Perioperative Considerations, Anesthetic Management and Transesophageal Echocardiographic Evaluation of Patients Undergoing the Ross Procedure
Abstract
1. Introduction
2. Anatomical Considerations
3. Preoperative Assessment
3.1. Routine Preoperative Investigations
- A complete blood count (CBC) is used to evaluate for anemia, infection, and platelet abnormalities, providing baseline hematologic status.
- A comprehensive metabolic panel (CMP) assesses renal function, glucose, and electrolyte balance to optimize perioperative management.
- Coagulation studies, including prothrombin time (PT) and activated partial thromboplastin time (aPTT), screen for bleeding or clotting risks that influence intraoperative anticoagulation and transfusion planning.
- Blood typing and crossmatching ensure that compatible blood products are available should transfusion be required.
- Electrocardiography (ECG) identifies arrhythmias, conduction abnormalities, or ischemic changes that may affect anesthetic and surgical risk.
- A chest radiograph (CXR) is obtained to evaluate the cardiac silhouette and pulmonary parenchyma and to identify any occult thoracic pathology.
- Transthoracic echocardiography (TTE) provides an initial noninvasive assessment of cardiac structure and function, including valvular morphology, ventricular performance, and chamber dimensions.
3.2. Ross Procedure-Specific Investigations
- Transthoracic echocardiography (TTE) continues to play a central role, offering initial information on the structure and function of both the aortic and pulmonary valves, as well as left ventricular systolic performance. Doppler imaging augments this evaluation by quantifying flow velocities and valvular properties [29].
- Although uncommon and avoided, if possible, transesophageal echocardiography (TEE) is an invasive modality that offers superior sonographic windows and spatial resolution, leading to more precise visualization of the aortic root, pulmonary valve, and ascending aorta in the case of suboptimal TTE.
- Computed tomography coronary angiography (CTCA) is used to scrutinize coronary artery architecture and identify variations such as a short left main or anomalous origin of the right coronary artery, which may complicate autograft harvesting and reimplantation [16].
- Further anatomic and functional detail is provided by cardiac magnetic resonance imaging (cMRI), which provides more advanced anatomical and functional insights and is particularly useful for characterizing valvular flow dynamics, ventricular function, perfusion, and anomalous coronary arteries [34].
- Cardiac computed tomography angiography (CTA) offers high-resolution cross-sectional imaging, which complements cMRI [29]. CTA is employed to examine the aortic root and ascending aorta, particularly in cases where dilation is suspected. Measurements exceeding 38–40 mm typically prompt consideration of concomitant surgical intervention [19].
4. Intraoperative Technical Considerations
4.1. Cannulation Strategies
4.2. Autograft Harvesting and Implantation
4.3. Right Ventricular Outflow Tract Reconstruction
5. Blood Conservation Techniques
5.1. Acute Normovolemic Hemodilution
5.2. Retrograde Autologous Priming
6. Anesthetic Considerations
6.1. Intraoperative Monitoring
6.2. Anesthesia and Analgesia
6.3. Regional Anesthesia
6.4. Separation from Cardiopulmonary Bypass and Hemodynamic Control
6.5. Early Extubation and Fast-Track Recovery After Cardiac Surgery
7. Intraoperative Transesophageal Echocardiographic Assessment
7.1. Pre-Cardiopulmonary Bypass TEE Assessment
- Inspection of the native AV
- Inspection of the native PV
- Evaluation of LV systolic function and RWMA
- Evaluation of RV systolic function
- Evaluation of the ascending aorta and aortic arch
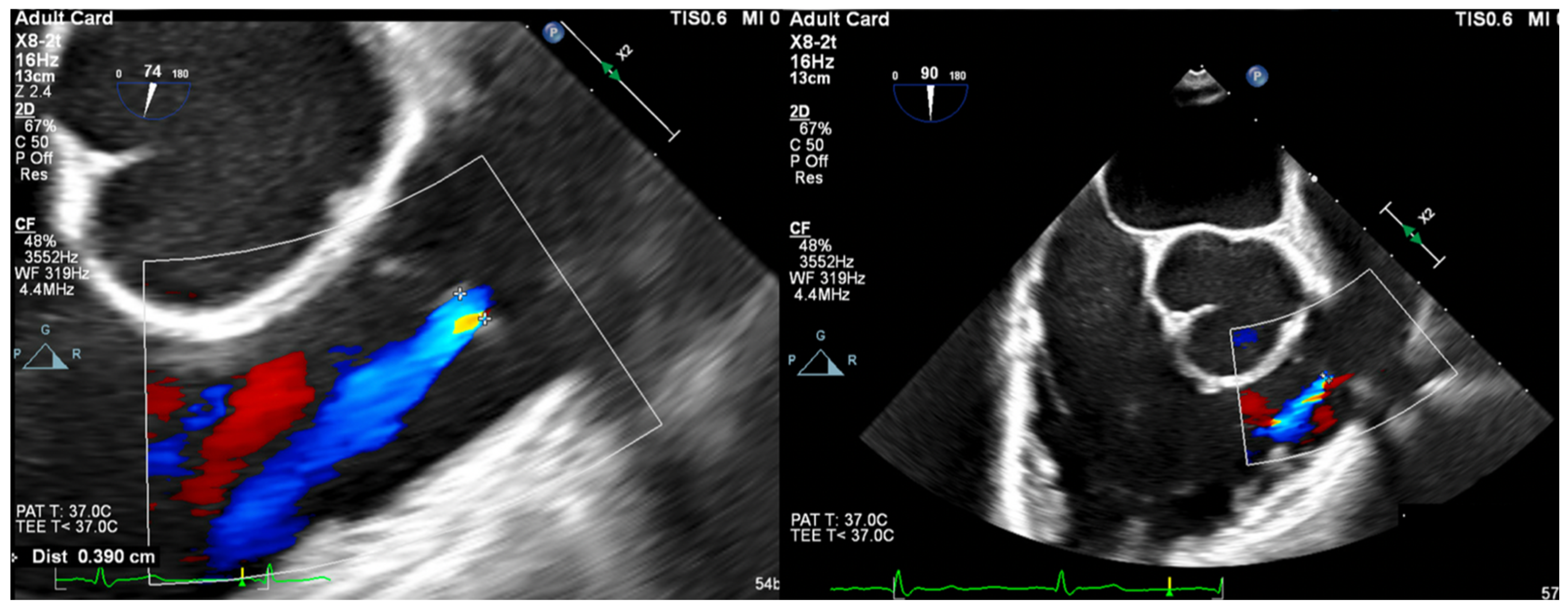
- Vena Contracta Width (VCW): Typically not used for multiple jets. Cutoffs for various grades of PR have not been validated, but most use AI cutoffs (Figure 1).
- VCW/PV ratio has been used with a value of >0.5 consistent with severe PR.
- PWD flow reversal in branched PA: Affected by PA compliance, so brief flow reversals are normal.
- CWD: Through the mPA, watch for envelope density. Dense is consistent with moderate to severe PR, faint is more likely to be mild.
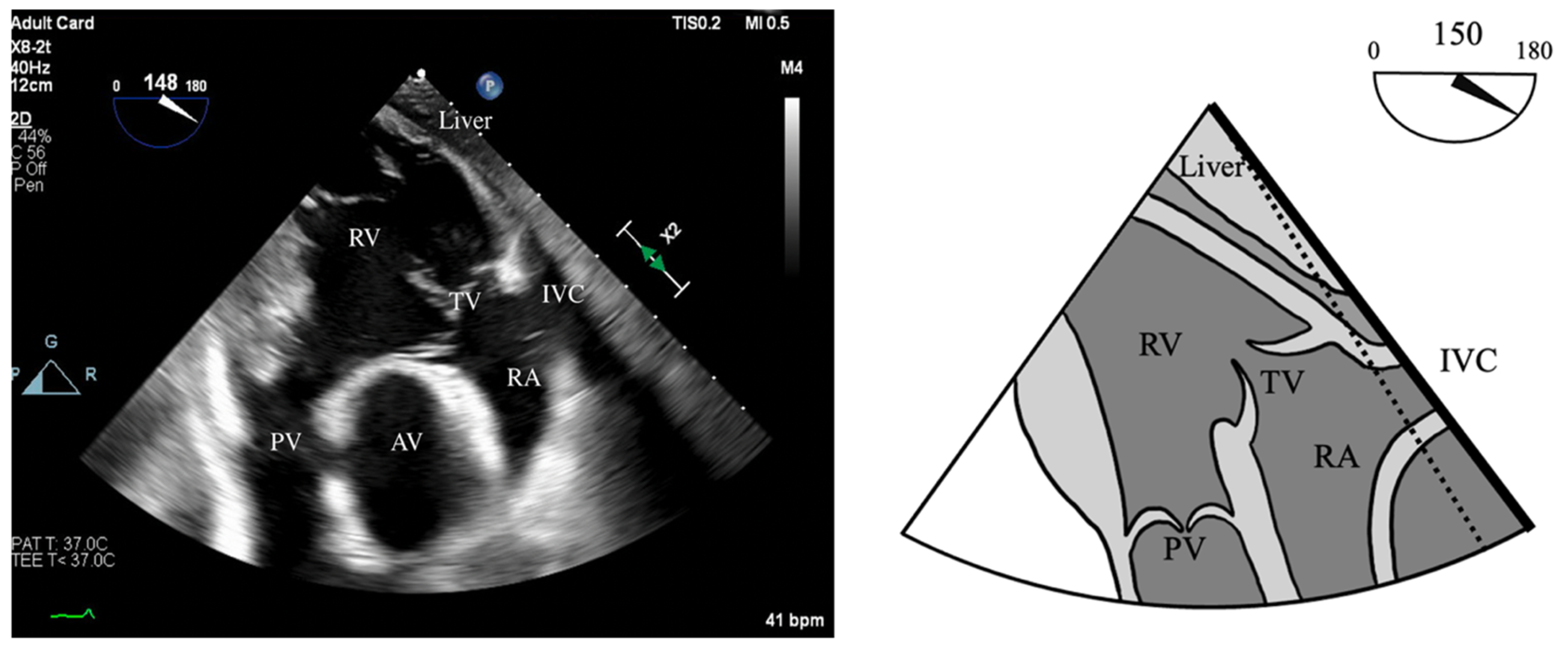
- PR Index: The ratio of PR duration and total diastolic time (regurgitation time/diastole); <0.77 is consistent with severe PR (Figure 2).
- PR Jet Width/RVOT Width: With >65% consistent with severe PR (Figure 3).
- RV Fractional Area Change (FAC): Measured in the ME-4C view, normal >35%.
- Tricuspid Annular Plane Systolic Excursion (TAPSE): ME-RV Inflow/Outflow with angle correction over lateral tricuspid annulus, normal >1.6 cm/s.
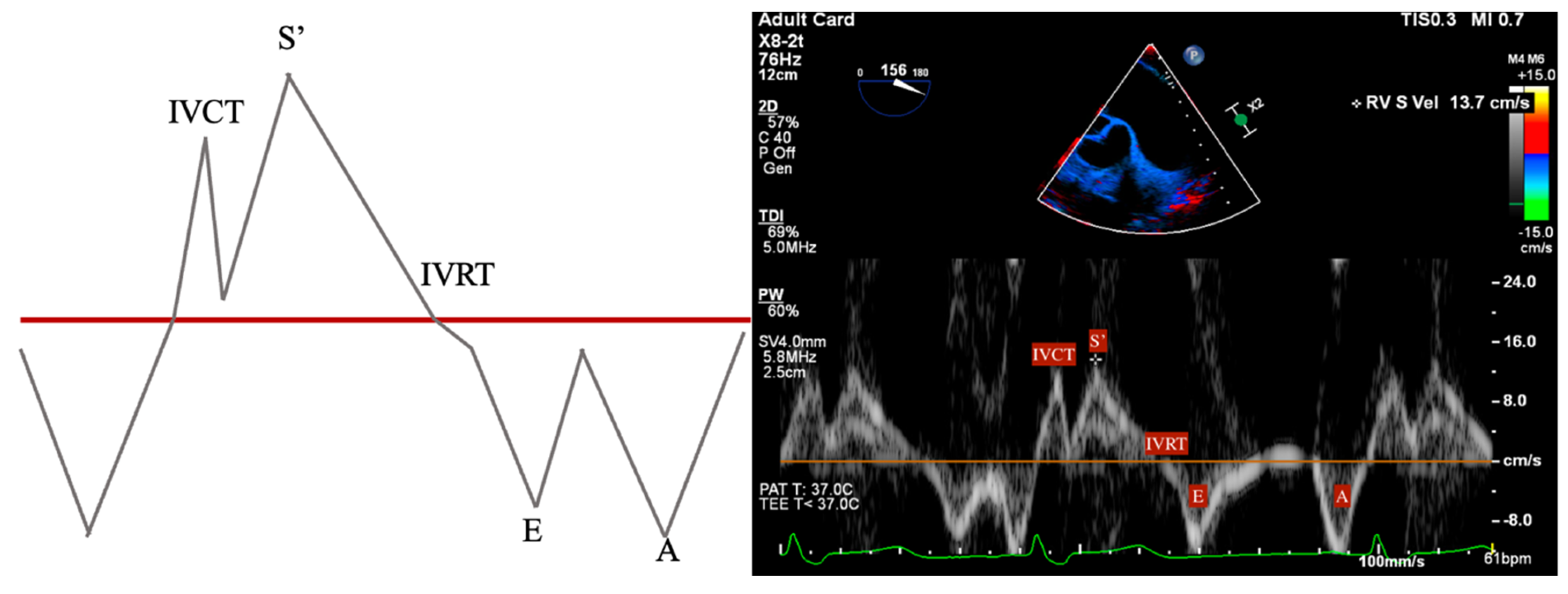
- S’: Measured from a non-standard TEE view. In the Deep Transgastric long axis (DTG-LAX) view, omniplane to 120–150° and turn right. The RV and lateral tricuspid annulus will be centered on the screen. Tissue Doppler Imaging (TDI) is then used to measure the systolic velocity of the lateral tricuspid valve annulus (S’) with >10 cm/s considered normal [79] (Figure 4 and Figure 5).
7.2. Post-Cardiopulmonary Bypass TEE Assessment
- Inspection of the neo-AV
- Inspection of the homograft PV
- Evaluation of LV systolic function and RWMA
- Evaluation of RV systolic function
- Evaluation of the ascending aorta and aortic arch (Figure 7).
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ross, D. Replacement of aortic and mitral valves with a pulmonary autograft. Lancet 1967, 290, 956–958. [Google Scholar] [CrossRef]
- Lower, R.R.; Stofer, R.C.; Shumway, N.E. Autotransplantation of the pulmonic valve into the aorta. J. Thorac. Cardiovasc. Surg. 1960, 39, 680–687. [Google Scholar] [CrossRef]
- Concha, M.; Aranda, P.J.; Casares, J.; Merino, C.; Alados, P.; Muñoz, I.; Gonzalez, J.R.; Ribes, R.; Villalba, R. The Ross procedure. J. Card. Surg. 2004, 19, 401–409. [Google Scholar]
- Ouzounian, M.; Mazine, A.; David, T.E. The Ross procedure is the best operation to treat aortic stenosis in young and middle-aged adults. J. Thorac. Cardiovasc. Surg. 2017, 154, 778–782. [Google Scholar] [CrossRef] [PubMed]
- Yacoub, M.H.; El-Hamamsy, I.; Sievers, H.-H.; Carabello, B.A.; Bonow, R.O.; Stelzer, P.; da Costa, F.D.A.; Schäfers, H.J.; Skillington, P.; Charitos, E.I.; et al. Under-use of the Ross operation—A lost opportunity. Lancet 2014, 384, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Gott, V.L.; Alejo, D.E.; Cameron, D.E. Mechanical heart valves: 50 years of evolution. Ann. Thorac. Surg. 2003, 76, S2230–S2239. [Google Scholar] [CrossRef]
- Matthews, A.M. The development of the Starr-Edwards heart valve. Tex. Heart Inst. J. 1998, 25, 282–293. [Google Scholar]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar]
- Yap, C.-H.; Yii, M. Allograft aortic valve replacement in the adult: A review. Heart Lung Circ. 2004, 13, 41–51. [Google Scholar] [CrossRef]
- Kostyunin, A.E.; Yuzhalin, A.E.; Rezvova, M.A.; Ovcharenko, E.A.; Glushkova, T.V.; Kutikhin, A.G. Degeneration of Bioprosthetic Heart Valves: Update 2020. J. Am. Heart Assoc. 2020, 9, e018506. [Google Scholar] [CrossRef]
- Tillquist, M.N.; Maddox, T. Cardiac crossroads: Deciding between mechanical or bioprosthetic heart valve replacement. Patient Prefer. Adherence 2011, 5, 91–99. [Google Scholar] [CrossRef]
- Stradins, P. Comparison of biomechanical and structural properties between human aortic and pulmonary valve. Eur. J. Cardio-Thorac. Surg. 2004, 26, 634–639. [Google Scholar] [CrossRef]
- Van Hoof, L.; Verbrugghe, P.; Jones, E.A.; Humphrey, J.D.; Janssens, S.; Famaey, N.; Rega, F. Understanding Pulmonary Autograft Remodeling After the Ross Procedure: Stick to the Facts. Front. Cardiovasc. Med. 2022, 9, 829120. [Google Scholar]
- Stelzer, P.; Weinrauch, S.; Tranbaugh, R.F. Ten years of experience with the modified Ross procedure. J. Thorac. Cardiovasc. Surg. 1998, 115, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Reece, T.B.; Welke, K.F.; O’brien, S.; Grau-Sepulveda, M.V.; Grover, F.L.; Gammie, J.S. Rethinking the Ross Procedure in Adults. Ann. Thorac. Surg. 2014, 97, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; El-Hamamsy, I.; Verma, S.; Peterson, M.D.; Bonow, R.O.; Yacoub, M.H.; David, T.E.; Bhatt, D.L. Ross Procedure in Adults for Cardiologists and Cardiac Surgeons: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2761–2777. [Google Scholar]
- Vojáček, J.; El-Hamamsy, I.; Ondrášek, J.; Žáček, P.; Fila, P.; Voborník, M.; Špatenka, J. Current status of the Ross procedure in aortic valve surgery. Cor Vasa 2017, 59, e71–e76. [Google Scholar] [CrossRef]
- David, T.E.; David, C.; Woo, A.; Manlhiot, C. The Ross procedure: Outcomes at 20 years. J. Thorac. Cardiovasc. Surg. 2014, 147, 85–94. [Google Scholar] [CrossRef]
- David, T.E.; Ouzounian, M.; David, C.M.; Lafreniere-Roula, M.; Manlhiot, C. Late results of the Ross procedure. J. Thorac. Cardiovasc. Surg. 2019, 157, 201–208. [Google Scholar] [CrossRef]
- Andreas, M.; Wiedemann, D.; Seebacher, G.; Rath, C.; Aref, T.; Rosenhek, R.; Heinze, G.; Eigenbauer, E.; Simon, P.; Ruetzler, K.; et al. The Ross procedure offers excellent survival compared with mechanical aortic valve replacement in a real-world setting. Eur. J. Cardio-Thorac. Surg. 2014, 46, 409–414. [Google Scholar] [CrossRef]
- Sharabiani, M.T.; Dorobantu, D.M.; Mahani, A.S.; Turner, M.; Tometzki, A.J.P.; Angelini, G.D.; Parry, A.J.; Caputo, M.; Stoica, S.C. Aortic Valve Replacement and the Ross Operation in Children and Young Adults. J. Am. Coll. Cardiol. 2016, 67, 2858–2870. [Google Scholar] [CrossRef] [PubMed]
- Aboud, A.; Charitos, E.I.; Fujita, B.; Stierle, U.; Reil, J.-C.; Voth, V.; Liebrich, M.; Andreas, M.; Holubec, T.; Bening, C.; et al. Long-Term Outcomes of Patients Undergoing the Ross Procedure. J. Am. Coll. Cardiol. 2021, 77, 1412–1422. [Google Scholar] [CrossRef] [PubMed]
- Liebrich, M.; Charitos, E.I.; Dingemann, C.; Roser, D.; Seeburger, J.; Hemmer, W.; Voth, V. The reinforced full-root technique for the Ross operation: Surgical considerations and operative insights. Ann. Cardiothorac. Surg. 2021, 10, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Mazine, A.; Ghoneim, A.; El-Hamamsy, I. The Ross Procedure: How I Teach It. Ann. Thorac. Surg. 2018, 105, 1294–1298. [Google Scholar] [CrossRef]
- Bouhout, I.; Ghoneim, A.; Tousch, M.; Stevens, L.M.; Semplonius, T.; Tarabzoni, M.; Poirier, N.; Cartier, R.; Demers, P.; Guo, L.; et al. Impact of a tailored surgical approach on autograft root dimensions in patients undergoing the Ross procedure for aortic regurgitation. Eur. J. Cardio-Thorac. Surg. 2019, 56, 959–967. [Google Scholar] [CrossRef]
- Tudorache, I.; Theodoridis, K.; Baraki, H.; Sarikouch, S.; Bara, C.; Meyer, T.; Höffler, K.; Hartung, D.; Hilfiker, A.; Haverich, A.; et al. Decellularized aortic allografts versus pulmonary autografts for aortic valve replacement in the growing sheep model: Haemodynamic and morphological results at 20 months after implantation. Eur. J. Cardio-Thorac. Surg. 2015, 49, 1228–1238. [Google Scholar] [CrossRef]
- Schmidtke, C.; Bechtel, J.F.; Noetzold, A.; Sievers, H.H. Up to seven years of experience with the Ross procedure in patients >60 years of age. J. Am. Coll. Cardiol. 2000, 36, 1173–1177. [Google Scholar] [CrossRef]
- Hage, A.; Hage, F.; Valdis, M.; Guo, L.; Chu, M.W.A. The Ross procedure is the optimal solution for young adults with unrepairable aortic valve disease. Ann. Cardiothorac. Surg. 2021, 10, 454–462. [Google Scholar] [CrossRef]
- Galzerano, D.; Kholaif, N.; Al Amro, B.; Al Admawi, M.; Eltayeb, A.; Alshammari, A.; Di Salvo, G.; Al-Halees, Z.Y. The Ross Procedure: Imaging, Outcomes and Future Directions in Aortic Valve Replacement. J. Clin. Med. 2024, 13, 630. [Google Scholar] [CrossRef]
- Jahanyar, J.; Tsai, P.I.; Arabkhani, B.; Aphram, G.; Mastrobuoni, S.; El Khoury, G.; de Kerchove, L. Functional and pathomorphological anatomy of the aortic valve and root for aortic valve sparing surgery in tricuspid and bicuspid aortic valves. Ann. Cardiothorac. Surg. 2023, 12, 179–193. [Google Scholar] [CrossRef]
- Sundjaja, J.; Bordoni, B. Anatomy, Thorax, Heart Pulmonic Valve. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wenn, P.; Zeltser, R. Aortic Valve Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Merlo, A.; Chen, K.; Deo, S.; Markowitz, A. Does routine preoperative computed tomography imaging provide clinical utility in patients undergoing primary cardiac surgery? Interact. Cardiovasc. Thorac. Surg. 2017, 25, 659–662. [Google Scholar] [PubMed]
- Brothers, J.A.; Kim, T.S.; Fogel, M.A.; Whitehead, K.K.; Morrison, T.M.; Paridon, S.M.; Harris, M.A. Cardiac magnetic resonance imaging characterizes stenosis, perfusion, and fibrosis preoperatively and postoperatively in children with anomalous coronary arteries. J. Thorac. Cardiovasc. Surg. 2016, 152, 205–210. [Google Scholar] [CrossRef]
- Poh, C.L.; Buratto, E.; Larobina, M.; Wynne, R.; O’keefe, M.; Goldblatt, J.; Tatoulis, J.; Skillington, P.D. The Ross procedure in adults presenting with bicuspid aortic valve and pure aortic regurgitation: 85% freedom from reoperation at 20 years. Eur. J. Cardio-Thorac. Surg. 2018, 54, 420–426. [Google Scholar] [CrossRef]
- Leyh, R.G.; Kofidis, T.; Fischer, S.; Kallenbach, K.; Harringer, W.; Haverich, A. Aortic root reimplantation for successful repair of an insufficient pulmonary autograft valve after the Ross procedure. J. Thorac. Cardiovasc. Surg. 2002, 124, 1048–1049. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.E.; El-Hamamsy, I. The Ross Procedure. Oper. Tech. Thorac. Cardiovasc. Surg. 2021, 26, 189–209. [Google Scholar] [CrossRef]
- Peng, R.; Ba, J.; Wang, C.; Lai, H.; Hu, K.; Shi, H. A New Venous Drainage Technique in Minimally Invasive Redo Tricuspid Surgery: Vacuum-Assist Venous Drainage via a Single Femoral Venous Cannula. Heart Lung Circ. 2017, 26, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.H.L.; Ho, J.Y.K.; Underwood, M.J. Chapter 48—Complex Reoperative Thoracic Aortic Surgery: Tactics and Techniques. In New Approaches to Aortic Diseases from Valve to Abdominal Bifurcation; Tintoiu, I.C., Elefteriades, J.A., Ursulescu, A., Underwood, M.J., Droc, I., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 515–528. [Google Scholar]
- Flynn, C.D.; De Bono, J.H.; Muston, B.; Rattan, N.; Tian, D.H.; Larobina, M.; O’keefe, M.; Skillington, P. Systematic review and meta-analysis of long-term outcomes in adults undergoing the Ross procedure. Ann. Cardiothorac. Surg. 2021, 10, 411–419. [Google Scholar] [CrossRef]
- Wang, X.; Bakhuis, W.; Veen, K.M.; Bogers, A.J.J.C.; Etnel, J.R.G.; van Der Ven, C.C.E.M.; Roos-Hesselink, J.W.; Andrinopoulou, E.-R.; Takkenberg, J.J.M. Outcomes after right ventricular outflow tract reconstruction with valve substitutes: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2022, 9, 897946. [Google Scholar] [CrossRef]
- Brown, K.N.; Kanmanthareddy, A. Ross Procedure for Aortic Valve Replacement. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- da Costa, F.D.A.; Etnel, J.R.G.; Torres, R.; Filho, E.M.B.; Torres, R.; Calixto, A.; Mulinari, L.A. Decellularized Allografts for Right Ventricular Outflow Tract Reconstruction in Children. World J. Pediatr. Congenit. Heart Surg. 2017, 8, 605–612. [Google Scholar] [CrossRef]
- Chauvette, V.; Bouhout, I.; Tarabzoni, M.; Pham, M.; Wong, D.; Whitlock, R.; Chu, M.W.; El-Hamamsy, I.; Lefebvre, L.; Poirier, N.; et al. Pulmonary homograft dysfunction after the Ross procedure using decellularized homografts—A multicenter study. J. Thorac. Cardiovasc. Surg. 2022, 163, 1296–1305.e3. [Google Scholar] [CrossRef]
- Barile, L.; Fominskiy, E.; Di Tomasso, N.; Castro, L.E.A.; Landoni, G.; De Luca, M.; Bignami, E.; Sala, A.; Zangrillo, A.; Monaco, F. Acute Normovolemic Hemodilution Reduces Allogeneic Red Blood Cell Transfusion in Cardiac Surgery: A Systematic Review and Meta-analysis of Randomized Trials. Anesth. Analg. 2017, 124, 743–752. [Google Scholar] [CrossRef]
- Weerasinghe, A.; Taylor, K.M. The platelet in cardiopulmonary bypass. Ann. Thorac. Surg. 1998, 66, 2145–2152. [Google Scholar] [PubMed]
- Greilich, P.E.; Carr, J.; Carr, S.L.; Chang, A.S. Reductions in platelet force development by cardiopulmonary bypass are associated with hemorrhage. Anesth. Analg. 1995, 80, 459–465. [Google Scholar]
- O’Carroll-Kuehn, B.U.; Meeran, H. Management of coagulation during cardiopulmonary bypass. Contin. Educ. Anaesth. Crit. Care Pain 2007, 7, 195–198. [Google Scholar]
- Monaco, F.; Guarracino, F.; Vendramin, I.; Lei, C.; Zhang, H.; Lomivorotov, V.; Osinsky, R.; Efremov, S.; Gürcü, M.E.; Mazzeffi, M.; et al. Acute normovolemic hemodilution in cardiac surgery: Rationale and design of a multicenter randomized trial. Contemp. Clin. Trials 2024, 143, 107605. [Google Scholar] [CrossRef] [PubMed]
- Murray, D. Acute normovolemic hemodilution. Eur. Spine J. 2004, 13 (Suppl. S1), S72–S75. [Google Scholar]
- Ranucci, M.; Biagioli, B.; Scolletta, S.; Grillone, G.; Cazzaniga, A.; Cattabriga, I.; Isgrò, G.; Giomarelli, P. Lowest hematocrit on cardiopulmonary bypass impairs the outcome in coronary surgery: An Italian Multicenter Study from the National Cardioanesthesia Database. Tex. Heart Inst. J. 2006, 33, 300–305. [Google Scholar]
- Hofmann, B.; Kaufmann, C.; Stiller, M.; Neitzel, T.; Wienke, A.; Silber, R.-E.; Treede, H. Positive impact of retrograde autologous priming in adult patients undergoing cardiac surgery: A randomized clinical trial. J. Cardiothorac. Surg. 2018, 13, 50. [Google Scholar] [CrossRef]
- Horvath, K.A.; Chang, H.; Bagiella, E.; Smith, P.K.; Iribarne, A.; Kron, I.L.; Lackner, P.; Argenziano, M.; Ascheim, D.D.; Gelijns, A.C.; et al. Blood Transfusion and Infection After Cardiac Surgery. Ann. Thorac. Surg. 2013, 95, 2194–2201. [Google Scholar] [CrossRef]
- Swaminathan, M.; Phillips-Bute, B.G.; Conlon, P.J.; Smith, P.K.; Newman, M.F.; Stafford-Smith, M. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann. Thorac. Surg. 2003, 76, 784–791. [Google Scholar] [CrossRef]
- Foreman, E.; Eddy, M.; Holdcombe, J.; Warren, P.; Gebicke, L.; Raney, P.; Clements, W.; Zellner, J. To RAP or Not to RAP: A Retrospective Comparison of the Effects of Retrograde Autologous Priming. J. Extracorpor. Technol. 2021, 53, 279–285. [Google Scholar] [CrossRef]
- Likhvantsev, V.V.; Landoni, G.; Levikov, D.I.; Grebenchikov, O.A.; Skripkin, Y.V.; Cherpakov, R.A. Sevoflurane Versus Total Intravenous Anesthesia for Isolated Coronary Artery Bypass Surgery With Cardiopulmonary Bypass: A Randomized Trial. J. Cardiothorac. Vasc. Anesth. 2016, 30, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Landoni, G.; Lomivorotov, V.V.; Neto, C.N.; Monaco, F.; Pasyuga, V.V.; Bradic, N.; Lembo, R.; Gazivoda, G.; Likhvantsev, V.V.; Lei, C.; et al. Volatile Anesthetics versus Total Intravenous Anesthesia for Cardiac Surgery. N. Engl. J. Med. 2019, 380, 1214–1225. [Google Scholar] [CrossRef]
- A Lobova, V.; Roll, J.M.; Roll, M.L.C. Intraoperative Methadone Use in Cardiac Surgery: A Systematic Review. Pain Med. 2021, 22, 2827–2834. [Google Scholar] [CrossRef]
- Wang, D.J.; Song, P.; Nault, K.M. Impact of intraoperative methadone use on postoperative opioid requirements after cardiac surgery. Am. J. Health Pharm. 2021, 79, 636–642. [Google Scholar] [CrossRef]
- Edwards, J.N.; Whitney, M.A.; Smith, B.B.; Fah, M.K.; Petty, S.A.B.; Durra, O.; Sell-Dottin, K.A.; Portner, E.; Wittwer, E.D.; Milam, A.J. The role of methadone in cardiac surgery for management of postoperative pain. BJA Open 2024, 10, 100270. [Google Scholar] [CrossRef]
- Murphy, G.S.; Szokol, J.W.; Avram, M.J.; Greenberg, S.B.; Marymont, J.H.; Shear, T.; Parikh, K.N.; Patel, S.S.; Gupta, D.K. Intraoperative Methadone for the Prevention of Postoperative Pain. Anesthesiology 2015, 122, 1112–1122. [Google Scholar] [CrossRef] [PubMed]
- Brinck, E.C.; Tiippana, E.; Heesen, M.; Bell, R.F.; Straube, S.; Moore, R.A.; Kontinen, V. Perioperative intravenous ketamine for acute postoperative pain in adults. Cochrane Database Syst. Rev. 2018, 12, CD012033. [Google Scholar] [CrossRef]
- Avidan, M.S.; Maybrier, H.R.; Ben Abdallah, A.; Jacobsohn, E.; E Vlisides, P.; O Pryor, K.; A Veselis, R.; Grocott, H.P.; A Emmert, D.; Rogers, E.M.; et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: An international, multicentre, double-blind, randomised clinical trial. Lancet 2017, 390, 267–275. [Google Scholar] [CrossRef]
- Mazzeffi, M.; Johnson, K.; Paciullo, C. Ketamine in adult cardiac surgery and the cardiac surgery Intensive Care Unit: An evidence-based clinical review. Ann. Card. Anaesth. 2015, 18, 202–209. [Google Scholar] [CrossRef]
- Mamoun, N.F.; Lin, P.; Zimmerman, N.M.; Mascha, E.J.; Mick, S.L.; Insler, S.R.; Sessler, D.I.; Duncan, A.E. Intravenous acetaminophen analgesia after cardiac surgery: A randomized, blinded, controlled superiority trial. J. Thorac. Cardiovasc. Surg. 2016, 152, 881–889.e1. [Google Scholar] [CrossRef]
- Young, A.M.; Strobel, R.J.; Rotar, E.P.; Kleiman, A.; McNeil, J.S.; Teman, N.R.; Hawkins, R.B.; Raphael, J.; Mehaffey, J.H. Perioperative acetaminophen is associated with reduced acute kidney injury after cardiac surgery. J. Thorac. Cardiovasc. Surg. 2024, 167, 1372–1380. [Google Scholar] [CrossRef]
- Krishna, S.N.; Chauhan, S.; Bhoi, D.; Kaushal, B.; Hasija, S.; Sangdup, T.; Bisoi, A.K. Bilateral Erector Spinae Plane Block for Acute Post-Surgical Pain in Adult Cardiac Surgical Patients: A Randomized Controlled Trial. J. Cardiothorac. Vasc. Anesth. 2019, 33, 368–375. [Google Scholar] [CrossRef]
- Kumar, A.K.; Chauhaan, S.; Bhoi, D.; Kaushal, B. Pectointercostal Fascial Block (PIFB) as a Novel Technique for Postoperative Pain Management in Patients Undergoing Cardiac Surgery. J Cardiothorac Vasc Anesth. 2021, 35, 116–122. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Chen, S. Bilateral transversus thoracis muscle plane block provides effective analgesia and enhances recovery after open cardiac surgery. J. Card. Surg. 2021, 36, 2818–2823. [Google Scholar] [CrossRef]
- Mansour, M.A.; Mahmoud, H.E.; Fakhry, D.M.; Kassim, D.Y. Comparison of the effects of transversus thoracic muscle plane block and pecto-intercostal fascial block on postoperative opioid consumption in patients undergoing open cardiac surgery: A prospective randomized study. BMC Anesthesiol. 2024, 24, 63. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, L.; Jiang, B.; Xin, L.; He, M.; Yang, W.; Zhao, Z.; Feng, Y. Effects of pecto-intercostal fascial block combined with rectus sheath block for postoperative pain management after cardiac surgery: A randomized controlled trial. BMC Anesthesiol. 2023, 23, 90. [Google Scholar] [CrossRef]
- Boyd, W.C.; Thomas, S.J. Pro: Magnesium should be administered to all coronary artery bypass graft surgery patients undergoing cardiopulmonary bypass. J. Cardiothorac. Vasc. Anesth. 2000, 14, 339–343. [Google Scholar]
- Miller, S.; Crystal, E.; Garfinkle, M.; Lau, C.; Lashevsky, I.; Connolly, S.J. Effects of magnesium on atrial fibrillation after cardiac surgery: A meta-analysis. Heart 2005, 91, 618–623. [Google Scholar] [CrossRef]
- Cadaroni, F.; Skillington, P.; O’Keefe, M.; Buratto, E.; Wynne, R. Twenty-five years of the ross operation in adults: The inclusion technique keeps up the expectations. J. Thorac. Cardiovasc. Surg. 2025, 19, S0022-5223(25)00029-7. [Google Scholar] [CrossRef]
- Grant, M.C.; Isada, T.; Ruzankin, P.; Whitman, G.; Lawton, J.S.; Dodd-O, J.; Barodka, V.; Ibekwe, S.; Mihocsa, A.B.; Gottschalk, A.; et al. Results from an enhanced recovery program for cardiac surgery. J. Thorac. Cardiovasc. Surg. 2020, 159, 1393–1402.e7. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.-T.; Lai, V.K.; Chee, Y.E.; Lee, A. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst. Rev. 2016, 2016, CD003587. [Google Scholar] [CrossRef] [PubMed]
- Shanewise, J.S.; Cheung, A.T.; Aronson, S.; Stewart, W.J.; Weiss, R.L.; Mark, J.B.; Savage, R.M.; Sears-Rogan, P.; Mathew, J.P.; Quiñones, M.A.; et al. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: Recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth. Analg. 1999, 89, 870–884. [Google Scholar] [PubMed]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Denault, A.Y.; Couture, P.; Buithieu, J.; Haddad, F.; Carrier, M.; Babin, D.; Levesque, S.; Tardif, J.-C. Left and right ventricular diastolic dysfunction as predictors of difficult separation from cardiopulmonary bypass. Can. J. Anaesth. 2006, 53, 1020–1029. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scorsese, G.; Yonel, B.; Schmalzried, E.; Solowinska, A.; Jin, Z.; Poppers, J. Perioperative Considerations, Anesthetic Management and Transesophageal Echocardiographic Evaluation of Patients Undergoing the Ross Procedure. J. Cardiovasc. Dev. Dis. 2025, 12, 126. https://doi.org/10.3390/jcdd12040126
Scorsese G, Yonel B, Schmalzried E, Solowinska A, Jin Z, Poppers J. Perioperative Considerations, Anesthetic Management and Transesophageal Echocardiographic Evaluation of Patients Undergoing the Ross Procedure. Journal of Cardiovascular Development and Disease. 2025; 12(4):126. https://doi.org/10.3390/jcdd12040126
Chicago/Turabian StyleScorsese, Giacomo, Brandon Yonel, Eric Schmalzried, Alexandra Solowinska, Zhaosheng Jin, and Jeremy Poppers. 2025. "Perioperative Considerations, Anesthetic Management and Transesophageal Echocardiographic Evaluation of Patients Undergoing the Ross Procedure" Journal of Cardiovascular Development and Disease 12, no. 4: 126. https://doi.org/10.3390/jcdd12040126
APA StyleScorsese, G., Yonel, B., Schmalzried, E., Solowinska, A., Jin, Z., & Poppers, J. (2025). Perioperative Considerations, Anesthetic Management and Transesophageal Echocardiographic Evaluation of Patients Undergoing the Ross Procedure. Journal of Cardiovascular Development and Disease, 12(4), 126. https://doi.org/10.3390/jcdd12040126






