Leaving Nothing Behind: Expanding the Clinical Frontiers of Drug-Coated Balloon Angioplasty in Coronary Artery Disease
Abstract
1. Introduction and Rationale
- Complex Lesion Subsets. Bifurcation side branches, long diffuse segments, and heavily calcified disease often pose increased technical and clinical risks for stenting. DCBs may simplify the procedure and reduce future reintervention complexity [1].
2. Methods
3. Procedural Considerations for Optimal Drug-Coated Balloon Angioplasty
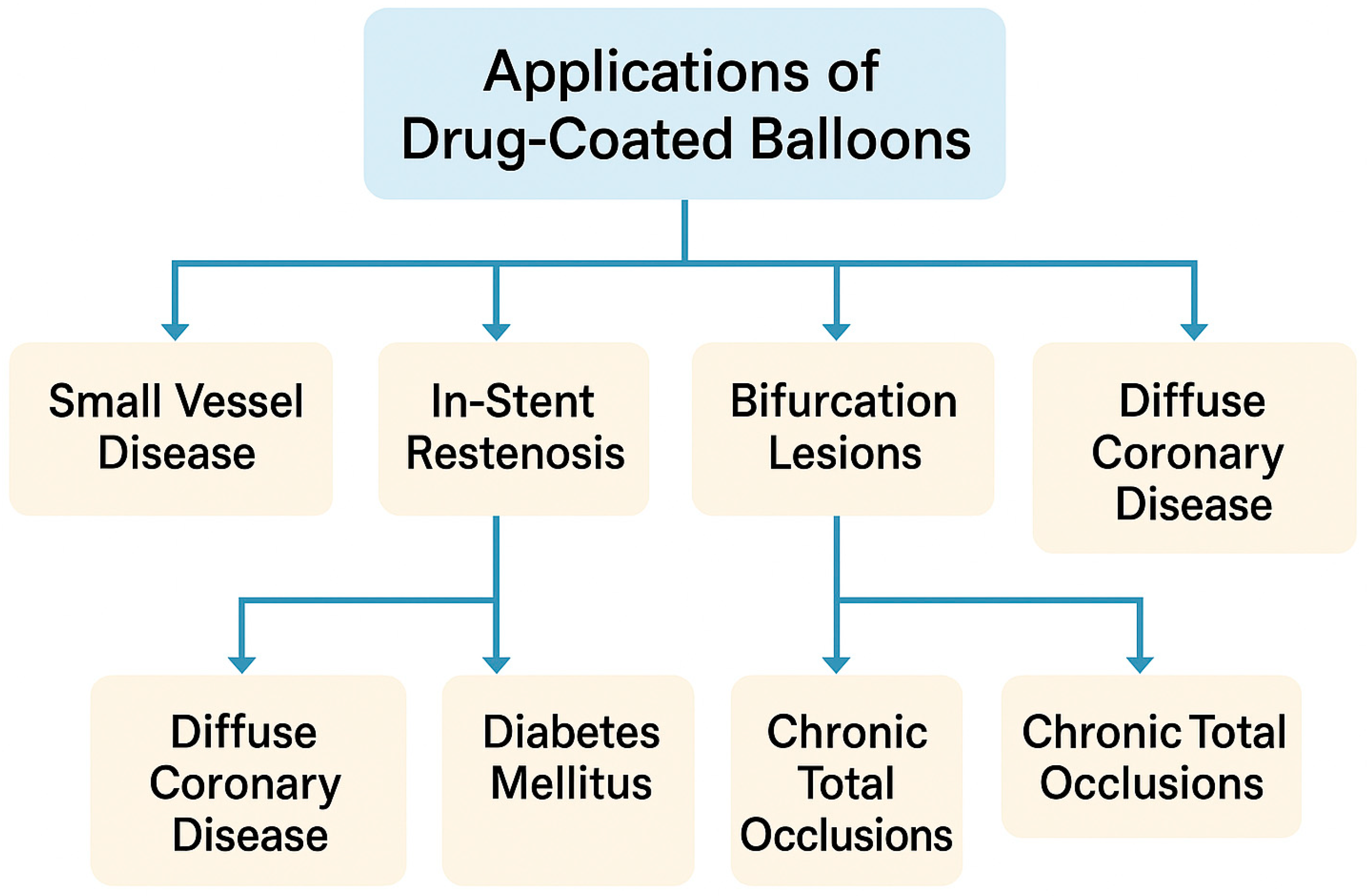
4. Clinical Applications of Drug-Coated Balloons: Lesion-Specific Evidence
4.1. Small-Vessel Disease
4.2. In-Stent Restenosis
4.3. Bifurcation Lesions
4.4. Diffuse Coronary Artery Disease
4.5. Chronic Total Occlusions (CTOs)
| Lesion Type | Study | Design | No. of Patients | Primary Endpoint | Follow-Up | Main Findings |
|---|---|---|---|---|---|---|
| Small-vessel disease | Basket Small-2 [15,18] | RCT | 758 | MACEs (CV death, MI, TVR) | 12 months | DCB non-inferior to DES (7.5% vs. 7.3%, p = 0.918) |
| Piccoleto Ii [8] | RCT | 232 | LLL, MACEs | 12 months | Superior to DES (0.04 vs. 0.17 mm, p = 0.03) | |
| Restore Svd China [19] | RCT | 230 | Diameter stenosis, TLF | 24 months | DCB and DES comparable | |
| In-stent restenosis | Ribs Iv [22] | RCT | 309 | In-segment LLL, TLR, MACEs | 36 months | Superior to POBA, comparable to DES |
| Isar-Desire 3 [21] | RCT | 402 | Diameter stenosis (%), TLR, MI | 36 months | Comparable to DES, superior to POBA | |
| Bifurcation lesions | Pepcad-Bif [32] | RCT | 64 | Angiographic LLL, TLR | 9 months | DCB superior vs. POBA (0.13 vs. 0.51 mm, p = 0.013) |
| Debside [33] | Observational | 52 | TLR | 12 months | Effective side-branch treatment, TLR 7.7% | |
| Hyper [34] | Observational | 210 | MACEs (CV death, MI), TLR | 12 months | Hybrid strategy effective, low TLR (5.5%) | |
| Diffuse coronary lesions | Spartan [39] | Observational | 1517 | All-cause mortality | NR | Similar mortality to DES |
| Leone et al. [40] | Observational | 93 | MACEs (CV death, MI), TLR | 12 months | High procedural success, low TLR (5.8%) | |
| Costopoulos et al. [38] | Observational | 212 | MACEs (CV death, MI), TLR | 12 months | Outcomes comparable to DES, less stenting | |
| Xu et al. [41] | Observational | 109 | MACEs, TLR | 12 months | Hybrid approach effective, low TLR | |
| Chronic total occlusions | Koln et al. [43] | Observational | 34 | MACEs (CV death, MI), TLR | 9 months | Safe, low MACE rate (11.8%), feasible |
| Wang et al. [44] | RCT | 591 | Angiographic LLL, MACEs | 3 years | Less LLL in DCB, comparable restenosis and MACEs |
5. Special Populations and Clinical Settings
5.1. High Bleeding Risk (HBR)
5.2. Acute Coronary Syndromes (ACSs)
5.3. Diabetes Mellitus
| Clinical Setting | Study | Design | No. of Patients | Primary Endpoint | Follow-Up | Main Findings |
|---|---|---|---|---|---|---|
| High bleeding risk | Debut [11] | RCT | 208 | MACEs (CV death, MI, TLR) | 12 months | DCB superior to BMS (1% vs. 14%, p < 0.001) |
| Panelux [12] | Observational | 432 | TLF, CV death, MI, TLR | 12 months | Low TLF (5.6%), safe short DAPT duration (median 33 days) | |
| Ultimate Iii [48] | RCT | 448 | In-segment LLL | 7 month | IVUS-guided DCB superior to angiography-guided | |
| Uskela et al. [47] | Observational | 301 | MACEs, BARC 2–5 bleeding | 12 months | Low TLR (1.4–2.8%), acceptable bleeding rate (5.9%) | |
| Rasanen et al. [10] | Observational | 114 | TLR, bleeding | 12 months | Low TLR (0–3%), acceptable bleeding with SAPT (10.5%) | |
| Diabetes mellitus | BASKET-SMALL 2 (DM Substudy) [56] | RCT (subanalysis) | 263 | MACEs (CV death, MI, TLR) | 12 months | DCB comparable vs. DES (10.1% vs. 13.7%, p = 0.52) |
| PICCOLETO II (DM Substudy) [57] | RCT (subanalysis) | 232 | MACEs, TLR | 36 month | Low TLR rates similar to DES, sustained benefit | |
| RESTORE SVD China (DM Substudy) [19] | RCT (subanalysis) | 230 | In-segment LLL, TLR | 9 months | Comparable outcomes for DCB vs. DES (12% vs. 11.1%) | |
| EASTBOURNE (DM Substudy) [58] | Observational | 424 | MACEs, TLR | 12 months | Low TLR (3.6%), favorable MACE (6.5%) | |
| EASTBOURNE-BIF (DM Substudy) [60] | Observational | 210 | MACEs, TLR | 12 months | Effective in bifurcation lesions, low TLR (4.5%) | |
| Acute Coronary Syndrome (NSTEMI) | Pepcad Nstemi [51] | RCT | 210 | LLL | 9 months | DCB non-inferior to DES |
| Besic et al. [52] | Observational | 120 | Restenosis rate, TLR | 12 months | Lower TLR (4.5% DCB + BMS vs. 7.9% BMS alone, p = 0.29) | |
| Acute Coronary syndrome (STEMI) | Deb-Ami [50] | RCT | 150 | LLL | 6 months | DES superior angiographically, similar clinical outcomes |
| Pappa [53] | Observational | 100 | MACEs (CV death, MI) | 6 months | Feasible DCB-only STEMI, low MACE rate (5%) | |
| Merinopoulos et al. [54] | Observational | 1139 | TLR, MACEs | 12 months | Comparable TLR and MACEs for DCB vs. DES (7.0% vs. 6.8%) |
6. Future Perspectives and Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACS | Acute Coronary Syndromes |
| BMS | Bare-Metal Stent |
| CAD | Coronary Artery Disease |
| CTO | Chronic Total Occlusion |
| DAPT | Dual Antiplatelet Therapy |
| DCB | Drug-Coated Balloon |
| DEB | Drug-Eluting Balloon |
| DES | Drug-Eluting Stent |
| DM | Diabetes Mellitus |
| HBR | High Bleeding Risk |
| ISR | In-Stent Restenosis |
| IVUS | Intravascular Ultrasound |
| LLL | Late Lumen Loss |
| MACE | Major Adverse Cardiovascular Event |
| NSTEMI | Non-ST Elevated Myocardial Infarction |
| NCB | Non-compliant Balloon |
| OCT | Optical Coherence Tomography |
| PCB | Paclitaxel-Coated Balloon |
| PCI | Percutaneous Coronary Intervention |
| RCT | Randomized Controlled Trial |
| SAPT | Single Antiplatelet Therapy |
| SCB | Sirolimus-Coated Balloon |
| STEMI | ST-Elevated Myocardial Infarction |
| SVD | Small-Vessel Disease |
| TLF | Target Lesion Failure |
| TLR | Target Lesion Revascularization |
| TVR | Target Vessel Revascularization |
References
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on Myocardial Revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [PubMed]
- Urban, P.; Meredith, I.T.; Abizaid, A.; Pocock, S.J.; Carrie, D.; Naber, C.; Lipiecki, J.; Richardt, G.; Iniguez, A.; Brunel, P.; et al. Polymer-Free Drug-Coated Coronary Stents in Patients at High Bleeding Risk. N. Engl. J. Med. 2015, 373, 2038–2047. [Google Scholar] [CrossRef]
- Ando, G.; De Santis, G.A.; Greco, A.; Pistelli, L.; Francaviglia, B.; Capodanno, D.; De Caterina, R.; Capranzano, P. P2Y(12) Inhibitor or Aspirin Following Dual Antiplatelet Therapy After Percutaneous Coronary Intervention: A Network Meta-Analysis. JACC Cardiovasc Interv 2022, 15, 2239–2249. [Google Scholar] [CrossRef]
- Scheller, B.; Hehrlein, C.; Bocksch, W.; Rutsch, W.; Haghi, D.; Dietz, U.; Speck, U. Treatment of Coronary In-Stent Restenosis with a Paclitaxel-Coated Balloon Catheter. N. Engl. J. Med. 2006, 355, 2113–2124. [Google Scholar] [CrossRef]
- Kereiakes, D.J. “Leave Nothing Behind”: Strategy of Choice for Small Coronaries? JACC Cardiovasc. Interv. 2020, 13, 2850–2852. [Google Scholar] [CrossRef] [PubMed]
- Rittger, H.; Brachmann, J.; Sinha, A.M.; Waliszewski, M.; Ohlow, M.; Brugger, A.; Thiele, H.; Birkemeyer, R.; Kurowski, V.; Breithardt, O.A.; et al. A randomized, multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with plain balloon angioplasty in drug-eluting stent restenosis: The PEPCAD-DES study. J. Am. Coll. Cardiol. 2012, 59, 1377–1382. [Google Scholar] [CrossRef]
- Cannon, L.A.; Simon, D.I.; Kereiakes, D.; Jones, J.; Mehran, R.; Kusano, H.; Zhang, Z.; Lombardi, W.; James Fleischhauer, F.; Costa, M.A. The XIENCE nano everolimus eluting coronary stent system for the treatment of small coronary arteries: The SPIRIT Small Vessel trial. Catheter. Cardiovasc. Interv. 2012, 80, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Di Palma, G.; Guimaraes, M.G.; Piraino, D.; Orrego, P.S.; Buccheri, D.; Rivero, F.; Perotto, A.; Zambelli, G.; Alfonso, F. Drug-Coated Balloon Versus Drug-Eluting Stent for Small Coronary Vessel Disease: PICCOLETO II Randomized Clinical Trial. JACC Cardiovasc. Interv. 2020, 13, 2840–2849. [Google Scholar] [CrossRef]
- Fezzi, S.; Giacoppo, D.; Fahrni, G.; Latib, A.; Alfonso, F.; Colombo, A.; Mahfoud, F.; Scheller, B.; Jeger, R.; Cortese, B. Individual patient data meta-analysis of paclitaxel-coated balloons vs. drug-eluting stents for small-vessel coronary artery disease: The ANDROMEDA study. Eur Heart J 2025, 46, 1586–1599. [Google Scholar] [CrossRef]
- Rasanen, A.; Karkkainen, J.M.; Eranti, A.; Eranen, J.; Rissanen, T.T. Percutaneous coronary intervention with drug-coated balloon-only strategy combined with single antiplatelet treatment in patients at high bleeding risk: Single center experience of a novel concept. Catheter. Cardiovasc. Interv. 2023, 101, 569–578. [Google Scholar] [CrossRef]
- Rissanen, T.T.; Uskela, S.; Eranen, J.; Mantyla, P.; Olli, A.; Romppanen, H.; Siljander, A.; Pietila, M.; Minkkinen, M.J.; Tervo, J.; et al. Drug-coated balloon for treatment of de-novo coronary artery lesions in patients with high bleeding risk (DEBUT): A single-blind, randomised, non-inferiority trial. Lancet 2019, 394, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Roncalli, J.; Godin, M.; Boughalem, K.; Shayne, J.; Piot, C.; Huret, B.; Belle, L.; Cayla, G.; Faurie, B.; Amor, M.; et al. Paclitaxel Drug-Coated Balloon After Bare-Metal Stent Implantation, an Alternative Treatment to Drug-Eluting Stent in High Bleeding Risk Patients (The Panelux Trial). J. Invasive Cardiol. 2019, 31, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Kereiakes, D.J.; Smits, P.C.; Kedhi, E.; Parise, H.; Fahy, M.; Serruys, P.W.; Stone, G.W. Predictors of death or myocardial infarction, ischaemic-driven revascularisation, and major adverse cardiovascular events following everolimus-eluting or paclitaxel-eluting stent deployment: Pooled analysis from the SPIRIT II, III, IV and COMPARE trials. EuroIntervention 2011, 7, 74–83. [Google Scholar] [CrossRef]
- van der Heijden, L.C.; Kok, M.M.; Danse, P.W.; Schramm, A.R.; Hartmann, M.; Lowik, M.M.; Linssen, G.C.; Stoel, M.G.; Doggen, C.J.; von Birgelen, C. Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: Insights from 2-year DUTCH PEERS (TWENTE II) randomized trial. Am. Heart J. 2016, 176, 28–35. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Mobius-Winkler, S.; Leibundgut, G.; Weilenmann, D.; Wohrle, J.; Richter, S.; Schreiber, M.; et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): An open-label randomised non-inferiority trial. Lancet 2018, 392, 849–856. [Google Scholar] [CrossRef]
- Unverdorben, M.; Kleber, F.X.; Heuer, H.; Figulla, H.R.; Vallbracht, C.; Leschke, M.; Cremers, B.; Hardt, S.; Buerke, M.; Ackermann, H.; et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter in the PEPCAD I study: Are lesions clinically stable from 12 to 36 months? EuroIntervention 2013, 9, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Latib, A.; Colombo, A.; Castriota, F.; Micari, A.; Cremonesi, A.; De Felice, F.; Marchese, A.; Tespili, M.; Presbitero, P.; Sgueglia, G.A.; et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: The BELLO (Balloon Elution and Late Loss Optimization) study. J. Am. Coll. Cardiol. 2012, 60, 2473–2480. [Google Scholar] [CrossRef]
- Jeger, R.V.; Farah, A.; Ohlow, M.A.; Mangner, N.; Mobius-Winkler, S.; Weilenmann, D.; Wohrle, J.; Stachel, G.; Markovic, S.; Leibundgut, G.; et al. Long-term efficacy and safety of drug-coated balloons versus drug-eluting stents for small coronary artery disease (BASKET-SMALL 2): 3-year follow-up of a randomised, non-inferiority trial. Lancet 2020, 396, 1504–1510. [Google Scholar] [CrossRef]
- Tang, Y.; Qiao, S.; Su, X.; Chen, Y.; Jin, Z.; Chen, H.; Xu, B.; Kong, X.; Pang, W.; Liu, Y.; et al. Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease: The RESTORE SVD China Randomized Trial. JACC Cardiovasc. Interv. 2018, 11, 2381–2392. [Google Scholar] [CrossRef]
- Byrne, R.A.; Joner, M.; Kastrati, A. Stent thrombosis and restenosis: What have we learned and where are we going? The Andreas Gruntzig Lecture ESC 2014. Eur. Heart J. 2015, 36, 3320–3331. [Google Scholar] [CrossRef]
- Byrne, R.A.; Neumann, F.J.; Mehilli, J.; Pinieck, S.; Wolff, B.; Tiroch, K.; Schulz, S.; Fusaro, M.; Ott, I.; Ibrahim, T.; et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): A randomised, open-label trial. Lancet 2013, 381, 461–467. [Google Scholar] [CrossRef]
- Alfonso, F.; Perez-Vizcayno, M.J.; Cardenas, A.; Garcia del Blanco, B.; Garcia-Touchard, A.; Lopez-Minguez, J.R.; Benedicto, A.; Masotti, M.; Zueco, J.; Iniguez, A.; et al. A Prospective Randomized Trial of Drug-Eluting Balloons Versus Everolimus-Eluting Stents in Patients With In-Stent Restenosis of Drug-Eluting Stents: The RIBS IV Randomized Clinical Trial. J. Am. Coll. Cardiol. 2015, 66, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Gao, R.; Wang, J.; Yang, Y.; Chen, S.; Liu, B.; Chen, F.; Li, Z.; Han, Y.; Fu, G.; et al. A prospective, multicenter, randomized trial of paclitaxel-coated balloon versus paclitaxel-eluting stent for the treatment of drug-eluting stent in-stent restenosis: Results from the PEPCAD China ISR trial. JACC Cardiovasc. Interv. 2014, 7, 204–211. [Google Scholar] [CrossRef]
- Yeh, R.W.; Shlofmitz, R.; Moses, J.; Bachinsky, W.; Dohad, S.; Rudick, S.; Stoler, R.; Jefferson, B.K.; Nicholson, W.; Altman, J.; et al. Paclitaxel-Coated Balloon vs Uncoated Balloon for Coronary In-Stent Restenosis: The AGENT IDE Randomized Clinical Trial. JAMA 2024, 331, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Verheye, S.; Vrolix, M.; Kumsars, I.; Erglis, A.; Sondore, D.; Agostoni, P.; Cornelis, K.; Janssens, L.; Maeng, M.; Slagboom, T.; et al. The SABRE Trial (Sirolimus Angioplasty Balloon for Coronary In-Stent Restenosis): Angiographic Results and 1-Year Clinical Outcomes. JACC Cardiovasc. Interv. 2017, 10, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Rissanen, T.T.; Farah, A.; Ohlow, M.A.; Mangner, N.; Wohrle, J.; Mobius-Winkler, S.; Weilenmann, D.; Leibundgut, G.; Cuculi, F.; et al. Drug-Coated Balloon for Small Coronary Artery Disease in Patients With and Without High-Bleeding Risk in the BASKET-SMALL 2 Trial. Circ. Cardiovasc. Interv. 2022, 15, e011569. [Google Scholar] [CrossRef]
- Scheller, B.; Mangner, N.; Abdul Kader, M.; Wan Ahmad, W.A.; Jeger, R.; Wohrle, J.; Ong, T.K.; Liew, H.B.; Gori, T.; Mahfoud, F.; et al. Combined Analysis of Two Parallel Randomized Trials of Sirolimus-Coated and Paclitaxel-Coated Balloons in Coronary In-Stent Restenosis Lesions. Circ. Cardiovasc. Interv. 2022, 15, e012305. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.J.; Richardt, G.; Tolg, R.; Erglis, A.; Skurk, C.; Jung, W.; Neumann, F.J.; Stangl, K.; Brachmann, J.; Fischer, D.; et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: The BIOLUX randomised controlled trial. EuroIntervention 2018, 14, 1096–1103. [Google Scholar] [CrossRef]
- De Filippo, O.; Wanha, W.; Sanavia, T.; Januszek, R.; Giacobbe, F.; Campo, G.; Pinxterhuis, T.H.; Capodanno, D.; Tomasiewicz, B.; Iannaccone, M.; et al. Treatment of in-stent restenosis with ultrathin-strut versus thin-strut drug-eluting stents or drug-eluting balloons: A multicentre registry. EuroIntervention 2024, 20, e1340–e1354. [Google Scholar]
- Wanha, W.; D’Ascenzo, F.; Kuzma, L.; Januszek, R.; Iwanczyk, S.; Tomasiewicz, B.; Brzozowski, P.; Niezgoda, P.; Kowalewski, M.; Jaguszewski, M.; et al. Long-term outcomes following paclitaxel-coated balloons versus thin-strut drug-eluting stents for treatment of in-stent restenosis in chronic coronary syndrome (CCS Dragon-Registry). Kardiol. Pol. 2024, 82, 749–759. [Google Scholar] [CrossRef]
- Lefevre, T.; Louvard, Y.; Morice, M.C.; Dumas, P.; Loubeyre, C.; Benslimane, A.; Premchand, R.K.; Guillard, N.; Piechaud, J.F. Stenting of bifurcation lesions: Classification, treatments, and results. Catheter. Cardiovasc. Interv. 2000, 49, 274–283. [Google Scholar] [CrossRef]
- Kleber, F.X.; Rittger, H.; Ludwig, J.; Schulz, A.; Mathey, D.G.; Boxberger, M.; Degenhardt, R.; Scheller, B.; Strasser, R.H. Drug eluting balloons as stand alone procedure for coronary bifurcational lesions: Results of the randomized multicenter PEPCAD-BIF trial. Clin. Res. Cardiol. 2016, 105, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Berland, J.; Lefevre, T.; Brenot, P.; Fajadet, J.; Motreff, P.; Guerin, P.; Dupouy, P.; Schandrin, C.; DEBSIDE trial investigators. DANUBIO—A new drug-eluting balloon for the treatment of side branches in bifurcation lesions: Six-month angiographic follow-up results of the DEBSIDE trial. EuroIntervention 2015, 11, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Buono, A.; Pellicano, M.; Regazzoli, D.; Donahue, M.; Tedeschi, D.; Loffi, M.; Zimbardo, G.; Reimers, B.; Danzi, G.; DE Blasio, G.; et al. Procedural and one-year outcomes following drug-eluting stent and drug-coated balloon combination for the treatment of de novo diffuse coronary artery disease: The HYPER Study. Minerva Cardiol. Angiol. 2024, 72, 163–171. [Google Scholar] [CrossRef]
- Kong, M.G.; Han, J.K.; Kang, J.H.; Zheng, C.; Yang, H.M.; Park, K.W.; Kang, H.J.; Koo, B.K.; Chae, I.H.; Kim, H.S.; et al. Clinical outcomes of long stenting in the drug-eluting stent era: Patient-level pooled analysis from the GRAND-DES registry. EuroIntervention 2021, 16, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Sharp, A.S.; Latib, A.; Ielasi, A.; Larosa, C.; Godino, C.; Saolini, M.; Magni, V.; Gerber, R.T.; Montorfano, M.; Carlino, M.; et al. Long-term follow-up on a large cohort of “full-metal jacket” percutaneous coronary intervention procedures. Circ. Cardiovasc. Interv. 2009, 2, 416–422. [Google Scholar] [CrossRef]
- Giustino, G.; Colombo, A.; Camaj, A.; Yasumura, K.; Mehran, R.; Stone, G.W.; Kini, A.; Sharma, S.K. Coronary In-Stent Restenosis: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 80, 348–372. [Google Scholar] [CrossRef]
- Costopoulos, C.; Latib, A.; Naganuma, T.; Sticchi, A.; Figini, F.; Basavarajaiah, S.; Carlino, M.; Chieffo, A.; Montorfano, M.; Naim, C.; et al. The role of drug-eluting balloons alone or in combination with drug-eluting stents in the treatment of de novo diffuse coronary disease. JACC Cardiovasc. Interv. 2013, 6, 1153–1159. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Wickramarachchi, U.; Richardson, P.; Maart, C.; Sreekumar, S.; Sawh, C.; Wistow, T.; Sarev, T.; Ryding, A.; et al. Long-term safety of paclitaxel drug-coated balloon-only angioplasty for de novo coronary artery disease: The SPARTAN DCB study. Clin. Res. Cardiol. 2021, 110, 220–227. [Google Scholar] [CrossRef]
- Leone, P.P.; Oliva, A.; Regazzoli, D.; Gitto, M.; Novelli, L.; Cozzi, O.; Stefanini, G.G.; Rossi, M.L.; Sticchi, A.; Tartaglia, F.; et al. Immediate and follow-up outcomes of drug-coated balloon angioplasty in de novo long lesions on large coronary arteries. EuroIntervention 2023, 19, e923–e925. [Google Scholar] [CrossRef]
- Xu, H.; Qiao, S.; Cui, J.; Yuan, J.; Yang, W.; Liu, R.; Wang, T.; Guan, H.; Tian, T.; Zhu, F.; et al. Drug-eluting stent and drug-coated balloon for the treatment of de novo diffuse coronary artery disease lesions: A retrospective case series study. Clin. Cardiol. 2023, 46, 1511–1518. [Google Scholar] [CrossRef]
- Brilakis, E.S.; Banerjee, S.; Karmpaliotis, D.; Lombardi, W.L.; Tsai, T.T.; Shunk, K.A.; Kennedy, K.F.; Spertus, J.A.; Holmes, D.R., Jr.; Grantham, J.A. Procedural outcomes of chronic total occlusion percutaneous coronary intervention: A report from the NCDR (National Cardiovascular Data Registry). JACC Cardiovasc. Interv. 2015, 8, 245–253. [Google Scholar] [CrossRef]
- Koln, P.J.; Scheller, B.; Liew, H.B.; Rissanen, T.T.; Ahmad, W.A.; Weser, R.; Hauschild, T.; Nuruddin, A.A.; Clever, Y.P.; Ho, H.H.; et al. Treatment of chronic total occlusions in native coronary arteries by drug-coated balloons without stenting—A feasibility and safety study. Int. J. Cardiol. 2016, 225, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, X.; Lu, W.; Pan, L.; Han, Z.; Pan, S.; Shan, Y.; Wang, X.; Zheng, X.; Li, R.; et al. Long-term outcomes of less drug-eluting stents by the use of drug-coated balloons in de novo coronary chronic total occlusion intervention: A multicenter observational study. Front. Cardiovasc. Med. 2023, 10, 1045859. [Google Scholar] [CrossRef]
- Natarajan, R.; Corballis, N.; Merinopoulos, I.; Tsampasian, V.; Vassiliou, V.S.; Eccleshall, S.C. A systematic review and meta-analysis of the use of drug-coated balloon angioplasty for treatment of both de novo and in-stent coronary chronic total occlusions. Clin. Res. Cardiol. 2025. [CrossRef] [PubMed]
- Gargiulo, G.; Carrara, G.; Frigoli, E.; Leonardi, S.; Vranckx, P.; Campo, G.; Varbella, F.; Calabro, P.; Zaro, T.; Bartolini, D.; et al. Post-Procedural Bivalirudin Infusion at Full or Low Regimen in Patients With Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2019, 73, 758–774. [Google Scholar] [CrossRef] [PubMed]
- Uskela, S.; Karkkainen, J.M.; Eranen, J.; Siljander, A.; Mantyla, P.; Mustonen, J.; Rissanen, T.T. Percutaneous coronary intervention with drug-coated balloon-only strategy in stable coronary artery disease and in acute coronary syndromes: An all-comers registry study. Catheter. Cardiovasc. Interv. 2019, 93, 893–900. [Google Scholar] [CrossRef]
- Gao, X.F.; Ge, Z.; Kong, X.Q.; Chen, X.; Han, L.; Qian, X.S.; Zuo, G.F.; Wang, Z.M.; Wang, J.; Song, J.X.; et al. Intravascular Ultrasound vs Angiography-Guided Drug-Coated Balloon Angioplasty: The ULTIMATE III Trial. JACC Cardiovasc. Interv. 2024, 17, 1519–1528. [Google Scholar] [CrossRef]
- Gragnano, F.; Branca, M.; Frigoli, E.; Leonardi, S.; Vranckx, P.; Di Maio, D.; Monda, E.; Fimiani, L.; Fioretti, V.; Chianese, S.; et al. Access-Site Crossover in Patients With Acute Coronary Syndrome Undergoing Invasive Management. JACC Cardiovasc. Interv. 2021, 14, 361–373. [Google Scholar] [CrossRef]
- Belkacemi, A.; Agostoni, P.; Nathoe, H.M.; Voskuil, M.; Shao, C.; Van Belle, E.; Wildbergh, T.; Politi, L.; Doevendans, P.A.; Sangiorgi, G.M.; et al. First results of the DEB-AMI (drug eluting balloon in acute ST-segment elevation myocardial infarction) trial: A multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in primary percutaneous coronary intervention with 6-month angiographic, intravascular, functional, and clinical outcomes. J. Am. Coll. Cardiol. 2012, 59, 2327–2337. [Google Scholar] [CrossRef]
- Scheller, B.; Ohlow, M.A.; Ewen, S.; Kische, S.; Rudolph, T.K.; Clever, Y.P.; Wagner, A.; Richter, S.; El-Garhy, M.; Bohm, M.; et al. Bare metal or drug-eluting stent versus drug-coated balloon in non-ST-elevation myocardial infarction: The randomised PEPCAD NSTEMI trial. EuroIntervention 2020, 15, 1527–1533. [Google Scholar] [CrossRef]
- Besic, K.M.; Strozzi, M.; Margetic, E.; Bulum, J.; Kolaric, B. Drug-eluting balloons in patients with non-ST elevation acute coronary syndrome. J. Cardiol. 2015, 65, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Vos, N.S.; van der Schaaf, R.J.; Amoroso, G.; Herrman, J.P.; Patterson, M.S.; Slagboom, T.; Vink, M.A. REVascularization with paclitaxEL-coated balloon angioplasty versus drug-eluting stenting in acute myocardial infarcTION-A randomized controlled trial: Rationale and design of the REVELATION trial. Catheter. Cardiovasc. Interv. 2016, 87, 1213–1221. [Google Scholar] [CrossRef]
- Merinopoulos, I.; Gunawardena, T.; Corballis, N.; Bhalraam, U.; Reinhold, J.; Wickramarachchi, U.; Maart, C.; Gilbert, T.; Richardson, P.; Sulfi, S.; et al. Assessment of Paclitaxel Drug-Coated Balloon Only Angioplasty in STEMI. JACC Cardiovasc. Interv. 2023, 16, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Machecourt, J.; Danchin, N.; Lablanche, J.M.; Fauvel, J.M.; Bonnet, J.L.; Marliere, S.; Foote, A.; Quesada, J.L.; Eltchaninoff, H.; Vanzetto, G.; et al. Risk factors for stent thrombosis after implantation of sirolimus-eluting stents in diabetic and nondiabetic patients: The EVASTENT Matched-Cohort Registry. J. Am. Coll. Cardiol. 2007, 50, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Wohrle, J.; Scheller, B.; Seeger, J.; Farah, A.; Ohlow, M.A.; Mangner, N.; Mobius-Winkler, S.; Weilenmann, D.; Stachel, G.; Leibundgut, G.; et al. Impact of Diabetes on Outcome With Drug-Coated Balloons Versus Drug-Eluting Stents: The BASKET-SMALL 2 Trial. JACC Cardiovasc. Interv. 2021, 14, 1789–1798. [Google Scholar] [CrossRef]
- Cortese, B.; Testa, G.; Rivero, F.; Erriquez, A.; Alfonso, F. Long-Term Outcome of Drug-Coated Balloon vs Drug-Eluting Stent for Small Coronary Vessels: PICCOLETO-II 3-Year Follow-Up. JACC Cardiovasc. Interv. 2023, 16, 1054–1061. [Google Scholar] [CrossRef]
- Caiazzo, G.; Oliva, A.; Testa, L.; Heang, T.M.; Lee, C.Y.; Milazzo, D.; Stefanini, G.; Pesenti, N.; Mangieri, A.; Colombo, A.; et al. Sirolimus-coated balloon in all-comer population of coronary artery disease patients: The EASTBOURNE DIABETES prospective registry. Cardiovasc. Diabetol. 2024, 23, 52. [Google Scholar] [CrossRef]
- Niezgoda, P.; Kasprzak, M.; Kubica, J.; Kuzma, L.; Januszek, R.; Iwanczyk, S.; Tomasiewicz, B.; Bil, J.; Kowalewski, M.; Jaguszewski, M.; et al. Drug-Eluting Balloons and Drug-Eluting Stents in Diabetic Patients Undergoing Percutaneous Coronary Intervention Due to Restenosis-DM-Dragon Registry. J. Clin. Med. 2024, 13, 4464. [Google Scholar] [CrossRef]
- Lazar, F.L.; Prvulovic, D.; Onea, H.L.; Cortese, B. The role of drug-coated balloons for coronary bifurcation management: Results from the prospective EASTBOURNE-BIF study. Minerva Cardiol Angiol 2024, 72, 346–354. [Google Scholar] [CrossRef]
- Buonpane, A.; Trimarchi, G.; Ciardetti, M.; Coceani, M.A.; Alagna, G.; Benedetti, G.; Berti, S.; Ando, G.; Burzotta, F.; De Caterina, A.R. Optical Coherence Tomography in Myocardial Infarction Management: Enhancing Precision in Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 5791. [Google Scholar] [CrossRef] [PubMed]
- Fezzi, S.; Scheller, B.; Cortese, B.; Alfonso, F.; Jeger, R.; Colombo, A.; Joner, M.; Shin, E.S.; Kleber, F.X.; Latib, A.; et al. Definitions and standardized endpoints for the use of drug-coated balloon in coronary artery disease: Consensus document of the Drug Coated Balloon Academic Research Consortium. Eur. Heart J. 2025. [CrossRef]
- Gao, C.; He, X.; Ouyang, F.; Zhang, Z.; Shen, G.; Wu, M.; Yang, P.; Ma, L.; Yang, F.; Ji, Z.; et al. Drug-coated balloon angioplasty with rescue stenting versus intended stenting for the treatment of patients with de novo coronary artery lesions (REC-CAGEFREE I): An open-label, randomised, non-inferiority trial. Lancet 2024, 404, 1040–1050. [Google Scholar] [CrossRef]
- Gurgoglione, F.L.; Gattuso, D.; Greco, A.; Benatti, G.; Niccoli, G.; Cortese, B. Predictors and Long-Term Prognostic Significance of Bailout Stenting During Percutaneous Coronary Interventions with Sirolimus-Coated Balloon: A Subanalysis of the Eastbourne Study. Am. J. Cardiol. 2025, 239, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Mok, K.H.; Wickramarachchi, U.; Watson, T.; Ho, H.H.; Eccleshall, S.; Ong, P.J.L. Safety of bailout stenting after paclitaxel-coated balloon angioplasty. Herz 2017, 42, 684–689. [Google Scholar] [CrossRef]
- Mitomo, S.; Jabbour, R.J.; Mangieri, A.; Ancona, M.; Regazzoli, D.; Tanaka, A.; Giannini, F.; Carlino, M.; Montorfano, M.; Chieffo, A.; et al. Mid-term clinical outcomes after bailout drug-eluting stenting for suboptimal drug-coated balloon results: Insights from a Milan registry. Int. J. Cardiol. 2018, 263, 17–23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetta, M.; Sasso, S.; Paragliola, V.; Maffi, V.; Chiricolo, G.; Massaro, G.; Russo, G.; Benedetto, D.; Muscoli, S.; Colonna, G.; et al. Leaving Nothing Behind: Expanding the Clinical Frontiers of Drug-Coated Balloon Angioplasty in Coronary Artery Disease. J. Cardiovasc. Dev. Dis. 2025, 12, 176. https://doi.org/10.3390/jcdd12050176
Marchetta M, Sasso S, Paragliola V, Maffi V, Chiricolo G, Massaro G, Russo G, Benedetto D, Muscoli S, Colonna G, et al. Leaving Nothing Behind: Expanding the Clinical Frontiers of Drug-Coated Balloon Angioplasty in Coronary Artery Disease. Journal of Cardiovascular Development and Disease. 2025; 12(5):176. https://doi.org/10.3390/jcdd12050176
Chicago/Turabian StyleMarchetta, Marcello, Stefano Sasso, Vincenzo Paragliola, Valerio Maffi, Gaetano Chiricolo, Gianluca Massaro, Giulio Russo, Daniela Benedetto, Saverio Muscoli, Giuseppe Colonna, and et al. 2025. "Leaving Nothing Behind: Expanding the Clinical Frontiers of Drug-Coated Balloon Angioplasty in Coronary Artery Disease" Journal of Cardiovascular Development and Disease 12, no. 5: 176. https://doi.org/10.3390/jcdd12050176
APA StyleMarchetta, M., Sasso, S., Paragliola, V., Maffi, V., Chiricolo, G., Massaro, G., Russo, G., Benedetto, D., Muscoli, S., Colonna, G., Mandurino-Mirizzi, A., Cortese, B., Sangiorgi, G. M., & Andò, G. (2025). Leaving Nothing Behind: Expanding the Clinical Frontiers of Drug-Coated Balloon Angioplasty in Coronary Artery Disease. Journal of Cardiovascular Development and Disease, 12(5), 176. https://doi.org/10.3390/jcdd12050176











