The Role of Cardiac Magnetic Resonance in Characterizing Atrial Cardiomyopathy and Guiding Substrate Ablation in Atrial Fibrillation: A Narrative Review
Abstract
1. Introduction
2. Methods
3. AtCM and AF: Which Came First?
4. Atrial CMR Imaging for LA Fibrosis Evaluation: A New Frontier
5. The Role of CMR in Planning AF Ablation
6. Substrate AF Ablation Using CMR-Guided Approach
7. Limitations of DE-CMR in Detecting Atrial Fibrosis
8. DE-CMR in Guiding AF Substrate Ablation: Beyond the DECAAF II Results
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [PubMed]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.; Shamloo, A.S.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. J. Interv. Card. Electrophysiol. 2024, 67, 921–1072. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Palombi, M.; Jabbour, J.P.; Pierucci, N.; Cipollone, P.; Piro, A.; Chimenti, C.; Miraldi, F.; Vizza, C.D.; Lavalle, C. Usefulness of empiric superior vena cava isolation in paroxysmal atrial fibrillation ablation: A meta-analysis of randomized clinical trials. J. Interv. Card. Electrophysiol. 2025, 68, 93–100. [Google Scholar] [CrossRef]
- Tsadok, M.A.; Jackevicius, C.A.; Essebag, V.; Eisenberg, M.J.; Rahme, E.; Humphries, K.H.; Tu, J.V.; Behlouli, H.; Pilote, L. Rhythm versus rate control therapy and subsequent stroke or transient ischemic attack in patients with atrial fibrillation. Circulation 2012, 126, 2680–2687. [Google Scholar]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA 2010, 303, 333–340. [Google Scholar]
- Mont, L.; Bisbal, F.; Hernández-Madrid, A.; Pérez-Castellano, N.; Viñolas, X.; Arenal, A.; Arribas, F.; Fernández-Lozano, I.; Bodegas, A.; Cobos, A.; et al. Catheter ablation vs. antiarrhythmic drug treatment of persistent atrial fibrillation: A multicentre, randomized, controlled trial (SARA study). Eur. Heart J. 2014, 35, 501–507. [Google Scholar]
- Goette, A.; Corradi, D.; Dobrev, D.; Aguinaga, L.; Cabrera, J.A.; Chugh, S.S.; de Groot, J.R.; Soulat-Dufour, L.; Fenelon, G.; Hatem, S.N.; et al. Atrial cardiomyopathy revisited-evolution of a concept: A clinical consensus statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), the Asian Pacific Heart Rhythm Society (APHRS), and the Latin American Heart Rhythm Society (LAHRS). EP Eur. 2024, 26, euae204. [Google Scholar]
- Masuda, M.; Matsuda, Y.; Uematsu, H.; Sugino, A.; Ooka, H.; Kudo, S.; Fujii, S.; Asai, M.; Okamoto, S.; Ishihara, T.; et al. Prognostic impact of atrial cardiomyopathy: Long-term follow-up of patients with and without low-voltage areas following atrial fibrillation ablation. Heart Rhythm 2024, 21, 378–386. [Google Scholar]
- Goette, A.; Kalman, J.M.; Aguinaga, L.; Akar, J.; Cabrera, J.A.; Chen, S.A.; Chugh, S.S.; Corradi, D.; D’Avila, A.; Dobrev, D.; et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: Definition, characterization, and clinical implication. Europace 2016, 18, 1455–1490. [Google Scholar]
- Yoneda, Z.T.; Anderson, K.C.; Quintana, J.A.; O’Neill, M.J.; Sims, R.A.; Glazer, A.M.; Shaffer, C.M.; Crawford, D.M.; Stricker, T.; Ye, F.; et al. Early-Onset Atrial Fibrillation and the Prevalence of Rare Variants in Cardiomyopathy and Arrhythmia Genes. JAMA Cardiol. 2021, 6, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, Z.T.; Anderson, K.C.; Ye, F.; Quintana, J.A.; O’Neill, M.J.; Sims, R.A.; Sun, L.; Glazer, A.M.; Davogustto, G.; El-Harasis, M.; et al. Mortality Among Patients With Early-Onset Atrial Fibrillation and Rare Variants in Cardiomyopathy and Arrhythmia Genes. JAMA Cardiol. 2022, 7, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Baman, J.R.; Cox, J.L.; McCarthy, P.M.; Kim, D.; Patel, R.B.; Passman, R.S.; Wilcox, J.E. Atrial fibrillation and atrial cardiomyopathies. J. Cardiovasc. Electrophysiol. 2021, 32, 2845–2853. [Google Scholar] [PubMed]
- Yao, C.; Veleva, T.; Scott, L., Jr.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Ninni, S.; Dombrowicz, D.; de Winther, M.; Staels, B.; Montaigne, D.; Nattel, S. Genetic Factors Altering Immune Responses in Atrial Fibrillation: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2024, 83, 1163–1176. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Aguilar, M.; Thorin, E.; Ferbeyre, G.; Nattel, S. The role of cellular senescence in cardiac disease: Basic biology and clinical relevance. Nat. Rev. Cardiol. 2022, 19, 250–264. [Google Scholar] [CrossRef]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Opstad, T.B.; Tveit, A.; Solheim, S.; Arnesen, H.; Seljeflot, I. Biomarkers of ageing and cardiac remodeling are associated with atrial fibrillation. Scand. Cardiovasc. J. 2021, 55, 213–219. [Google Scholar] [CrossRef]
- Davies, S.S.; May-Zhang, L.S.; Boutaud, O.; Amarnath, V.; Kirabo, A.; Harrison, D.G. Isolevuglandins as mediators of disease and the development of dicarbonyl scavengers as pharmaceutical interventions. Pharmacol. Ther. 2020, 205, 107418. [Google Scholar] [CrossRef]
- Wong, C.X.; Abed, H.S.; Molaee, P.; Nelson, A.J.; Brooks, A.G.; Sharma, G.; Leong, D.P.; Lau, D.H.; Middeldorp, M.E.; Roberts-Thomson, K.C.; et al. Pericardial fat is associated with atrial fibrillation severity and ablation outcome. J. Am. Coll. Cardiol. 2011, 57, 1745–1751. [Google Scholar]
- Tsao, H.M.; Hu, W.C.; Wu, M.H.; Tai, C.T.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Wu, T.J.; et al. Quantitative analysis of quantity and distribution of epicardial adipose tissue surrounding the left atrium in patients with atrial fibrillation and effect of recurrence after ablation. Am. J. Cardiol. 2011, 107, 1498–1503. [Google Scholar]
- Wijffels, M.C.; Kirchhof, C.J.; Dorland, R.; Allessie, M.A. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [PubMed]
- Nattel, S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin. Electrophysiol. 2017, 3, 425–435. [Google Scholar] [PubMed]
- Sohns, C.; Marrouche, N.F. Atrial fibrillation and cardiac fibrosis. Eur. Heart J. 2020, 41, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.G.; Newton-Cheh, C.; Almgren, P.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Platonov, P.G.; Hedblad, B.; Engström, G.; Wang, T.J.; et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J. Am. Coll. Cardiol. 2010, 56, 1712–1719. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: The DECAAF study. JAMA 2014, 311, 498–506. [Google Scholar]
- McGann, C.; Akoum, N.; Patel, A.; Kholmovski, E.; Revelo, P.; Damal, K.; Wilson, B.; Cates, J.; Harrison, A.; Ranjan, R.; et al. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ. Arrhythmia Electrophysiol. 2014, 7, 23–30. [Google Scholar] [CrossRef]
- Kuppahally, S.S.; Akoum, N.; Badger, T.J.; Burgon, N.S.; Haslam, T.; Kholmovski, E.; Macleod, R.; McGann, C.; Marrouche, N.F. Echocardiographic left atrial reverse remodeling after catheter ablation of atrial fibrillation is predicted by preablation delayed enhancement of left atrium by magnetic resonance imaging. Am. Heart J. 2010, 160, 877–884. [Google Scholar] [CrossRef]
- Lee, H.G.; Shim, J.; Choi, J.I.; Kim, Y.H.; Oh, Y.W.; Hwang, S.H. Use of Cardiac Computed Tomography and Magnetic Resonance Imaging in Case Management of Atrial Fibrillation with Catheter Ablation. Korean J. Radiol. 2019, 20, 695–708. [Google Scholar]
- Fonseca, A.C.; Alves, P.; Inácio, N.; Marto, J.P.; Viana-Baptista, M.; Pinho-E-Melo, T.; Ferro, J.M.; Almeida, A.G. Patients With Undetermined Stroke Have Increased Atrial Fibrosis: A Cardiac Magnetic Resonance Imaging Study. Stroke 2018, 49, 734–737. [Google Scholar]
- Yaghi, S.; Kamel, H.; Elkind, M.S.V. Atrial cardiopathy: A mechanism of cryptogenic stroke. Expert Rev. Cardiovasc. Ther. 2017, 15, 591–599. [Google Scholar]
- Ferró, E.; Pérez, N.; Althoff, T.; Guasch, E.; Prat, S.; Doltra, A.; Borrás, R.; Tolosana, J.M.; Arbelo, E.; Sitges, M.; et al. Magnetic resonance detection of advanced atrial cardiomyopathy increases the risk for atypical atrial flutter occurrence following atrial fibrillation ablation. Europace 2023, 25, euad276. [Google Scholar] [PubMed]
- Guglielmo, M.; Baggiano, A.; Muscogiuri, G.; Fusini, L.; Andreini, D.; Mushtaq, S.; Conte, E.; Annoni, A.; Formenti, A.; Mancini, E.M.; et al. Multimodality imaging of left atrium in patients with atrial fibrillation. J. Cardiovasc. Comput. Tomogr. 2019, 13, 340–346. [Google Scholar] [PubMed]
- Anselmino, M.; Sillano, D.; Casolati, D.; Ferraris, F.; Scaglione, M.; Gaita, F. A new electrophysiology era: Zero fluoroscopy. J. Cardiovasc. Med. 2013, 14, 221–227. [Google Scholar]
- Njeim, M.; Desjardins, B.; Bogun, F. Multimodality Imaging for Guiding EP Ablation Procedures. JACC Cardiovasc. Imaging 2016, 9, 873–886. [Google Scholar]
- Kottkamp, H. Human atrial fibrillation substrate: Towards a specific fibrotic atrial cardiomyopathy. Eur. Heart J. 2013, 34, 2731–2738. [Google Scholar]
- Quinto, L.; Cozzari, J.; Benito, E.; Alarcón, F.; Bisbal, F.; Trotta, O.; Caixal, G.; San Antonio, R.; Garre, P.; Prat-Gonzalez, S.; et al. Magnetic resonance-guided re-ablation for atrial fibrillation is associated with a lower recurrence rate: A case-control study. Europace 2020, 22, 1805–1811. [Google Scholar]
- Akoum, N.; Morris, A.; Perry, D.; Cates, J.; Burgon, N.; Kholmovski, E.; MacLeod, R.; Marrouche, N. Substrate Modification Is a Better Predictor of Catheter Ablation Success in Atrial Fibrillation Than Pulmonary Vein Isolation: An LGE-MRI Study. Clin. Med. Insights Cardiol. 2015, 9, 25–31. [Google Scholar]
- Bisbal, F.; Benito, E.; Teis, A.; Alarcón, F.; Sarrias, A.; Caixal, G.; Villuendas, R.; Garre, P.; Soto, N.; Cozzari, J.; et al. Magnetic Resonance Imaging-Guided Fibrosis Ablation for the Treatment of Atrial Fibrillation: The ALICIA Trial. Clin. Med. Insights Cardiol. 2015, 9, 25–31. [Google Scholar]
- Marrouche, N.F.; Wazni, O.; McGann, C.; Greene, T.; Dean, J.M.; Dagher, L.; Kholmovski, E.; Mansour, M.; Marchlinski, F.; Wilber, D.; et al. DECAAF II Investigators. Effect of MRI-Guided Fibrosis Ablation vs. Conventional Catheter Ablation on Atrial Arrhythmia Recurrence in Patients With Persistent Atrial Fibrillation: The DECAAF II Randomized Clinical Trial. JAMA 2022, 327, 2296–2305. [Google Scholar]
- Akoum, N.; Wilber, D.; Hindricks, G.; Jais, P.; Cates, J.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. MRI Assessment of Ablation-Induced Scarring in Atrial Fibrillation: Analysis from the DECAAF Study. J. Cardiovasc. Electrophysiol. 2015, 26, 473–480. [Google Scholar]
- Chelu, M.G.; King, J.B.; Kholmovski, E.G.; Ma, J.; Gal, P.; Marashly, Q.; AlJuaid, M.A.; Kaur, G.; Silver, M.A.; Johnson, K.A.; et al. Atrial fibrosis by late gadolinium enhancement magnetic resonance imaging and catheter ablation of atrial fibrillation: 5-year follow-up data. J. Am. Heart Assoc. 2018, 7, e006313. [Google Scholar] [PubMed]
- Eichenlaub, M.; Mueller-Edenborn, B.; Minners, J.; Figueras, I.; Ventura, R.M.; Forcada, B.R.; Colomer, A.V.; Hein, M.; Ruile, P.; Lehrmann, H.; et al. Comparison of various late gadolinium enhancement magnetic resonance imaging methods with high-definition voltage and activation mapping for detection of atrial cardiomyopathy. Europace 2022, 24, 1102–1111. [Google Scholar] [PubMed]
- Oakes, R.S.; Badger, T.J.; Kholmovski, E.G.; Akoum, N.; Burgon, N.S.; Fish, E.N.; Blauer, J.J.; Rao, S.N.; DiBella, E.V.; Segerson, N.M.; et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation 2009, 119, 1758–1767. [Google Scholar] [PubMed]
- Benito, E.M.; Carlosena-Remirez, A.; Guasch, E.; Prat-González, S.; Perea, R.J.; Figueras, R.; Borràs, R.; Andreu, D.; Arbelo, E.; Tolosana, J.M.; et al. Left atrial fibrosis quantification by late gadolinium-enhanced magnetic resonance: A new method to standardize the thresholds for reproducibility. Europace 2017, 19, 1272–1279. [Google Scholar] [CrossRef]
- Khurram, I.M.; Beinart, R.; Zipunnikov, V.; Dewire, J.; Yarmohammadi, H.; Sasaki, T.; Spragg, D.D.; Marine, J.E.; Berger, R.D.; Halperin, H.R.; et al. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm 2014, 11, 85–92. [Google Scholar] [CrossRef]
- Zghaib, T.; Keramati, A.; Chrispin, J.; Huang, D.; Balouch, M.A.; Ciuffo, L.; Berger, R.D.; Marine, J.E.; Ashikaga, H.; Calkins, H.; et al. Multimodal examination of atrial fibrillation substrate: Correlation of left atrial bipolar voltage using multi-electrode fast automated mapping, point-by-point mapping, and magnetic resonance image intensity ratio. JACC Clin. Electrophysiol. 2018, 4, 59–68. [Google Scholar]
- Maesen, B.; Verheule, S.; Zeemering, S.; La Meir, M.; Nijs, J.; Lumeij, S.; Lau, D.H.; Granier, M.; Crijns, H.J.; Maessen, J.G.; et al. Endomysial fibrosis, rather than overall connective tissue content, is the main determinant of conduction disturbances in human atrial fibrillation. Europace 2022, 24, 1015–1024. [Google Scholar]
- Hopman, L.H.G.A.; Bhagirath, P.; Mulder, M.J.; Eggink, I.N.; van Rossum, A.C.; Allaart, C.P.; Götte, M.J.W. Extent of left atrial fibrosis correlates with descending aorta proximity at 3D late gadolinium enhancement cardiac MRI in patients with atrial fibrillation. Radiol. Cardiothorac. Imaging 2022, 4, e210192. [Google Scholar]
- Masuda, M.; Asai, M.; Iida, O.; Okamoto, S.; Ishihara, T.; Nanto, K.; Kanda, T.; Tsujimura, T.; Matsuda, Y.; Okuno, S.; et al. Additional low-voltage-area ablation in patients with paroxysmal atrial fibrillation: Results of the randomized controlled VOLCANO trial. J. Am. Heart Assoc. 2020, 9, e015927. [Google Scholar]
- Matteucci, A.; Bonanni, M.; Versaci, F.; Frati, G.; Peruzzi, M.; Sangiorgi, G.; Biondi-Zoccai, G.; Massaro, G. Cardiovascular medicine: A year in review. Minerva Cardiol. Angiol. 2022, 70, 40–55. [Google Scholar]
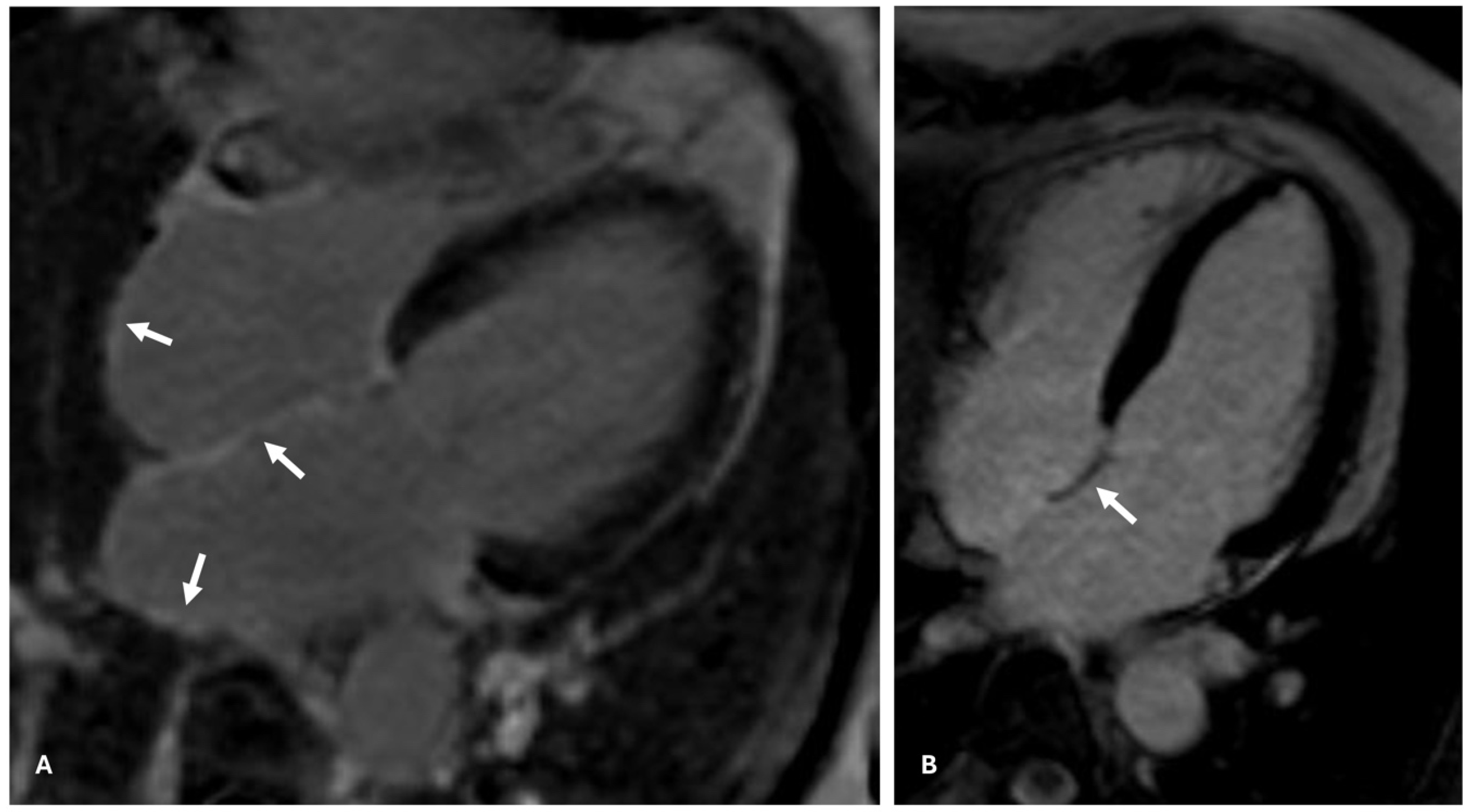
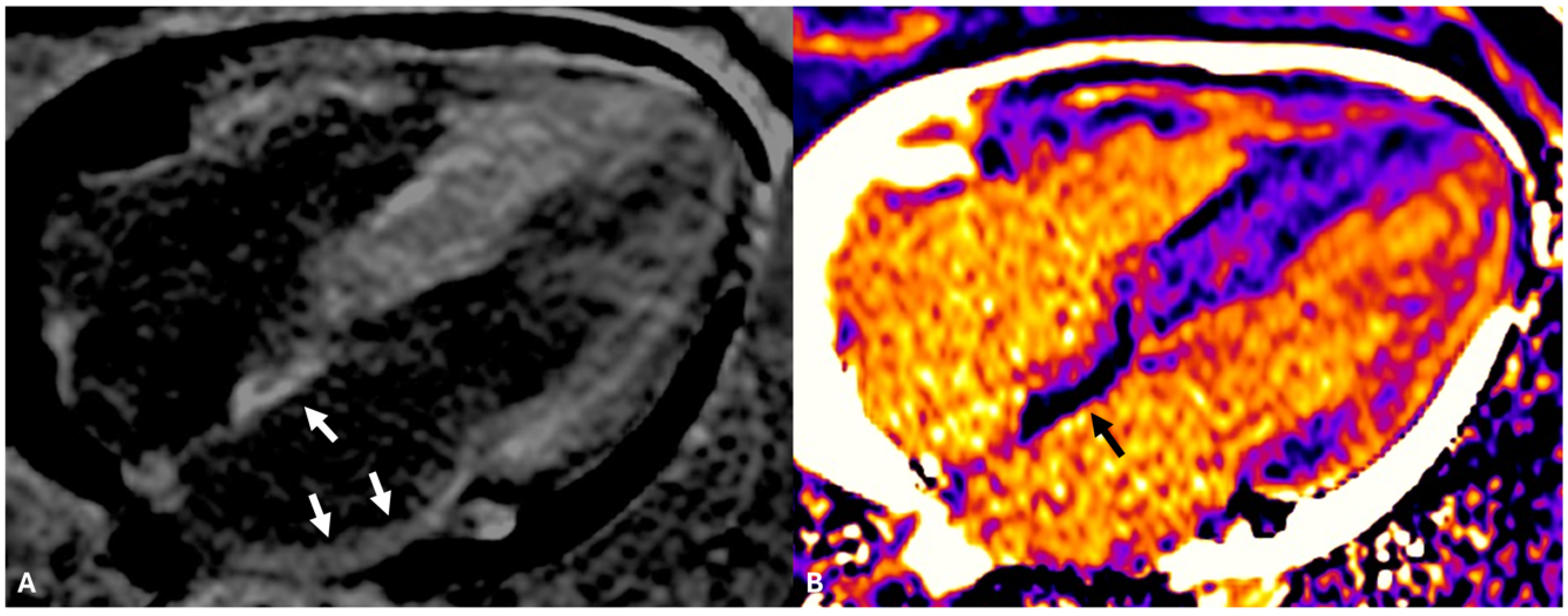
| Study/Year | Methodology | Disease | Age; % Male; Patients | Comments | Results |
|---|---|---|---|---|---|
| Quinto et al./2020 [36] | Retrospective case–control | Paroxysmal AF (69%) | 53 ± 9; 71%; 35 subjects | Shorter procedures and better clinical outcomes in repeated AF ablation procedures when DE-CMR is used in substrate characterization of anatomical veno-atrial gaps. | Positive |
| Bisbal et al./2020 (ALICIA) [38] | RCT | Paroxysmal AF (58%) | 59; 72.4%; 76 subjects | CMR-guided substrate ablation did not improve the recurrence rate at 1 year of follow-up in the AF patient population undergoing first or repeat ablation procedures. | Negative |
| Marrouche et al./2022 (DECAAF II) [39] | RCT | Persistent AF (100%) | 62.2; 79%; 421 subjects | CMR-guided fibrosis-targeted ablation with PVI did not significantly improve atrial arrhythmia recurrence at follow-up compared with PVI alone in persistent AF subjects. | Negative |
| Akoum et al./2015 [37] | Retrospective observational | Paroxysmal AF (53%) | 66 ± 11; 64.5%; 172 subjects | Procedural outcomes are better anticipated by baseline atrial fibrosis and ablation-induced substrate alteration evaluated by CMR. | Positive |
| Akoum et al. /2015 [40] | Prospective observational | Paroxysmal AF (65%) | 65.6%; 177 subjects | Baseline and residual atrial fibrosis uncovered by ablation scarring are associated with recurrent arrhythmia. | Positive |
| Marrouche et al./2014 [25] | Prospective observational | Paroxysmal AF (65%) | 64.6%, 260 subjects | Baseline atrial fibrosis estimated by DE-CMR is associated with arrhythmia recurrence. | Positive |
| Ferrò et al./2023 [31] | Retrospective observational | Paroxysmal AF (66%) | 57 ± 11, 72%, 166 subjects | Advanced atrial cardiomyopathy detected by DE-CMR is independently associated with the recurrence of arrhythmia, particularly atypical atrial flutter, after AF ablation. | Positive |
| Chelu et al./2018 [41] | Retrospective observational | Paroxysmal AF (40%) | 65 ± 12, 60%, 308 subjects | The extent of left atrial fibrosis predicts the 5-year outcome of atrial fibrillation ablation. | Positive |
| First Author/Year/Design of the Study | CMR Post-Processing Protocol |
|---|---|
| Marrouche/2014/The DECAAF study: a multicenter, prospective, observational cohort study [25] | Following data acquisition, CMR data were evaluated and processed using the Coreview image processing software (MARREK, Salt Lake City, UT, USA) for the processing of the LA wall segmentation, fibrosis identification, and export of 3D models. |
| Marrouche/2022/The DECAAF II study: RCT [39] | Patients received a DE-MRI within 30 days before the ablation procedure, using the Merisight protocol from MARREK Inc., to quantify left atrial fibrosis in each case. Reviewers who evaluated the quality of the MRIs were blinded to the patients’ randomized treatment groups. MARREK Inc provided support for image segmentation, processing, and the quantification of left atrial fibrosis. After the ablation, follow-up DE-MRIs were conducted between 90 and 180 days later to assess the formation of scar tissue related to the procedure. The reviewers classified the amount of fibrosis on a five-level scale. |
| Bisbal/2020/The ALICIA study: RCT [38] | Image post-processing of DE-CMR images was conducted using the dedicated ADAS software to obtain a 3D reconstruction of the LA incorporating fibrosis information. Experienced operators manually outlined the LA mid-wall contours in the axial plane to obtain the initial 3D model; further shell deformations were performed, if necessary. Fibrosis identification was based on voxel signal intensity and the mean signal intensity of the LA blood pool was automatically calculated; then, the image intensity ratio value for each voxel was calculated (voxel signal intensity/mean pixel intensity of blood pool) and projected to the 3D model to obtain the final fibrosis map. The thresholds for fibrosis visualization were 1.20 (native fibrosis) and 1.32 (dense scar). |
| Akoum/2015/monocentric, retrospective observational study [37] | Left atrial wall volumes were manually segmented from the LGE-MRI by expert observers using the Coreview image processing software (Marrek Inc.); the resulting LA wall segmentation included the 3D extent of both the LA wall and the antral regions of the PVs. Fibrosis regions in the LGE-MRI were identified based on an intensity threshold established through expert evaluation. A volume-rendering tool in Corview enabled operators to visualize enhancement distribution in 3D; a custom transfer function was employed to define enhancement gradations while suppressing blood and normal tissue. Fibrosis and scarring were reported as a percentage of the atrial wall; patients were categorized into four stages (Utah stages) based on their baseline fibrosis. |
| Quinto/2020/case–control study [36] | All DE-CMR images were segmented by experienced observers with ADAS3D software (ADAS3D Medical, Barcelona, Catalonia, Spain). The atrial wall was manually traced in axial slices, and the blood pool was automatically calculated. Color-coded pixel signal intensity maps were projected to the 3D shell of the atrium. Pixel signal intensity maps were normalized to the mean intensity of the blood pool signal, and the resulting value was plotted. Previously validated fibrosis thresholds were used, with an IIR > 1.32 determining dense scar areas and an IIR between 1.2 and 1.32 determining interstitial fibrosis. The resulting processed 3D model of the LA DE-CMR was integrated with the electro-anatomical map of the navigation system (CARTO 3, Biosense Webster, Diamond Bar, CA, USA) to guide the ablation procedure. |
| Ferrò/2023/retrospective observational study [31] | For the reconstruction of LA and the right atrium, the atrial walls were manually traced. LGE was defined using an IIR > 1.2, indicative of fibrotic tissue. LA was automatically divided into seven standardized regions. |
| Chelu/2018/retrospective observational [41] | The relative extent of fibrosis was quantified within the LA wall with a threshold-based algorithm. The algorithm automatically determines a threshold intensity likely to correspond to the enhanced or fibrotic voxels of the left atrium by estimating the mean and standard deviation of “normal” tissue. “Normal” tissue is defined as the lower region of the pixel intensity histogram, ranging from 2% to 40% of the maximum intensity within the region of interest (e.g., the left atrial wall). The threshold for enhanced/fibrotic tissue is then set at two to four standard deviations above the mean of the “normal” tissue, covering approximately 95% to 99.994% of a Gaussian distribution. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabbour, J.P.; Palombi, M.; Bonanni, M.; Matteucci, A.; Arcari, L.; Pierucci, N.; La Fazia, V.M.; Lavalle, C.; Mariani, M.V. The Role of Cardiac Magnetic Resonance in Characterizing Atrial Cardiomyopathy and Guiding Substrate Ablation in Atrial Fibrillation: A Narrative Review. J. Cardiovasc. Dev. Dis. 2025, 12, 114. https://doi.org/10.3390/jcdd12040114
Jabbour JP, Palombi M, Bonanni M, Matteucci A, Arcari L, Pierucci N, La Fazia VM, Lavalle C, Mariani MV. The Role of Cardiac Magnetic Resonance in Characterizing Atrial Cardiomyopathy and Guiding Substrate Ablation in Atrial Fibrillation: A Narrative Review. Journal of Cardiovascular Development and Disease. 2025; 12(4):114. https://doi.org/10.3390/jcdd12040114
Chicago/Turabian StyleJabbour, Jean Pierre, Marta Palombi, Michela Bonanni, Andrea Matteucci, Luca Arcari, Nicola Pierucci, Vincenzo Mirco La Fazia, Carlo Lavalle, and Marco Valerio Mariani. 2025. "The Role of Cardiac Magnetic Resonance in Characterizing Atrial Cardiomyopathy and Guiding Substrate Ablation in Atrial Fibrillation: A Narrative Review" Journal of Cardiovascular Development and Disease 12, no. 4: 114. https://doi.org/10.3390/jcdd12040114
APA StyleJabbour, J. P., Palombi, M., Bonanni, M., Matteucci, A., Arcari, L., Pierucci, N., La Fazia, V. M., Lavalle, C., & Mariani, M. V. (2025). The Role of Cardiac Magnetic Resonance in Characterizing Atrial Cardiomyopathy and Guiding Substrate Ablation in Atrial Fibrillation: A Narrative Review. Journal of Cardiovascular Development and Disease, 12(4), 114. https://doi.org/10.3390/jcdd12040114







