A Narrative Review of the Evidence for Transcatheter Aortic Valve Implants
Abstract
1. Background
2. Methods
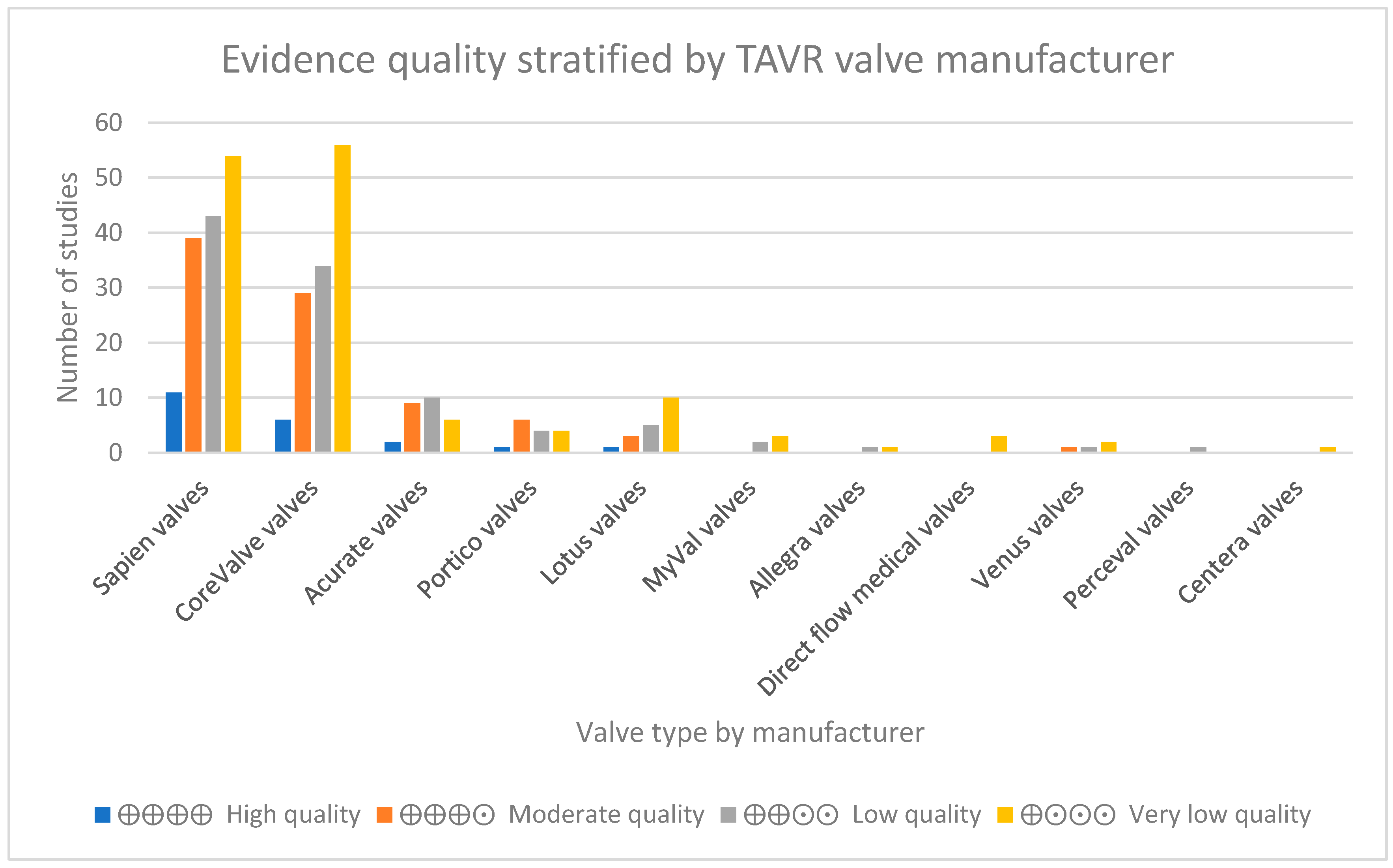
3. Results
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Search Strategy 1
- (((((‘transcatheter aortic valve replacement’/exp OR ‘transcatheter aortic valve replacement’ OR ((‘transcatheter’/exp OR transcatheter) AND aortic AND (‘valve’/exp OR valve) AND (‘replacement’/exp OR replacement)) OR ‘transcatheter aortic valve implantation’/exp OR ‘transcatheter aortic valve implantation’ OR ((‘transcatheter’/exp OR transcatheter) AND aortic AND (‘valve’/exp OR valve) AND (‘implantation’/exp OR implantation))) NOT mitral NOT pulmonary NOT reintervention NOT redo) AND (aortic AND stenosis NOT mitral)) AND ([2000–2024]/py NOT ’valve in valve’)) AND (evolut OR acurate OR portico OR navitor OR hydra OR allegra OR trilogy OR ‘j valve’ OR ‘venus a’ OR vita OR taurus OR sapien OR myval)) AND ((‘clinical article’/de OR ‘clinical study’/de OR ‘clinical trial’/de OR ‘clinical trial topic’/de OR ‘cohort analysis’/de OR ‘comparative effectiveness’/de OR ‘comparative study’/de OR ‘controlled clinical trial’/de OR ‘controlled study’/de OR ‘device comparison’/de OR ‘diagnostic test accuracy study’/de OR ‘evidence based medicine’/de OR ‘feasibility study’/de OR ‘human tissue’/de OR ‘intention to treat analysis’/de OR ‘intermethod comparison’/de OR ‘intervention study’/de OR ‘major clinical study’/de OR ‘medical record review’/de OR ‘model’/de OR ‘multicenter study’/de OR ‘multicenter study topic’/de OR ‘observational study’/de OR ‘open study’/de OR ‘pilot study’/de OR ‘proportional hazards model’/de OR ‘prospective study’/de OR ‘questionnaire’/de OR ‘randomized controlled trial’/de OR ‘randomized controlled trial topic’/de OR ‘retrospective study’/de OR ‘sample size’/de OR ‘simulation’/de OR ‘trend study’/de)).
Appendix A.2. Outcomes Figure

Appendix A.3. Journal Quality
| Top 10 Journals | Quartile | H-Index | Number of Publications | |
|---|---|---|---|---|
| Sapien valves Q1: 101 Q2: 33 Q3: 7 Q4: 6 | The New England Journal of Medicine The Lancet Circulation Journal of the American College of Cardiology European Heart Journal Scientific Reports American Journal of Cardiology Annals of Thoracic Surgery Journal of Thoracic and Cardiovascular Surgery Heart | 1 1 1 1 1 1 1 1 1 1 | 1184 895 674 493 348 315 237 222 218 207 | 4 1 4 11 7 1 12 2 2 1 |
| CoreValve valves Q1: 82 Q2: 26 Q3: 9 Q4: 6 | The New England Journal of Medicine The Lancet Circulation Journal of the American College of Cardiology European Heart Journal Scientific reports American Journal of Cardiology Annals of Thoracic Surgery Journal of Thoracic and Cardiovascular Surgery Heart | 1 1 1 1 1 1 1 1 1 1 | 1184 895 674 493 348 315 237 222 218 207 | 4 1 5 8 6 1 11 1 1 3 |
| Acurate valves Q1: 18 Q2: 7 Q3: 1 Q4: 0 | The Lancet Circulation European Heart Journal Circulation Scientific reports JACC: Cardiovascular Interventions International Journal of Cardiology Catheterization and Cardiovascular Interventions Circulation: Cardiovascular Interventions Journal of Clinical Medicine EuroIntervention | 1 1 1 1 1 1 1 1 1 1 | 895 674 348 315 154 146 130 116 113 95 | 1 1 1 1 1 3 2 2 2 2 |
| Perceval valves | Thoracic and Cardiovascular Surgeon | 2 | 58 | 1 |
| Centera valves | Journal of Thoracic Disease | 2 | 88 | 1 |
| Portico valves Q1: 9 Q2: 6 Q3: 0 Q4: 0 | The Lancet Scientific reports American Journal of Cardiology International Journal of Cardiology Catheterization and Cardiovascular Interventions Circulation: Cardiovascular Interventions Journal of Clinical Medicine Clinical Research in Cardiology Revista Espanola de Cardiologia Journal of Invasive Cardiology | 1 1 1 1 1 1 1 1 2 2 | 895 315 237 146 130 116 113 82 76 64 | 1 1 1 1 1 1 1 2 2 2 |
| Lotus valves Q1: 10 Q2: 6 Q3: 1 Q4: 2 | Circulation Journal of the American College of Cardiology Scientific reports Internal Medicine Journal International Journal of Cardiology Eurospace Internal Medicine Journal Circulation: Cardiovascular Interventions EuroIntervention Clinical Research in Cardiology | 1 1 1 1 1 1 1 1 1 1 | 674 493 315 188 146 128 188 116 95 82 | 1 2 1 1 1 1 1 2 1 1 |
| MyVal valves | International Journal of Cardiology Journal of Clinical Medicine Revista Espanola de Cardiologia | 1 1 2 | 146 113 76 | 2 2 1 |
| Allegra valves | International Journal of Cardiology Revista Espanola de Cardiologia | 1 2 | 146 76 | 1 1 |
| Direct flow medical valves | International Journal of Cardiology Journal of Cardiology Anatolian Journal of Cardiology | 1 2 3 | 146 63 37 | 1 1 1 |
| Venus valves | Catheterization and Cardiovascular Interventions Annals of Translational Medicine International Heart Journal Academic Journal of Second Military Medical University | 1 1 3 4 | 130 75 54 11 | 1 1 1 1 |
References
- Strange, G.; Scalia, G.M.; Playford, D.; Simon, S. Uncovering the treatable burden of severe aortic stenosis in Australia: Current and future projections within an ageing population. BMC Health Serv. Res. 2021, 21, 790. [Google Scholar] [CrossRef]
- Cao, C.; Ang, S.C.; Indraratna, P.; Manganas, C.; Bannon, P.; Black, D.; Tian, D.; Yan, T.D. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann. Cardiothorac. Surg. 2013, 2, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Afoakwah, C.; Chan, Y.K.; Strom, J.B.; Playford, D.; Strange, G.A. Counting the cost of premature mortality with progressively worse aortic stenosis in Australia: A clinical cohort study. Lancet Healthy Longev. 2022, 3, e599–e606. [Google Scholar] [CrossRef] [PubMed]
- Straiton, N.; Jin, K.; Bhindi, R.; Gallagher, R. Functional capacity and health-related quality of life outcomes post transcatheter aortic valve replacement: A systematic review and meta-analysis. Age Ageing 2018, 47, 478–482. [Google Scholar] [CrossRef]
- Medical Servise Advisory Committee. 1635—Transcatheter Aortic Valve Implantation via Transfemoral Delivery Using the Balloon-Expandable Valve System for Patients at Low Risk for Surgery. Available online: https://www.msac.gov.au/applications/1635 (accessed on 21 March 2025).
- Harvey, J.E., 3rd; Kapadia, S.R.; Cohen, D.J.; Kalra, A.; Irish, W.; Gunnarsson, C.; Ryan, M.; Chikermane, S.G.; Thompson, C.; Puri, R. Trends in Complications Among Patients Undergoing Aortic Valve Replacement in the United States. J. Am. Heart Assoc. 2024, 13, e031461. [Google Scholar] [CrossRef]
- Arora, S.; Strassle, P.D.; Kolte, D.; Ramm, C.J.; Falk, K.; Jack, G.; Caranasos, T.G.; Cavender, M.A.; Rossi, J.S.; Vavalle, J.P. Length of Stay and Discharge Disposition After Transcatheter Versus Surgical Aortic Valve Replacement in the United States. Circ. Cardiovasc. Interv. 2018, 11, e006929. [Google Scholar] [CrossRef]
- Vinayak, M.; Leone, P.P.; Tanner, R.; Dhulipala, V.; Camaj, A.; Makhija, R.R.K.; Hooda, A.; Kini, A.S.; Sharma, S.K.; Khera, S. Transcatheter Aortic Valve Replacement: Current Status and Future Indications. J. Clin. Med. 2024, 13, 0373. [Google Scholar] [CrossRef]
- Wang, B.; Mei, Z.; Ge, X.; Li, Y.; Zhou, Q.; Meng, X.; An, G. Comparison of outcomes of self-expanding versus balloon-expandable valves for transcatheter aortic valve replacement: A meta-analysis of randomized and propensity-matched studies. BMC Cardiovasc. Disord. 2023, 23, 382. [Google Scholar] [CrossRef]
- Therapeutic Goods Administration (TGA). Medical Devices Overview. 2024. Available online: https://www.tga.gov.au/products/medical-devices (accessed on 21 March 2025).
- Kim, H.; Byrnes, J.; Goodall, S.; ISPOR Australia Chapter Executive Committee. Health Technology Assessment in Australia: The Pharmaceutical Benefits Advisory Committee and Medical Services Advisory Committee. Value Health Reg. Issues 2021, 24, 6–11. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Aged Care. The Prostheses List. 2024. Available online: https://www.health.gov.au/resources/publications/prostheses-list-post-listing-review-consultants-report?language=en (accessed on 21 March 2025).
- Aromataris, E.L.C.; Porritt, K.; Pilla, B.; Jordan, Z. JBI Manual for Evidence Synthesis. JBI 2024. [Google Scholar] [CrossRef]
- SJR. Scimago Journal & Country Rank. Available online: https://www.scimagojr.com/ (accessed on 21 March 2025).
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, H.C.; Mehran, R.; Blackman, D.J.; Bailey, S.; Mollmann, H.; Abdel-Wahab, M.; Ben Ali, W.; Mahoney, P.D.; Ruge, H.; Wood, D.A.; et al. Self-Expanding or Balloon-Expandable TAVR in Patients with a Small Aortic Annulus. N. Engl. J. Med. 2024, 390, 1959–1971. [Google Scholar] [CrossRef]
- Genereux, P.; Schwartz, A.; Oldemeyer, J.B.; Pibarot, P.; Cohen, D.J.; Blanke, P.; Lindman, B.R.; Babaliaros, V.; Fearon, W.F.; Daniels, D.V.; et al. Transcatheter Aortic-Valve Replacement for Asymptomatic Severe Aortic Stenosis. N. Engl. J. Med. 2024, 392, 217–227. [Google Scholar] [CrossRef]
- Blankenberg, S.; Seiffert, M.; Vonthein, R.; Baumgartner, H.; Bleiziffer, S.; Borger, M.A.; Choi, Y.H.; Clemmensen, P.; Cremer, J.; Czerny, M.; et al. Transcatheter or Surgical Treatment of Aortic-Valve Stenosis. N. Engl. J. Med. 2024, 390, 1572–1583. [Google Scholar] [CrossRef]
- Wei, L.; Wang, B.; Yang, Y.; Dong, L.; Chen, X.; Bramlage, P.; Wang, Y. Transcatheter aortic valve replacement in China—A review of the available evidence. AsiaIntervention 2024, 10, 110–118. [Google Scholar] [CrossRef]
- Xu, H.; Liu, Q.; Cao, K.; Ye, Y.; Zhang, B.; Li, Z.; Hao, J.; Qi, X.; Zhao, Q.; Liu, S.; et al. Distribution, Characteristics, and Management of Older Patients With Valvular Heart Disease in China: China-DVD Study. JACC Asia 2022, 2, 354–365. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sivaprakasam, M.C.; PaulPandi, V.K.; Oomman, A.; Mahilmaran, A.; Kalarickal, M.S.; Sadhasivam, V.S.; Chandrasekaran, G.A.; Kanthallu Narayanamoorthy, S.; Karaimbil Puthukavi, P.K.; et al. Transcatheter aortic valve replacement in India-Early experience, challenges, and outcomes from a single center. Indian. Heart J. 2018, 70 (Suppl. S3), S347–S352. [Google Scholar] [CrossRef]
- Yildirim, E.; Yuksel, U.C.; Celik, M.; Bugan, B.; Gokoglan, Y.; Yasar, S.; Gormel, S.; Asil, S.; Murat, E.; Yener, S.; et al. Evaluation of the Transcatheter Aortic Valve Replacement Results in Patients with Borderline Aortic Annulus and the Impact of Under- or Oversizing the Valve. Anatol. J. Cardiol. 2023, 27, 189–196. [Google Scholar] [CrossRef]
- Smith, C.R.; Leon, M.B.; Mack, M.J.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N. Engl. J. Med. 2011, 364, 2187–2198. [Google Scholar] [CrossRef]
- Makkar, R.R.; Fontana, G.P.; Jilaihawi, H.; Kapadia, S.; Pichard, A.D.; Douglas, P.S.; Thourani, V.H.; Babaliaros, V.C.; Webb, J.G.; Herrmann, H.C.; et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N. Engl. J. Med. 2012, 366, 1696–1704. [Google Scholar] [CrossRef]
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef]
- Tay, E.L.; Gurvitch, R.; Wijeysinghe, N.; Nietlispach, F.; Leipsic, J.; Wood, D.A.; Yong, G.; Cheung, A.; Ye, J.; Lichtenstein, S.V.; et al. Outcome of patients after transcatheter aortic valve embolization. JACC Cardiovasc. Interv. 2011, 4, 228–234. [Google Scholar] [CrossRef]
- Rodes-Cabau, J.; Webb, J.G.; Cheung, A.; Ye, J.; Dumont, E.; Feindel, C.M.; Osten, M.; Natarajan, M.K.; Velianou, J.L.; Martucci, G.; et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: Acute and late outcomes of the multicenter Canadian experience. J. Am. Coll. Cardiol. 2010, 55, 1080–1090. [Google Scholar] [CrossRef]
- Moat, N.E.; Ludman, P.; de Belder, M.A.; Bridgewater, B.; Cunningham, A.D.; Young, C.P.; Thomas, M.; Kovac, J.; Spyt, T.; MacCarthy, P.A.; et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: The U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J. Am. Coll. Cardiol. 2011, 58, 2130–2138. [Google Scholar] [CrossRef]
- Kenny, D.; Hijazi, Z.M.; Kar, S.; Rhodes, J.; Mullen, M.; Makkar, R.; Shirali, G.; Fogel, M.; Fahey, J.; Heitschmidt, M.G.; et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: Early phase 1 results from an international multicenter clinical trial. J. Am. Coll. Cardiol. 2011, 58, 2248–2256. [Google Scholar] [CrossRef]
- Cribier, A.; Eltchaninoff, H.; Tron, C.; Bauer, F.; Agatiello, C.; Nercolini, D.; Tapiero, S.; Litzler, P.Y.; Bessou, J.P.; Babaliaros, V. Treatment of calcific aortic stenosis with the percutaneous heart valve: Mid-term follow-up from the initial feasibility studies: The French experience. J. Am. Coll. Cardiol. 2006, 47, 1214–1223. [Google Scholar] [CrossRef]
- Bosmans, J.M.; Kefer, J.; De Bruyne, B.; Herijgers, P.; Dubois, C.; Legrand, V.; Verheye, S.; Rodrigus, I.; Belgian, T.R.P. Procedural, 30-day and one year outcome following CoreValve or Edwards transcatheter aortic valve implantation: Results of the Belgian national registry. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 762–767. [Google Scholar] [CrossRef]
- Cribier, A.; Litzler, P.Y.; Eltchaninoff, H.; Godin, M.; Tron, C.; Bauer, F.; Bessou, J.P. Technique of transcatheter aortic valve implantation with the Edwards-Sapien heart valve using the transfemoral approach. Herz 2009, 34, 347–356. [Google Scholar] [CrossRef]
- Piazza, N.; Grube, E.; Gerckens, U.; den Heijer, P.; Linke, A.; Luha, O.; Ramondo, A.; Ussia, G.; Wenaweser, P.; Windecker, S.; et al. Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: Results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntervention 2008, 4, 242–249. [Google Scholar] [CrossRef]
- Panico, R.A.; Giannini, C.; De Carlo, M.; Angelillis, M.; Spontoni, P.; Pieroni, A.; Costa, G.; Bertini, P.; Guarracino, F.; Petronio, A.S. Long-term results and durability of the CoreValve transcatheter aortic bioprosthesis: Outcomes beyond five years. EuroIntervention 2019, 14, 1639–1647. [Google Scholar] [CrossRef]
- Ussia, G.P.; Barbanti, M.; Petronio, A.S.; Tarantini, G.; Ettori, F.; Colombo, A.; Violini, R.; Ramondo, A.; Santoro, G.; Klugmann, S.; et al. Transcatheter aortic valve implantation: 3-year outcomes of self-expanding CoreValve prosthesis. Eur. Heart J. 2012, 33, 969–976. [Google Scholar] [CrossRef]
- Eltchaninoff, H.; Prat, A.; Gilard, M.; Leguerrier, A.; Blanchard, D.; Fournial, G.; Iung, B.; Donzeau-Gouge, P.; Tribouilloy, C.; Debrux, J.L.; et al. Transcatheter aortic valve implantation: Early results of the FRANCE (FRench Aortic National CoreValve and Edwards) registry. Eur. Heart J. 2011, 32, 191–197. [Google Scholar] [CrossRef]
- Thomas, M.; Schymik, G.; Walther, T.; Himbert, D.; Lefevre, T.; Treede, H.; Eggebrecht, H.; Rubino, P.; Michev, I.; Lange, R.; et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation 2010, 122, 62–69. [Google Scholar] [CrossRef]
- Reynolds, M.R.; Magnuson, E.A.; Lei, Y.; Leon, M.B.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Babaliaros, V.C.; Bowers, B.S.; Fearon, W.F.; et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation 2011, 124, 1964–1972. [Google Scholar] [CrossRef]
- Grube, E.; Laborde, J.C.; Gerckens, U.; Felderhoff, T.; Sauren, B.; Buellesfeld, L.; Mueller, R.; Menichelli, M.; Schmidt, T.; Zickmann, B.; et al. Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: The Siegburg first-in-man study. Circulation 2006, 114, 1616–1624. [Google Scholar] [CrossRef]
- Jilaihawi, H.; Chakravarty, T.; Weiss, R.E.; Fontana, G.P.; Forrester, J.; Makkar, R.R. Meta-analysis of complications in aortic valve replacement: Comparison of Medtronic-Corevalve, Edwards-Sapien and surgical aortic valve replacement in 8,536 patients. Catheter. Cardiovasc. Interv. 2012, 80, 128–138. [Google Scholar] [CrossRef]
- Umscheid, C.A.; Margolis, D.J.; Grossman, C.E. Key concepts of clinical trials: A narrative review. Postgrad. Med. 2011, 123, 194–204. [Google Scholar] [CrossRef]
- The Pharmaceutical Benefits Advisory Committee. Guidelines for Preparing Submissions to the Pharmaceutical Benefits Advisory Committee (PBAC). Available online: https://pbac.pbs.gov.au/ (accessed on 21 March 2025).
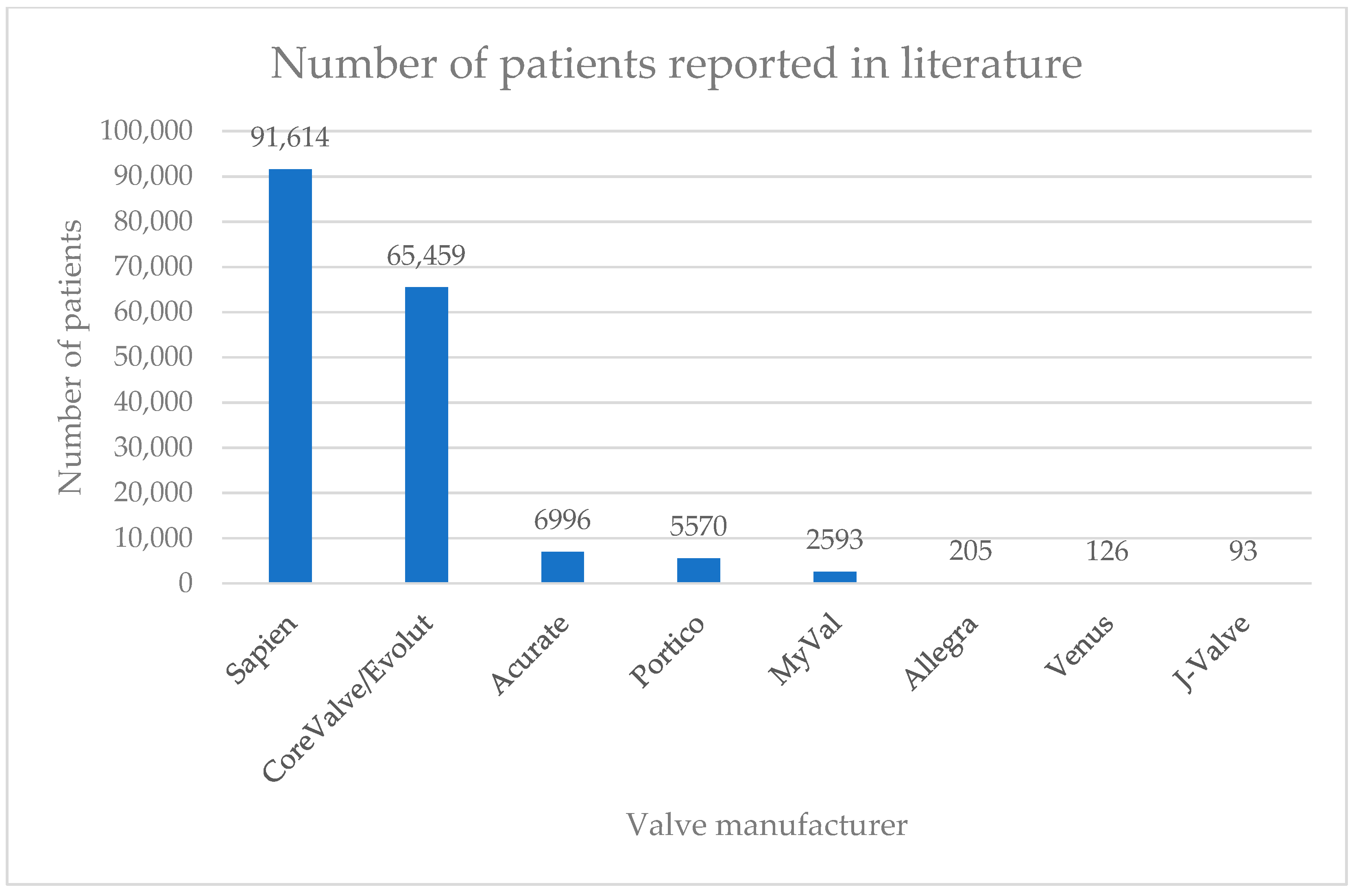
| Self-Expandable Valves | ||||||
| Medtronic, Minneapolis, MN, USA | ||||||
| Number of studies | CoreValve | Evolut R | Evolut PRO | Evolut PRO+ | Evolut FX | |
| 94 | 43 | 23 | 4 | 1 | ||
| Boston Scientific, Marlborough, MA, USA | ||||||
| Number of studies | Acurate Neo | Acurate Neo 2 | ||||
| 27 | 4 | |||||
| Abbott, Chicago, IL, USA | ||||||
| Number of studies | Portico | Navitor | ||||
| 11 | 0 | |||||
| New Valve Technology, Hechingen, Germany | ||||||
| Number of studies | Allegra | |||||
| 1 | ||||||
| JC Medical Inc., Burlingame, CA, USA | ||||||
| Number of studies | J-valve | |||||
| 5 | ||||||
| Venus Medtech Inc., Hangzhou, China | ||||||
| Number of studies | Venus-A | Venus-A-Plus | Venus-A-Pro | |||
| 4 | 0 | 0 | ||||
| Balloon-expandable valves | ||||||
| Edwards Lifesciences, Irvine, CA, USA | ||||||
| Number of studies | Sapien | Sapien XT | Sapien 3 | Sapien 3 Ultra | Sapien 3 Ultra Resilia | Sapien X4 |
| 64 | 44 | 65 | 6 | 1 | 0 | |
| Meril, Vapi, Gujarat, India | ||||||
| MyVal | MyVal Octacor | |||||
| Number of studies | 5 | 0 | ||||
| Anteris Technologies, Toowong, Australia | ||||||
| DurAVR | ||||||
| Number of studies | 0 | |||||
| Indication | Valve Type (Approval Date) |
|---|---|
| Symptomatic severe aortic stenosis (all risk categories) | Sapien 3 (26 March 2019), Sapien 3 Ultra (18 September 2020), Evolut R (9 July 2020), Evolut R+ (31 October 2019), Evolut PRO (14 July 2020), Evolut FX (7 December 2023) |
| Symptomatic severe aortic stenosis at high risk only | Portico (4 January 2017), Navitor (several different models: 23 November 2022, 21 May 2024, 6 June 2024) |
| Symptomatic severe aortic stenosis at extreme risk only | Navitor (several different models: 23 November 2022, 21 May 2024, 6 June 2024) |
| Valve replacement (valve-in-valve, secondary) | Sapien 3 (26 March 2019), Sapien 3 Ultra (18 September 2020) |
| Valve replacement (surgical, secondary) | Sapien 3 (26 March 2019), Sapien 3 Ultra (18 September 2020), Evolut R (9 July 2020), Evolut R+ (31 October 2019), Evolut PRO (14 July 2020), Evolut FX (7 December 2023) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, L.; Chan, B.M.-W.; Spencer, M.; Leung, J.; Liew, D.; Kim, H. A Narrative Review of the Evidence for Transcatheter Aortic Valve Implants. J. Cardiovasc. Dev. Dis. 2025, 12, 113. https://doi.org/10.3390/jcdd12040113
Lee L, Chan BM-W, Spencer M, Leung J, Liew D, Kim H. A Narrative Review of the Evidence for Transcatheter Aortic Valve Implants. Journal of Cardiovascular Development and Disease. 2025; 12(4):113. https://doi.org/10.3390/jcdd12040113
Chicago/Turabian StyleLee, Leonard, Brendan Min-Wei Chan, Melinda Spencer, Jovi Leung, Danny Liew, and Hansoo Kim. 2025. "A Narrative Review of the Evidence for Transcatheter Aortic Valve Implants" Journal of Cardiovascular Development and Disease 12, no. 4: 113. https://doi.org/10.3390/jcdd12040113
APA StyleLee, L., Chan, B. M.-W., Spencer, M., Leung, J., Liew, D., & Kim, H. (2025). A Narrative Review of the Evidence for Transcatheter Aortic Valve Implants. Journal of Cardiovascular Development and Disease, 12(4), 113. https://doi.org/10.3390/jcdd12040113






