Neuroprotective Strategies in Coronary Artery Disease Interventions
Abstract
1. Mechanism of Cardiac Surgery Related Brain Damage
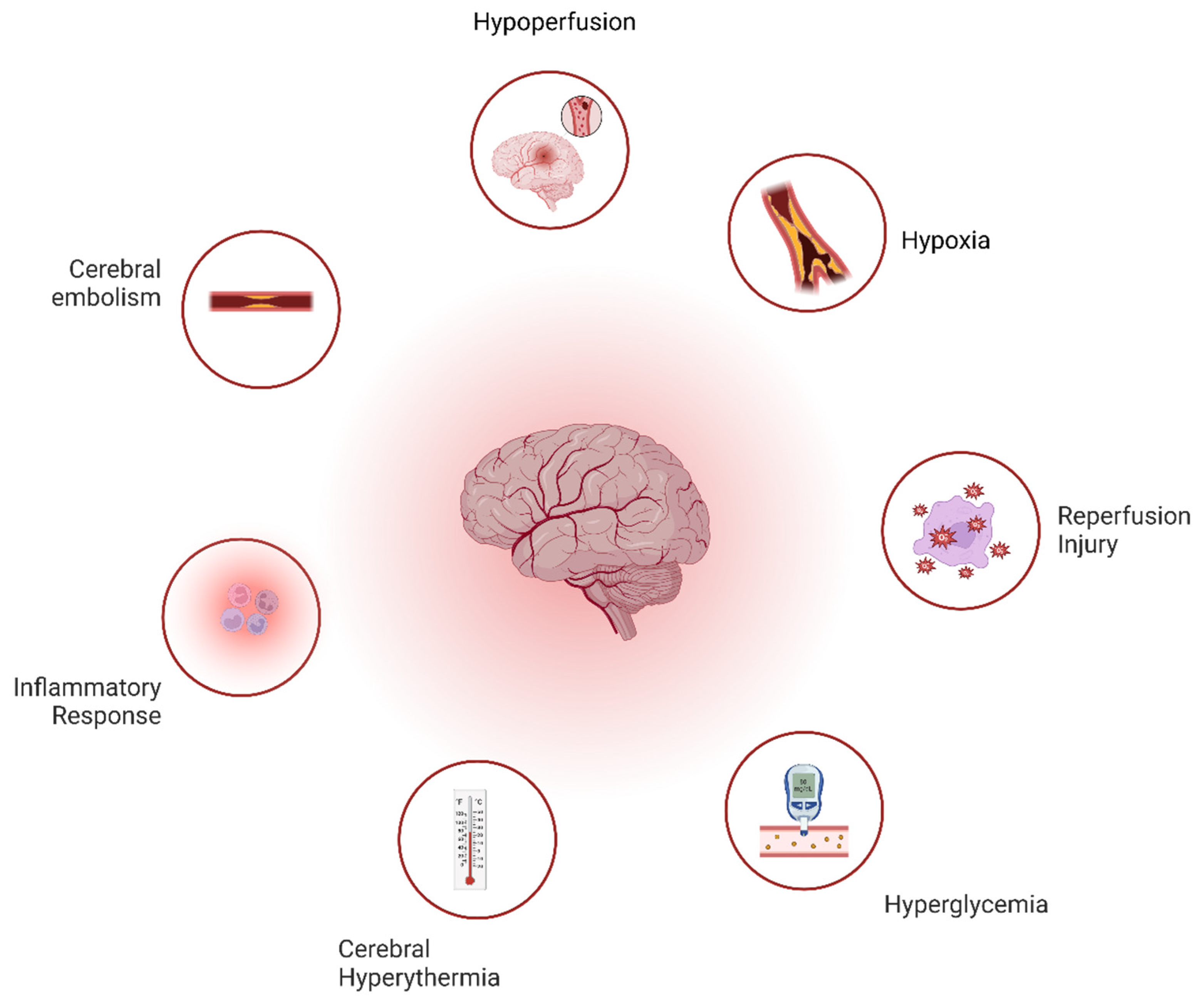
- Altered Cerebral Perfusion: Cerebral hypoperfusion during cardiopulmonary bypass (CPB) leads to ischemic injury, exacerbated by a reduced clearance of micro-emboli. Reperfusion generates reactive oxygen species (ROS), causing neuronal death [1].
- Hypoxia-Related Injury: Hypoperfusion-induced hypoxia triggers molecular pathways (e.g., HIF activation), ATP depletion, ion pump failure, and cell swelling, culminating in neuronal injury [1].
- Cerebral Embolism: Macro- and micro-emboli, arising from atherosclerotic plaques, gaseous emboli, or surgical debris obstruct cerebral blood flow, contributing to cognitive dysfunction [1].
- Inflammatory Response: CPB triggers systemic inflammation, causing blood–brain barrier leakage, cerebral edema, and neuronal damage.
- Cerebral Hyperthermia: Brain overheating during CPB exacerbates neuronal death, particularly in ischemic regions [2].
2. Neurological Risk Associated with Different Types of Coronary Artery Surgery
2.1. Neurological Complications in Coronary Artery Bypass Grafting (CABG)
2.1.1. Stroke
2.1.2. Encephalopathy
2.1.3. Seizures
2.1.4. Peripheral Nerve Injury
2.2. Neurological Complications in Totally Endoscopic Coronary Artery Bypass (TECAB)
2.3. Neurological Complications in Hybrid Coronary Revascularization (HCR)
3. Pharmacological Brain Protection Strategies
3.1. Barbiturates
3.2. Volatile Anesthetics
3.3. Lidocaine
3.4. N-Methyl-D-aspartate (NMDA) Receptor Antagonist
3.5. Magnesium
3.6. Nimodipine
3.7. Corticosteroids
3.8. Aprotinin
3.9. Deep Hypothermic Circulatory Arrest (DHCA)
4. Intra-Operative Neurological Risk Management in TECAB, and CABG
4.1. Risk Mitigation Strategies
4.1.1. Cerebral Perfusion Monitoring
4.1.2. Embolic Protection Strategies
4.1.3. Off-Pump CABG (OPCAB)
4.1.4. Neuroprotective Anesthesia and Pharmacology
4.1.5. Therapeutic Hypothermia
5. Pre- and Postoperative Neuroprotective Strategies in CABG
5.1. Preoperative Strategies
5.2. Intraoperative Techniques
5.3. Postoperative Management
5.4. Long-Term Follow-Up
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CABG | Coronary artery bypass grafting |
| TECAB | Totally endoscopic coronary artery bypass |
| CPB | Cardiopulmonary bypass |
| HCR | Hybrid coronary revasculariztion |
| CMRO2 | Cerebral metabolic oxygen demand |
| DHCA | Deep hypothermic circulatory arrest |
| OPCAB | Off-pump CABG |
References
- Hogue, C.W.; Gottesman, R.F.; Stearns, J. Mechanisms of cerebral injury from cardiac surgery. Crit. Care Clin. 2008, 24, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Hogue, C.W.; Levine, A.; Hudson, A.; Lewis, C. Clinical Applications of Near-infrared Spectroscopy Monitoring in Cardiovascular Surgery. Anesthesiology 2021, 134, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Breuer, A.C.; Furlan, A.J.; Hanson, M.R.; Lederman, R.J.; Loop, F.D.; Cosgrove, D.M.; Greenstreet, R.L.; Estafanous, F.G. Central nervous system complications of coronary artery bypass graft surgery: Prospective analysis of 421 patients. Stroke 1983, 14, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Roach, G.W.; Kanchuger, M.; Mangano, C.M.; Newman, M.; Nussmeier, N.; Wolman, R.; Aggarwal, A.; Marschall, K.; Graham, S.H.; Ley, C.; et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N. Engl. J. Med. 1996, 335, 1857–1863. [Google Scholar] [CrossRef]
- Tarakji, K.G.; Sabik, J.F.; Bhudia, S.K.; Batizy, L.H.; Blackstone, E.H. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. Jama 2011, 305, 381–390. [Google Scholar] [CrossRef]
- Barbut, D.; Yao, F.S.; Hager, D.N.; Kavanaugh, P.; Trifiletti, R.R.; Gold, J.P. Comparison of Transcranial Doppler Ultrasonography and Transesophageal Echocardiography to Monitor Emboli During Coronary Artery Bypass Surgery. Stroke 1996, 27, 87–90. [Google Scholar] [CrossRef]
- McKhann, G.M.; Grega, M.A.; Borowicz, L.M., Jr.; Bechamps, M.; Selnes, O.A.; Baumgartner, W.A.; Royall, R.M. Encephalopathy and stroke after coronary artery bypass grafting: Incidence, consequences, and prediction. Arch. Neurol. 2002, 59, 1422–1428. [Google Scholar] [CrossRef]
- McKhann, G. Predictors of stroke risk in coronary artery bypass. Ann. Surg. 1997, 63, 16–21. [Google Scholar] [CrossRef]
- Hogue, C.W., Jr.; Barzilai, B.; Pieper, K.S.; Coombs, L.P.; DeLong, E.R.; Kouchoukos, N.T.; Dávila-Román, V.G. Sex Differences in Neurological Outcomes and Mortality After Cardiac Surgery: A Society of Thoracic Surgery National Database Report. Circulation 2001, 103, 2133–2137. [Google Scholar] [CrossRef]
- Hogue, C.W.; Murphy, S.F.; Schechtman, K.B.; Dávila-Román, V.G. Risk Factors for Early or Delayed Stroke After Cardiac Surgery. Circulation 1999, 100, 642–647. [Google Scholar]
- Grocott, H.P.; White, W.D.; Morris, R.W.; Podgoreanu, M.V.; Mathew, J.P.; Nielsen, D.M.; Schwinn, D.A.; Newman, M.F. Genetic Polymorphisms and the Risk of Stroke After Cardiac Surgery. Stroke 2005, 36, 1854–1858. [Google Scholar] [CrossRef] [PubMed]
- Tardiff, B.E.; Newman, M.F.; Strittmatter, W.J.; White, W.D.; Davis, R.D., Jr.; Roses, A.D.; Reves, J.G. Preliminary report of a genetic basis for cognitive decline after cardiac operations. Ann. Thorac. Surg. 1997, 64, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Steed, L.; Kong, R.; Stygall, J.; Acharya, J.; Bolla, M.; Harrison, M.J.; Humphries, S.E.; Newman, S.P. The role of apolipoprotein E in cognitive decline after cardiac operation. Ann. Thorac. Surg. 2001, 71, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Leary, M.C.; Caplan, L.R. Technology insight: Brain MRI and cardiac surgery—Detection of postoperative brain ischemia. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 379–388. [Google Scholar] [CrossRef]
- Kornfeld, D.S.; Heller, S.S.; Frank, K.A.; Edie, R.N.; Barsa, J. Delirium after coronary artery bypass surgery. J. Thorac. Cardiovasc. Surg. 1978, 76, 93–96. [Google Scholar] [CrossRef]
- Manji, R.A.; Grocott, H.P.; Leake, J.; Ariano, R.E.; Manji, J.S.; Menkis, A.H.; Jacobsohn, E. Seizures following cardiac surgery: The impact of tranexamic acid and other risk factors. Can. J. Anesth. 2012, 59, 6–13. [Google Scholar] [CrossRef]
- Guptill, J.T.; Mehta, R.H.; Armstrong, P.W.; Horton, J.; Laskowitz, D.; James, S.; Granger, C.B.; Lopes, R.D. Stroke After Primary Percutaneous Coronary Intervention in Patients with ST-Segment Elevation Myocardial Infarction: Timing, Characteristics, and Clinical Outcomes. Circ.: Cardiovasc. Interv. 2013, 6, 176–183. [Google Scholar] [CrossRef]
- Myles, P.S.; Smith, J.A.; Forbes, A.; Silbert, B.; Jayarajah, M.; Painter, T.; Cooper, J.; Marasco, S.; McNeil, J.; Bussières, J.S.; et al. Stopping vs. Continuing Aspirin before Coronary Artery Surgery. N. Engl. J. Med. 2016, 374, 728–737. [Google Scholar] [CrossRef]
- Welch, M.B.; Brummett, C.M.; Welch, T.D.; Tremper, K.K.; Shanks, A.M.; Guglani, P.; Mashour, G.A. Perioperative peripheral nerve injuries: A retrospective study of 380,680 cases during a 10-year period at a single institution. Anesthesiology 2009, 111, 490–497. [Google Scholar] [CrossRef]
- Lederman, R.J.; Breuer, A.C.; Hanson, M.R.; Furlan, A.J.; Loop, F.D.; Cosgrove, D.M.; Estafanous, F.G.; Greenstreet, R.L. Peripheral nervous system complications of coronary artery bypass graft surgery. Ann. Neurol. 1982, 12, 297–301. [Google Scholar] [CrossRef]
- Llinas, R.; Barbut, D.; Caplan, L.R. Neurologic complications of cardiac surgery. Prog. Cardiovasc. Dis. 2000, 43, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Claessens, J.; Packlé, L.; Oosterbos, H.; Smeets, E.; Geens, J.; Gielen, J.; Van Genechten, S.; Heuts, S.; Maessen, J.G.; Yilmaz, A. Totally endoscopic coronary artery bypass grafting: Experience in 1500 patients. Interdiscip. CardioVascular Thorac. Surg. 2024, 39, ivae159. [Google Scholar] [CrossRef] [PubMed]
- Stessel, B.; Nijs, K.; Pelckmans, C.; Vandenbrande, J.; Ory, J.P.; Yilmaz, A.; Starinieri, P.; Van Tornout, M.; De Klippel, N.; Dendale, P. Neurological outcome after minimally invasive coronary artery bypass surgery (NOMICS): An observational prospective cohort study. PLoS ONE 2020, 15, e0242519. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Kappert, U.; Tugtekin, S.M.; Cichon, R.; Braun, M.; Matschke, K. Robotic totally endoscopic coronary artery bypass: A word of caution implicated by a five-year follow-up. J. Thorac. Cardiovasc. Surg. 2008, 135, 857–862. [Google Scholar] [CrossRef][Green Version]
- Gadelkarim, I.; Borger, M.A.; Davierwala, P.M. Hybrid coronary revascularization. AME Med. J. 2020, 5. Available online: https://amj.amegroups.org/article/view/5716 (accessed on 26 November 2024). [CrossRef]
- Nisivaco, S.M.; Kitahara, H.; Abutaleb, A.R.; Nathan, S.; Balkhy, H.H. Hybrid Coronary Revascularization: Early Outcomes and Midterm Follow-Up in Patients Undergoing Single or Multivessel Robotic TECAB and PCI. Innovations 2022, 17, 513–520. [Google Scholar] [CrossRef]
- Sanetra, K.; Buszman, P.P.; Jankowska-Sanetra, J.; Cisowski, M.; Fil, W.; Gorycki, B.; Bochenek, A.; Slabon-Turska, M.; Konopko, M.; Kaźmierczak, P.; et al. One-stage hybrid coronary revascularization for the treatment of multivessel coronary artery disease—Periprocedural and long-term results from the “HYBRID-COR” feasibility study. Front. Cardiovasc. Med. 2022, 9, 1016255. [Google Scholar] [CrossRef]
- Sultan, S.; Acharya, Y.; Barrett, N.; Hynes, N. A pilot protocol and review of triple neuroprotection with targeted hypothermia, controlled induced hypertension, and barbiturate infusion during emergency carotid endarterectomy for acute stroke after failed tPA or beyond 24-hour window of opportunity. Ann. Transl. Med. 2020, 8, 1275. [Google Scholar] [CrossRef]
- Bilotta, F.; Stazi, E.; Zlotnik, A.; Gruenbaum, S.E.; Rosa, G. Neuroprotective effects of intravenous anesthetics: A new critical perspective. Curr. Pharm. Des. 2014, 20, 5469–5475. [Google Scholar] [CrossRef]
- Nussmeier, N.A.; Arlund, C.; Slogoff, S. Neuropsychiatric complications after cardiopulmonary bypass: Cerebral protection by a barbiturate. Anesthesiology 1986, 64, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, T.; Kameda, T.; Kumamoto, T.; Shirota, S.; Yamano, M. Protective effect of thiopental against cerebral ischemia during circulatory arrest. Thorac. Cardiovasc. Surg. 1999, 47, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, Y.; Shu, S.; Yao, S.; Lynch, C.; Bayliss, D.A.; Chen, X. TASK channels contribute to neuroprotective action of inhalational anesthetics. Sci. Rep. 2017, 7, 44203. [Google Scholar]
- Hudetz, J.A.; Iqbal, Z.; Gandhi, S.D.; Patterson, K.M.; Byrne, A.J.; Hudetz, A.G.; Pagel, P.S.; Warltier, D.C. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol. Scand. 2009, 53, 864–872. [Google Scholar]
- Arrowsmith, J.E.; Harrison, M.J.; Newman, S.P.; Stygall, J.; Timberlake, N.; Pugsley, W.B. Neuroprotection of the brain during cardiopulmonary bypass: A randomized trial of remacemide during coronary artery bypass in 171 patients. Stroke 1998, 29, 2357–2362. [Google Scholar]
- Koerner, I.P.; Brambrink, A.M. Brain protection by anesthetic agents. Curr. Opin. Anaesthesiol. 2006, 19, 481–486. [Google Scholar]
- Verbrugghe, P.; Van de Velde, M.; Meuris, B.; Meyfroidt, G.; Milisen, K.; Fieuws, S.; Rex, S. Intraoperative xenon for prevention of delirium after on-pump cardiac surgery: A randomised, observer-blind, controlled clinical trial. Br. J. Anaesth. 2020, 124, 454–462. [Google Scholar]
- Ohira, T.; Peacock, J.M.; Iso, H.; Chambless, L.E.; Rosamond, W.D.; Folsom, A.R. Serum and dietary magnesium and risk of ischemic stroke: The Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2009, 169, 1437–1444. [Google Scholar]
- Wolf, F.I.; Trapani, V.; Simonacci, M.; Ferré, S.; Maier, J.A.M. Magnesium deficiency and endothelial dysfunction: Is oxidative stress involved? Magnes. Res. 2008, 21, 58–64. [Google Scholar]
- Bhudia, S.K.; Cosgrove, D.M.; Naugle, R.I.; Rajeswaran, J.; Lam, B.K.; Walton, E.; Petrich, J.; Palumbo, R.C.; Gillinov, A.M.; Apperson-Hansen, C.; et al. Magnesium as a neuroprotectant in cardiac surgery: A randomized clinical trial. J. Thorac. Cardiovasc. Surg. 2006, 131, 853–861. [Google Scholar]
- Pearce, A.; Lockwood, C.; van den Heuvel, C.; Pearce, J. The use of therapeutic magnesium for neuroprotection during global cerebral ischemia associated with cardiac arrest and cardiac surgery in adults: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 86–118. [Google Scholar] [CrossRef] [PubMed]
- Wadworth, A.N.; McTavish, D. Nimodipine. A review of its pharmacological properties, and therapeutic efficacy in cerebral disorders. Drugs Aging 1992, 2, 262–286. [Google Scholar] [CrossRef] [PubMed]
- Kazda, S.; Towart, R. Nimodipine: A new calcium antagonistic drug with a preferential cerebrovascular action. Acta Neurochir. 1982, 63, 259–265. [Google Scholar] [CrossRef]
- Forsman, M.; Olsnes, B.T.; Semb, G.; Steen, P.A. Effects of nimodipine on cerebral blood flow and neuropsychological outcome after cardiac surgery. Br. J. Anaesth. 1990, 65, 514–520. [Google Scholar] [CrossRef]
- Motshabi-Chakane, P.; Mogane, P.; Moutlana, J.; Leballo-Mothibi, G.; Dingezweni, S.; Mpanya, D.; Tsabedze, N. Contemporary Neuroprotection Strategies during Cardiac Surgery: State of the Art Review. Int. J. Environ. Res. Public Health 2021, 18, 12747. [Google Scholar] [CrossRef]
- Shum-Tim, D.; Tchervenkov, C.I.; Jamal, A.M.; Nimeh, T.; Luo, C.Y.; Chedrawy, E.; Laliberte, E.; Philip, A.; Rose, C.P.; Lavoie, J. Systemic steroid pretreatment improves cerebral protection after circulatory arrest. Ann. Thorac. Surg. 2001, 72, 1465–1472. [Google Scholar] [CrossRef]
- Demir, T.; Demir, H.; Tansel, T.; Kalko, Y.; Tireli, E.; Dayioglu, E.; Barlas, S.; Onursal, E. Influence of methylprednisolone on levels of neuron-specific enolase in cardiac surgery: A corticosteroid derivative to decrease possible neuronal damage. J. Card. Surg. 2009, 24, 397–403. [Google Scholar] [CrossRef]
- Anttila, V.; Hagino, I.; Iwata, Y.; Mettler, B.A.; Lidov, H.G.; Zurakowski, D.; Jonas, R.A. Aprotinin improves cerebral protection: Evidence from a survival porcine model. J. Thorac. Cardiovasc. Surg. 2006, 132, 948–953. [Google Scholar] [CrossRef]
- Ziganshin, B.A.; Elefteriades, J.A. Deep hypothermic circulatory arrest. Ann. Cardiothorac. Surg. 2013, 2, 303–315. [Google Scholar]
- Tian, D.H.; Wan, B.; Bannon, P.G.; Misfeld, M.; LeMaire, S.A.; Kazui, T.; Kouchoukos, N.T.; Elefteriades, J.A.; Bavaria, J.; Coselli, J.S.; et al. A meta-analysis of deep hypothermic circulatory arrest versus moderate hypothermic circulatory arrest with selective antegrade cerebral perfusion. Ann. Cardiothorac. Surg. 2013, 2, 148–158. [Google Scholar]
- Fleseriu, C.M.; Sultan, I.; Brown, J.A.; Mina, A.; Frenchman, J.; Crammond, D.J.; Balzer, J.; Anetakis, K.M.; Subramaniam, K.; Shandal, V.; et al. Role of Intraoperative Neurophysiological Monitoring in Preventing Stroke After Cardiac Surgery. Ann. Thorac. Surg. 2023, 116, 623–629. [Google Scholar] [CrossRef]
- Göbölös, L.; Ramahi, J.; Obeso, A.; Bartel, T.; Hogan, M.; Traina, M.; Edris, A.; Hasan, F.; Banna, M.E.; Tuzcu, E.M.; et al. Robotic Totally Endoscopic Coronary Artery Bypass Grafting: Systematic Review of Clinical Outcomes from the Past two Decades. Innovations 2019, 14, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Gocoł, R.; Hudziak, D.; Bis, J.; Mendrala, K.; Morkisz, Ł.; Podsiadło, P.; Kosiński, S.; Piątek, J.; Darocha, T. The Role of Deep Hypothermia in Cardiac Surgery. Int. J. Environ. Res. Public Health 2021, 18, 7061. [Google Scholar] [CrossRef] [PubMed]
- Halkos, M.E.; Anderson, A.; Binongo, J.N.G.; Stringer, A.; Lasanajak, Y.; Thourani, V.H.; Lattouf, O.M.; Guyton, R.A.; Baio, K.T.; Sarin, E.; et al. Operative strategies to reduce cerebral embolic events during on- and off-pump coronary artery bypass surgery: A stratified, prospective randomized trial. J. Thorac. Cardiovasc. Surg. 2017, 154, 1278–1285.e1. [Google Scholar] [CrossRef]
- Pan, E.; Nielsen, S.J.; Mennander, A.; Björklund, E.; Martinsson, A.; Lindgren, M.; Hansson, E.C.; Pivodic, A.; Jeppsson, A. Statins for secondary prevention and major adverse events after coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2022, 164, 1875–1886.e4. [Google Scholar] [CrossRef]
- Jeppsson, A.; Rocca, B.; Hansson, E.C.; Gudbjartsson, T.; James, S.; Kaski, J.K. 2024 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur. J. Cardiothorac. Surg. 2024, 10, ezae355. [Google Scholar]
- Andersen, N.D.; Hart, S.A.; Devendra, G.P.; Kim, E.S.; Johnston, D.R.; Schroder, J.N.; Krasuski, R.A. Atheromatous disease of the aorta and perioperative stroke. J. Thorac. Cardiovasc. Surg. 2018, 155, 508–516. [Google Scholar] [CrossRef]
- Haider, Z.; Jalal, A.; Alamgir, A.R.; Rasheed, I. Neurological complications are avoidable during CABG. Pak. J. Med. Sci. 2018, 34, 5–9. [Google Scholar] [CrossRef]
- Deja, M.A.; Kargul, T.; Domaradzki, W.; Stącel, T.; Mazur, W.; Wojakowski, W.; Gocoł, R.; Gaszewska-Żurek, E.; Żurek, P.; Pytel, A.; et al. Effects of preoperative aspirin in coronary artery bypass grafting: A double-blind, placebo-controlled, randomized trial. J. Thorac. Cardiovasc. Surg. 2012, 144, 204–209. [Google Scholar] [CrossRef]
- Dobrev, D.; Aguilar, M.; Heijman, J.; Guichard, J.B.; Nattel, S. Postoperative atrial fibrillation: Mechanisms, manifestations and management. Nat. Rev. Cardiol. 2019, 16, 417–436. [Google Scholar] [CrossRef]
- Greenberg, J.W.; Lancaster, T.S.; Schuessler, R.B.; Melby, S.J. Postoperative atrial fibrillation following cardiac surgery: A persistent complication. Eur. J. Cardiothorac. Surg. 2017, 52, 665–672. [Google Scholar] [PubMed]
- Kaw, R.; Hernandez, A.V.; Masood, I.; Gillinov, A.M.; Saliba, W.; Blackstone, E.H. Short- and long-term mortality associated with new-onset atrial fibrillation after coronary artery bypass grafting: A systematic review and meta-analysis. J. Thorac. Cardiovasc. Surg. 2011, 141, 1305–1312. [Google Scholar] [PubMed]
- Steinberg, B.A.; Zhao, Y.; He, X.; Hernandez, A.F.; Fullerton, D.A.; Thomas, K.L.; Mills, R.; Klaskala, W.; Peterson, E.D.; Piccini, J.P. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: Insights from the Society of Thoracic Surgeons CAPS-Care Atrial Fibrillation Registry. Clin Cardiol. 2014, 37, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, K.A.; Yusuf, A.M.; Crystal, E.; Healey, J.S.; Morillo, C.A.; Nair, G.M.; Whitlock, R.P. Interventions for preventing post-operative atrial fibrillation in patients undergoing heart surgery. Cochrane Database Syst. Rev. 2013, 2013, CD003611. [Google Scholar]
- Blessberger, H.; Lewis, S.R.; Pritchard, M.W.; Fawcett, L.J.; Domanovits, H.; Schlager, O.; Wildner, B.; Kammler, J.; Steinwender, C.; Cochrane Anaesthesia Group. Perioperative beta-blockers for preventing surgery-related mortality and morbidity in adults undergoing cardiac surgery. Cochrane Database Syst. Rev. 2019, 9, CD013435. [Google Scholar]
- Kim, S.H.; Jang, M.J.; Hwang, H.Y. Perioperative Beta-Blocker for Atrial Fibrillation after Cardiac Surgery: A Meta-Analysis. Thorac. Cardiovasc. Surg. 2021, 69, 133–140. [Google Scholar]
- da Graca, B.; Filardo, G.; Sass, D.M.; Edgerton, J.R. Preoperative β-Blockers for Isolated Coronary Artery Bypass Graft. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e005027. [Google Scholar] [CrossRef]
- White, C.M.; Caron, M.F.; Kalus, J.S. Intravenous plus oral amiodarone, atrial septal pacing, or both strategies to prevent post-cardiothoracic surgery atrial fibrillation: The Atrial Fibrillation Suppression Trial II (AFIST II). Circulation 2003, 108 (Suppl. S1), 200–206. [Google Scholar]
| Strategy/Factor | Application in Coronary Interventions | Rationale/Mechanism | Key Considerations |
|---|---|---|---|
| Surgical Approach | CABG, TECAB, HCR, PCI | Minimizing aortic manipulation (e.g., “no-touch” technique) reduces risk of embolization and stroke | Patient selection, preoperative imaging (e.g., epiaortic ultrasound), and careful graft planning |
| Off-Pump CABG (OPCAB) | CABG | Avoids cardiopulmonary bypass, reducing embolic load and inflammatory response | Suitable for patients at high neurological risk; surgeon expertise required |
| Epiaortic Ultrasound | CABG | Identifies aortic plaque burden and guides safe cannulation/anastomosis sites | Reduces stroke risk by preventing plaque dislodgment |
| Deep Hypothermic Circulatory Arrest (DHCA) | Complex aortic/arch procedures rarely involving coronary surgery | Lowers cerebral metabolism and protects the brain in scenarios with no blood flow (e.g., arch aneurysm repair) | Not routine for standard coronary procedures; reserved for complex aortic reconstructions |
| Pharmacologic Agents | CABG, PCI, TECAB, HCR | Reduce inflammation, excitotoxicity, and metabolic demand | Agents include volatile anesthetics, barbiturates, lidocaine, NMDA antagonists, magnesium, nimodipine, corticosteroids, aprotinin |
| Anesthetic Management | All coronary interventions | Neuroprotective anesthetics (e.g., volatile agents, dexmedetomidine) stabilize hemodynamics and reduce cerebral metabolic rate | Tailor anesthetic choice to patient comorbidities and risk profile |
| Therapeutic Hypothermia | High-risk CABG/PCI scenarios | Decreases cerebral metabolic demand, limits ischemic injury | Typically mild/moderate hypothermia; must balance benefits against potential coagulopathy and arrhythmias |
| Cerebral Perfusion Monitoring | CABG, TECAB | NIRS, transcranial Doppler to ensure adequate cerebral blood flow and oxygenation | Early detection of ischemia allows prompt intervention (adjusting perfusion, BP, etc.) |
| Preoperative Medical Optimization | CABG, PCI | Controlling hypertension, diabetes, optimizing lipids (statins, ezetimibe) reduces stroke risk | Follow guidelines for continuation of statins, careful initiation of new therapies pre-surgery |
| Antiplatelet Management | CABG, PCI | ASA and/or P2Y12 inhibitors reduce thrombotic events; timing is critical to balance bleeding and ischemic risks | Tailored per guidelines (e.g., DAPT bridging, test platelet function if recently discontinued) |
| Arrhythmia Prophylaxis | CABG, PCI | Beta-blockers, amiodarone reduce incidence of postoperative AF and associated stroke risk | Start/continue BBs if already on therapy; carefully initiate in naïve patients |
| Postoperative Therapies | CABG, PCI | Early ASA (within 6 h), resuming DAPT in ACS/PCI patients reduce graft occlusion and ischemic events | Monitor bleeding risk, ensure stable hemodynamics, and address delirium prevention |
| Long-Term Follow-Up | All interventions | Ongoing risk factor management, neurocognitive assessments, and lipid control support long-term neuroprotection | Regular follow-up imaging, medications adherence, lifestyle interventions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, M.; Bazarbaev, A.; Rana, A.; Khurshid, R.; Effiom, V.; Bajwa, N.K.; Nasir, A.; Candelario, K.; Tabraiz, S.A.; Colon, S.; et al. Neuroprotective Strategies in Coronary Artery Disease Interventions. J. Cardiovasc. Dev. Dis. 2025, 12, 143. https://doi.org/10.3390/jcdd12040143
Fatima M, Bazarbaev A, Rana A, Khurshid R, Effiom V, Bajwa NK, Nasir A, Candelario K, Tabraiz SA, Colon S, et al. Neuroprotective Strategies in Coronary Artery Disease Interventions. Journal of Cardiovascular Development and Disease. 2025; 12(4):143. https://doi.org/10.3390/jcdd12040143
Chicago/Turabian StyleFatima, Maurish, Akbar Bazarbaev, Asama Rana, Ruman Khurshid, Victory Effiom, Nyle Khalid Bajwa, Afsheen Nasir, Katherine Candelario, Sair Ahmad Tabraiz, Samantha Colon, and et al. 2025. "Neuroprotective Strategies in Coronary Artery Disease Interventions" Journal of Cardiovascular Development and Disease 12, no. 4: 143. https://doi.org/10.3390/jcdd12040143
APA StyleFatima, M., Bazarbaev, A., Rana, A., Khurshid, R., Effiom, V., Bajwa, N. K., Nasir, A., Candelario, K., Tabraiz, S. A., Colon, S., Lee, C., Dankwa, S., & Hameed, I. (2025). Neuroprotective Strategies in Coronary Artery Disease Interventions. Journal of Cardiovascular Development and Disease, 12(4), 143. https://doi.org/10.3390/jcdd12040143





