti-B2GPI Antibody Is Associated with Stroke. Stroke 2018, 49, 2770–2772. [Google Scholar] [CrossRef]
- Di Minno, M.N.D.; Scalera, A.; Tufano, A.; Ambrosino, P.; Bettiol, A.; Silvestri, E.; Emmi, G.; Prisco, D. The Association of Adjusted Global AntiphosPholipid Syndrome Score (AGAPSS) with Cardiovascular Disease in Subjects with Antiphospholipid Antibodies. Atherosclerosis 2018, 278, 60–65. [Google Scholar] [CrossRef]
- Radin, M.; Schreiber, K.; Cecchi, I.; Roccatello, D.; Cuadrado, M.J.; Sciascia, S. The Risk of Ischaemic Stroke in Primary Antiphospholipid Syndrome Patients: A Prospective Study. Eur. J. Neurol. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Gašperšič, N.; Zaletel, M.; Kobal, J.; Žigon, P.; Čučnik, S.; Šemrl, S.S.; Tomšič, M.; Ambrožič, A. Stroke and Antiphospholipid Syndrome-Antiphospholipid Antibodies Are a Risk Factor for an Ischemic Cerebrovascular Event. Clin. Rheumatol. 2019, 38, 379–384. [Google Scholar] [CrossRef]
- Saidi, S.; Mahjoub, T.; Almawi, W.Y. Lupus Anticoagulants and Anti-Phospholipid Antibodies as Risk Factors for a First Episode of Ischemic Stroke. J. Thromb. Haemost. 2009, 7, 1075–1080. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, Y.; Bu, X.; Peng, H.; Xu, T.; Wang, A.; Guo, L.; Liu, J.; Zhang, J.; Li, D.; et al. Antiphospholipid Antibodies Predict Post-Stroke Depression after Acute Ischemic Stroke. J. Affect. Disord. 2019, 257, 160–165. [Google Scholar] [CrossRef]
- Mehta, T.; Hussain, M.; Sheth, K.; Ding, Y.; McCullough, L.D. Risk of Hemorrhagic Transformation after Ischemic Stroke in Patients with Antiphospholipid Antibody Syndrome. Neurol. Res. 2017, 39, 477–483. [Google Scholar] [CrossRef]
- Ricarte, I.F.; Dutra, L.A.; Barsottini, O.G.P.; de Souza, A.W.S.; de Andrade, D.C.O.; Mangueira, C.; Silva, G.S. Transcranial Doppler Findings in Antiphospholipid Syndrome. Lupus 2019, 28, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Gavriilaki, E.; Brodsky, R.A. Complementopathies and Precision Medicine. J. Clin. Investig. 2020, 130, 2152–2163. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Braunstein, E.M.; Yuan, X.; Yu, J.; Alexander, A.; Chen, H.; Gavriilaki, E.; Alluri, R.; Streiff, M.B.; Petri, M.; et al. Complement Activity and Complement Regulatory Gene Mutations Are Associated with Thrombosis in APS and CAPS. Blood 2020, 135, 239–251. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Braunstein, E.M.; Brodsky, R.A. Antiphospholipid Syndrome: Complement Activation, Complement Gene Mutations, and Therapeutic Implications. J. Thromb. Haemost. 2021, 19, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Brodsky, R.A.; McCrae, K.R. Complement in the Pathophysiology of the Antiphospholipid Syndrome. Front. Immunol. 2019, 10, 449. [Google Scholar] [CrossRef]
- Gavriilaki, E.; Yuan, X.; Ye, Z.; Ambinder, A.J.; Shanbhag, S.P.; Streiff, M.B.; Kickler, T.S.; Moliterno, A.R.; Sperati, C.J.; Brodsky, R.A. Modified Ham Test for Atypical Hemolytic Uremic Syndrome. Blood 2015, 125, 3637–3646. [Google Scholar] [CrossRef]
- Yuan, X.; Yu, J.; Gerber, G.; Chaturvedi, S.; Cole, M.; Chen, H.; Metjian, A.; Sperati, C.J.; Braunstein, E.M.; Brodsky, R.A. Ex Vivo Assays to Detect Complement Activation in Complementopathies. Clin. Immunol. 2020, 221, 108616. [Google Scholar] [CrossRef]
- Sikorska, M.; Chmiel, J.; Papuga-Szela, E.; Broniatowska, E.; Undas, A. Apixaban Versus Vitamin K Antagonists in Patients with Antiphospholipid Syndrome: A Cohort Study. J. Cardiovasc. Pharmacol. 2024, 84, 36–44. [Google Scholar] [CrossRef]
- Ordi-Ros, J.; Sáez-Comet, L.; Pérez-Conesa, M.; Vidal, X.; Riera-Mestre, A.; Castro-Salomó, A.; Cuquet-Pedragosa, J.; Ortiz-Santamaria, V.; Mauri-Plana, M.; Solé, C.; et al. Rivaroxaban Versus Vitamin K Antagonist in Antiphospholipid Syndrome: A Randomized Noninferiority Trial. Ann. Intern. Med. 2019, 171, 685–694. [Google Scholar] [CrossRef]
- Bala, M.M.; Celinska-Lowenhoff, M.; Szot, W.; Padjas, A.; Kaczmarczyk, M.; Swierz, M.J.; Undas, A. Antiplatelet and Anticoagulant Agents for Secondary Prevention of Stroke and Other Thromboembolic Events in People with Antiphospholipid Syndrome. Cochrane Database Syst. Rev. 2020, 10, CD012169. [Google Scholar] [CrossRef]
- Laureano, M.; Crowther, M.A. Higher-Risk APS: Do We Dare to DOAC? Blood 2018, 132, 1357–1358. [Google Scholar] [CrossRef] [PubMed]
- Rosa, R.F.; Ugolini-Lopes, M.R.; Gandara, A.P.R.; Vendramini, M.B.G.; Campanholo, K.R.; Dutra, L.; de Andrade, D.C.O. Cognitive Dysfunction and Serum Levels of Brain-Derived Neurotrophic Factor (BDNF) in Primary Anti-Phospholipid Syndrome (PAPS). Rheumatology 2021, 60, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Iseki, T.; Yamashita, Y.; Ueno, Y.; Hira, K.; Miyamoto, N.; Yamashiro, K.; Tsunemi, T.; Teranishi, K.; Yatomi, K.; Nakajima, S.; et al. Cerebral Artery Dissection Secondary to Antiphospholipid Syndrome: A Report of Two Cases and a Literature Review. Lupus 2021, 30, 118–124. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).