Figure 1.
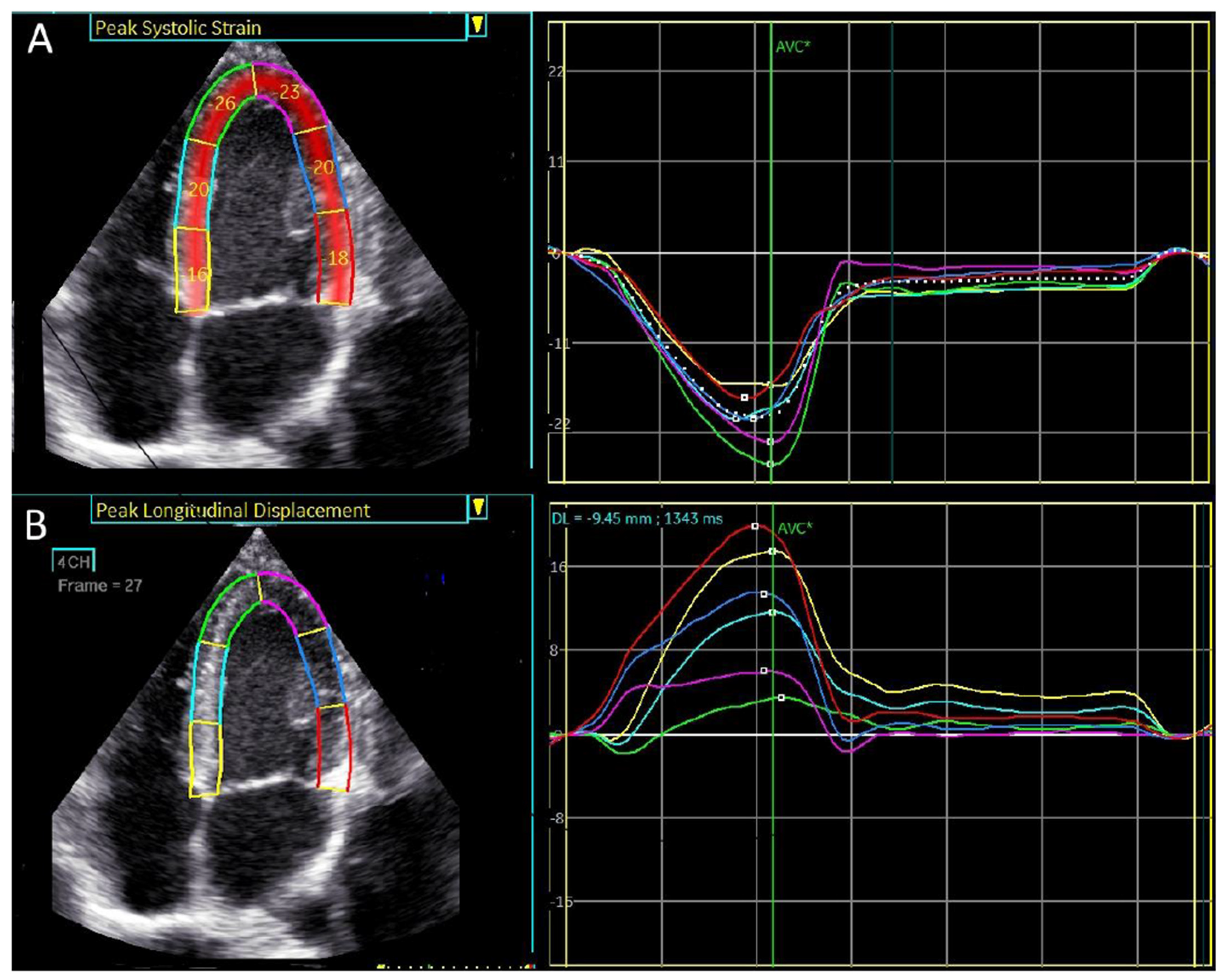
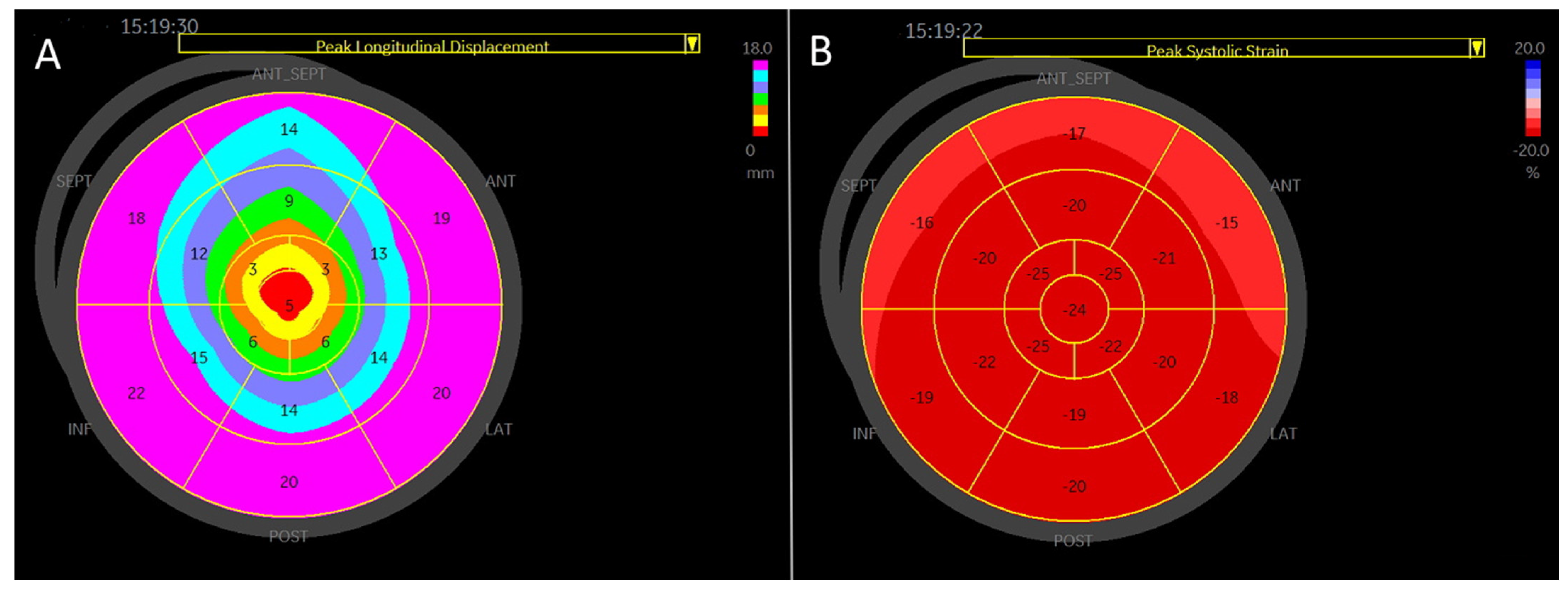
4-chamber apical view. Regional longitudinal strain and displacement of a normal subject. (A) Regional peak systolic longitudinal strain has a negative direction. (B) Regional peak systolic longitudinal displacement has a positive direction.
Figure 1.
4-chamber apical view. Regional longitudinal strain and displacement of a normal subject. (A) Regional peak systolic longitudinal strain has a negative direction. (B) Regional peak systolic longitudinal displacement has a positive direction.
Figure 2.
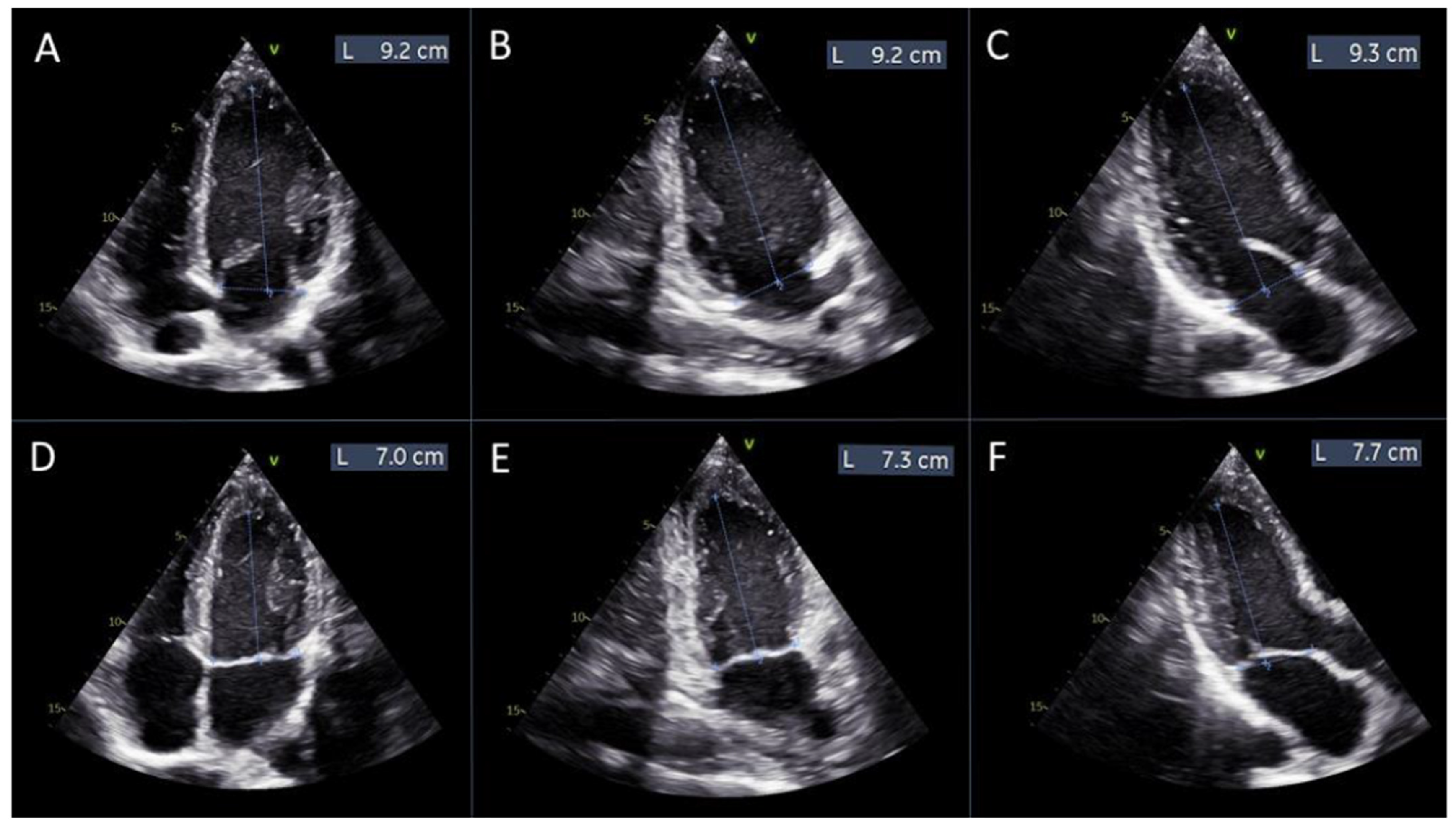
Measurements of left ventricular length for manual global longitudinal strain calculation. Panels (A–C) display the measurements of the left ventricular length obtained from the apical 4-chamber, 2-chamber, and 3-chamber views, respectively, at diastole. Panels (D–F) show the corresponding measurements from the same apical views at systole.
Figure 2.
Measurements of left ventricular length for manual global longitudinal strain calculation. Panels (A–C) display the measurements of the left ventricular length obtained from the apical 4-chamber, 2-chamber, and 3-chamber views, respectively, at diastole. Panels (D–F) show the corresponding measurements from the same apical views at systole.
Figure 3.
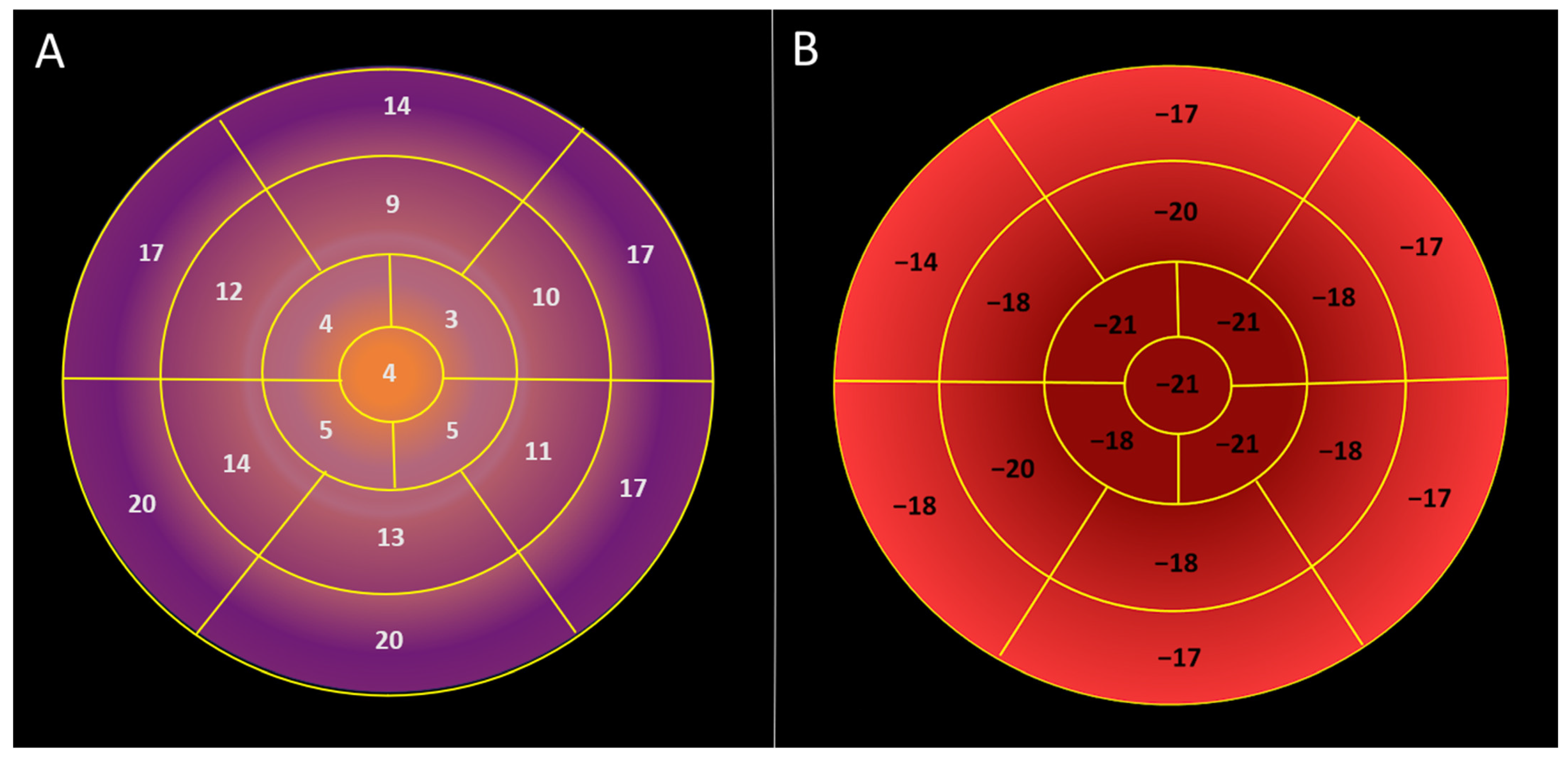
Visual plot of regional longitudinal displacement and strain over the left ventricle across the healthy study population [Group 1]. (A) Regional peak systolic longitudinal displacement over the left ventricle is highest in the basal segments and lowest in the apical segments. (B) Regional peak systolic longitudinal strain is lowest in the basal segments and highest in the apical segments.
Figure 3.
Visual plot of regional longitudinal displacement and strain over the left ventricle across the healthy study population [Group 1]. (A) Regional peak systolic longitudinal displacement over the left ventricle is highest in the basal segments and lowest in the apical segments. (B) Regional peak systolic longitudinal strain is lowest in the basal segments and highest in the apical segments.
Figure 4.
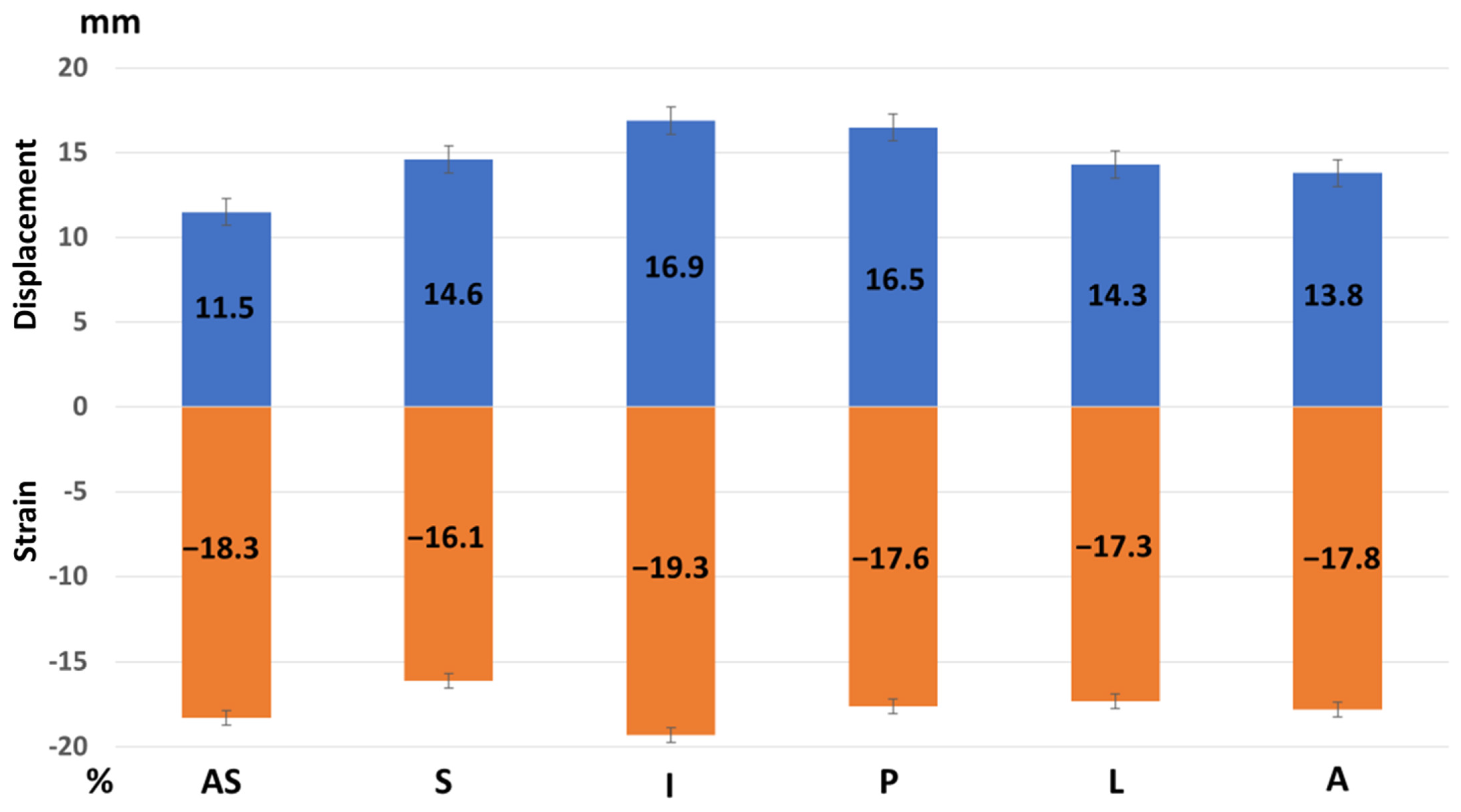
Regional peak systolic strain and longitudinal displacement of the left ventricle in a healthy study population [Group 1]. AS—antero-septal wall, S—septum, I—inferior wall, P—posterior wall, L—lateral wall, and A—anterior wall. Longitudinal displacement is highest in the inferior and posterior wall and lowest in the anterior septum. Longitudinal strain is highest in the inferior wall.
Figure 4.
Regional peak systolic strain and longitudinal displacement of the left ventricle in a healthy study population [Group 1]. AS—antero-septal wall, S—septum, I—inferior wall, P—posterior wall, L—lateral wall, and A—anterior wall. Longitudinal displacement is highest in the inferior and posterior wall and lowest in the anterior septum. Longitudinal strain is highest in the inferior wall.
Figure 5.
A visual plot of the normal peak longitudinal displacement and strain of 27-year-old football player. (A) Peak longitudinal displacement is highest in the basal segments and lowest in the apex. (B) Peak longitudinal strain is lowest in the basal segment and highest in the apex.
Figure 5.
A visual plot of the normal peak longitudinal displacement and strain of 27-year-old football player. (A) Peak longitudinal displacement is highest in the basal segments and lowest in the apex. (B) Peak longitudinal strain is lowest in the basal segment and highest in the apex.
Figure 6.
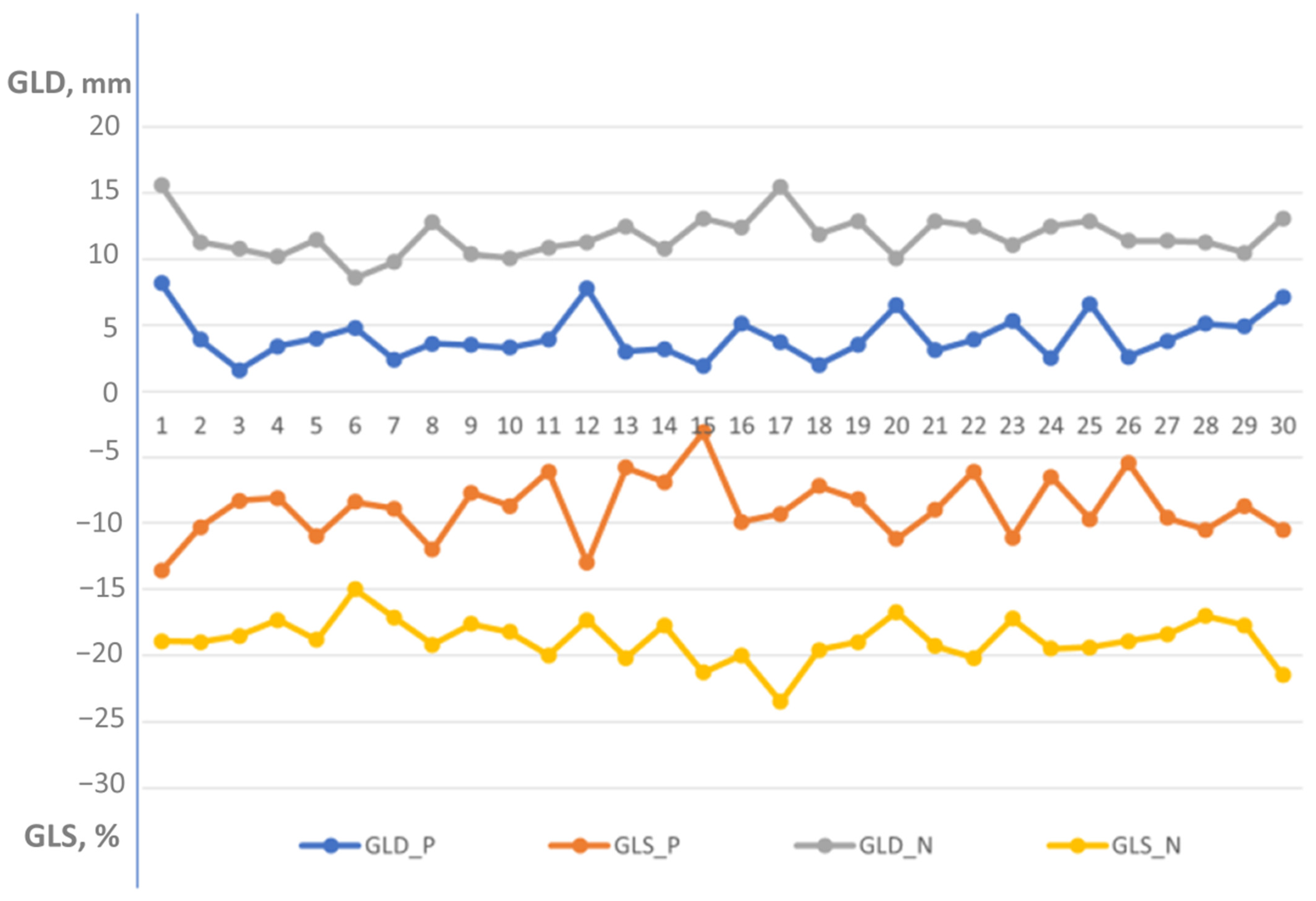
Distribution of global longitudinal displacement and global longitudinal strain in patients with left ventricular dysfunction [Group 2] versus healthy individuals [Group 1]. GLD-P, blue—global longitudinal displacement in patient group; GLS-P, orange—global longitudinal strain in patient group; GLD-N, gray—global longitudinal displacement in normal group; and GLS-N, yellow—global longitudinal strain in normal group. Global longitudinal displacement and strain were significantly lower in the patient group compared to the normal healthy individuals (4.4 ± 1.7 mm vs. 11.7 ± 1.5 mm, p < 0.000001 and −8.2 ± 2.3% vs. −18.8 ± 1.6%, p < 0.000001, respectively).
Figure 6.
Distribution of global longitudinal displacement and global longitudinal strain in patients with left ventricular dysfunction [Group 2] versus healthy individuals [Group 1]. GLD-P, blue—global longitudinal displacement in patient group; GLS-P, orange—global longitudinal strain in patient group; GLD-N, gray—global longitudinal displacement in normal group; and GLS-N, yellow—global longitudinal strain in normal group. Global longitudinal displacement and strain were significantly lower in the patient group compared to the normal healthy individuals (4.4 ± 1.7 mm vs. 11.7 ± 1.5 mm, p < 0.000001 and −8.2 ± 2.3% vs. −18.8 ± 1.6%, p < 0.000001, respectively).
Figure 7.
A visual plot of peak longitudinal displacement and strain in a 48-year-old man with anterior myocardial infarction and an ejection fraction of 35%. (A) Peak longitudinal displacement is very low (around 0) in the apical region (red), consistent with extensive apical myocardial infarction and involvement of other myocardial walls. Higher displacement is observed in the infero-posterolateral segments (green and brown). The average global longitudinal displacement is 3.4 mm. (B) Peak longitudinal strain is also very low in the corresponding apical segments, with better strain observed in the postero-lateral wall. The global longitudinal strain is −8.1%.
Figure 7.
A visual plot of peak longitudinal displacement and strain in a 48-year-old man with anterior myocardial infarction and an ejection fraction of 35%. (A) Peak longitudinal displacement is very low (around 0) in the apical region (red), consistent with extensive apical myocardial infarction and involvement of other myocardial walls. Higher displacement is observed in the infero-posterolateral segments (green and brown). The average global longitudinal displacement is 3.4 mm. (B) Peak longitudinal strain is also very low in the corresponding apical segments, with better strain observed in the postero-lateral wall. The global longitudinal strain is −8.1%.
Figure 8.
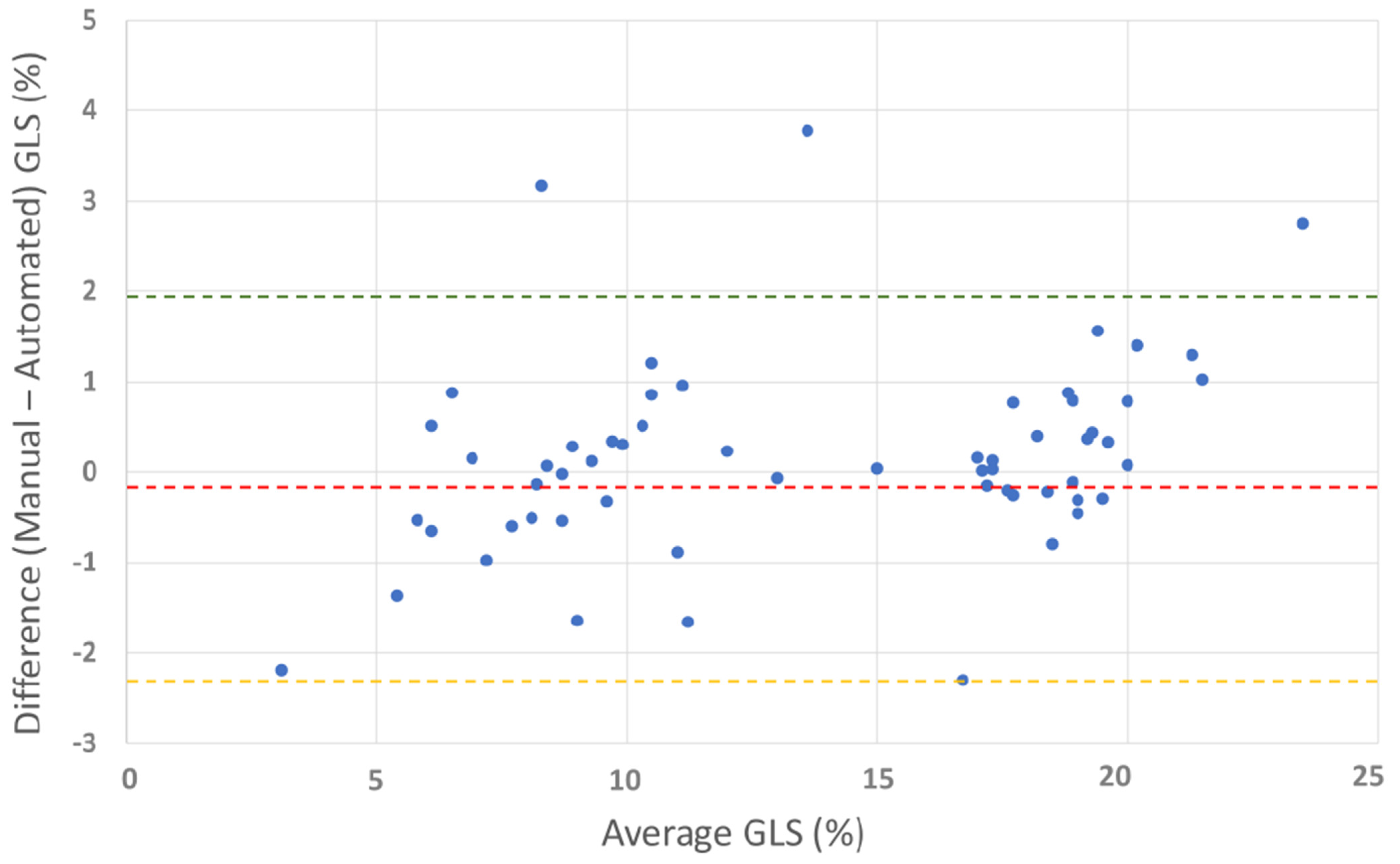
Bland–Altman plot for manual vs. automated global longitudinal strain. The plot shows the agreement between manual and automated GLS measurements. Red line: mean difference (−0.18%), green line: upper limit of agreement (1.92%), and orange line: lower limit of agreement (−2.29%). The Bland–Altman plot illustrates good overall agreement between manual and automated GLS methods, with a small mean difference (−0.18%). Most differences fall within the limits of agreement, confirming acceptable variability.
Figure 8.
Bland–Altman plot for manual vs. automated global longitudinal strain. The plot shows the agreement between manual and automated GLS measurements. Red line: mean difference (−0.18%), green line: upper limit of agreement (1.92%), and orange line: lower limit of agreement (−2.29%). The Bland–Altman plot illustrates good overall agreement between manual and automated GLS methods, with a small mean difference (−0.18%). Most differences fall within the limits of agreement, confirming acceptable variability.
Table 1.
Demographic and echocardiography characteristics of healthy athletes [Group 1]. BSA—body surface area, LAVi—left atrial volume index, LVEDD—left ventricular end diastolic diameter, LVESD—left ventricular end systolic diameter, IVS—interventricular septum, PW—posterior wall, LVMi—left ventricular mass index, EF—ejection fraction, GLS—global longitudinal strain, and GLD—global longitudinal displacement.
Table 1.
Demographic and echocardiography characteristics of healthy athletes [Group 1]. BSA—body surface area, LAVi—left atrial volume index, LVEDD—left ventricular end diastolic diameter, LVESD—left ventricular end systolic diameter, IVS—interventricular septum, PW—posterior wall, LVMi—left ventricular mass index, EF—ejection fraction, GLS—global longitudinal strain, and GLD—global longitudinal displacement.
| Age, years | 24.6 ± 4.6 |
| Height, cm | 180.4 ± 8.3 |
| Weight, kg | 75 ± 6.3 |
| BSA, m2 | 1.9 ± 0.1 |
| LAVi, mL/m2 | 33.9 ± 8.7 |
| LVEDD, cm | 5.0 ± 0.4 |
| LVESD, cm | 3.1 ± 0.4 |
| IVS, cm | 1 ± 0.1 |
| PW, cm | 0.9 ± 0.1 |
| LVMi, g/m2 | 84.5 ± 15.8 |
| EF, % | 58.9 ± 2.8 |
| GLS, % | −18.8 ± 1.6 |
| GLD, mm | 11.7 ± 1.5 |
| E/A ratio | 1.9 ± 0.5 |
| E deceleration, ms | 168.1 ± 46.9 |
| E/e’ ratio | 5 ± 0.8 |
Table 2.
Displacement and strain at different left ventricular levels in Group 1. Displacement is highest in basal segments and lowest in apical segments, consistent with reversed basal to apical gradient. Strain is lowest in basal segments and highest in the apical segments.
Table 2.
Displacement and strain at different left ventricular levels in Group 1. Displacement is highest in basal segments and lowest in apical segments, consistent with reversed basal to apical gradient. Strain is lowest in basal segments and highest in the apical segments.
| LV Segments | Displacement, mm | p-Value | Strain, % | p-Value |
|---|
| Basal segments | 17.6 ± 3.5 | <0.000001 | −16.3 ± 4.7 | <0.000001 |
| Mid segments | 11.5 ± 2.9 | <0.000001 | −19 ± 2.5 | <0.000001 |
| Apical segments | 4.22 ± 1.7 | <0.000001 | −21.1 ± 4.6 | <0.000001 |
Table 3.
Regional peak longitudinal displacement versus longitudinal strain in Group 1. Displacement is highest in the inferior and posterior wall and lowest in the antero-septal wall. Strain is highest in the inferior wall and otherwise is similar over the left ventricular walls.
Table 3.
Regional peak longitudinal displacement versus longitudinal strain in Group 1. Displacement is highest in the inferior and posterior wall and lowest in the antero-septal wall. Strain is highest in the inferior wall and otherwise is similar over the left ventricular walls.
| Left Ventricle Wall | Antero-Septal | Septal | Inferior | Posterior | Lateral | Anterior |
|---|
| Displacement, mm | 11.5 ± 3.4 | 14.6 ± 3.8 | 16.9 ± 4.3 | 16.5 ± 4.3 | 14.3 ± 4.3 | 13.8 ± 4.2 |
| p-value | 0.001 | <0.000001 | <0002 | 0.555 | 0.007 | 0.469 |
| Strain, % | −18.3 ± 2.5 | −16.1 ± 4.7 | −19.3 ± 2.4 | −17.6 ± 3.4 | −17.3 ± 3.1 | −17.8 ± 5.5 |
| p-value | 0.510 | <0.002 | <0.0001 | 0.002 | 0.678 | 0.557 |
Table 4.
Demographic and echocardiography characteristics of patients with left ventricular dysfunction [Group 2]. BSA—body surface area, LAVi—left atrial volume index, LVEDD—left ventricular end diastolic diameter, LVESD—left ventricular end systolic diameter, IVS—interventricular septum, PW—posterior wall, LVMi—left ventricular mass index, EF—ejection fraction, WMSi—wall motion score index, GLS—global longitudinal strain, and GLD—global longitudinal displacement.
Table 4.
Demographic and echocardiography characteristics of patients with left ventricular dysfunction [Group 2]. BSA—body surface area, LAVi—left atrial volume index, LVEDD—left ventricular end diastolic diameter, LVESD—left ventricular end systolic diameter, IVS—interventricular septum, PW—posterior wall, LVMi—left ventricular mass index, EF—ejection fraction, WMSi—wall motion score index, GLS—global longitudinal strain, and GLD—global longitudinal displacement.
| Age, years | 69.1 ± 14.1 |
| Gender, m | 23 (77%) |
| Height, cm | 169.9 ± 7.3 |
| Weight, kg | 76.9 ± 15.0 |
| BSA, m2 | 1.9 ± 0.2 |
| LAVi, mL/m2 | 37.7 ± 12.3 |
| LVEDD, cm | 4.7± 0.8 |
| LVESD, cm | 3.1 ± 0.8 |
| IVS, cm | 1.2 ± 0.3 |
| PW, cm | 1.0 ± 0.2 |
| LVMi, g/m2 | 102 ± 31.4 |
| EF, % | 33.9 ± 3.2 |
| WMSi | 2 ± 0.3 |
| GLS, % | 8.2 ± 2.3 |
| GLD, mm | 4.4 ± 1.7 |
| E/A | 1.3 ± 0.9 |
| E deceleration, ms | 178.9 ± 62.5 |
| E/E’ | 12.8 ± 5.5 |
Table 5.
Comparison of segmental longitudinal displacement. AS—anterior septum, S—septum, I—inferior segments, P—posterior segments, L—lateral segments, A—anterior segments, Ap—apex, 1—basal segments, 2—mid-ventricular segments, and 3—apical segments.
Table 5.
Comparison of segmental longitudinal displacement. AS—anterior septum, S—septum, I—inferior segments, P—posterior segments, L—lateral segments, A—anterior segments, Ap—apex, 1—basal segments, 2—mid-ventricular segments, and 3—apical segments.
| Segment | Displacement (mm) Healthy Subjects | Displacement (mm) LV Dysfunction | Lower Limit of Normal (5th percentile), mm | Abnormal Displacement Rate in LV Dysfunction Patients (%) |
|---|
| AS1 | 14.1 ± 2.4 | 6.5 ± 3.1 | 9.3 | 83 |
| S1 | 17.3 ± 2.9 | 7.8 ± 3.1 | 11.5 | 90 |
| I1 | 20.2 ± 3.0 | 9.0 ± 3.1 | 14.2 | 96.7 |
| P1 | 19.6 ± 3.2 | 8.7 ± 3.9 | 13.2 | 90 |
| L1 | 17.4 ± 3.1 | 6.6 ± 3.8 | 11.2 | 96.7 |
| A1 | 17.2 ± 2.7 | 6.0 ± 3.8 | 11.8 | 96.7 |
| AS2 | 8.9 ± 2.0 | 3.7 ± 2.5 | 4.9 | 63.3 |
| S2 | 11.8 ± 2.2 | 4.5 ± 2.7 | 7.4 | 86.7 |
| I2 | 13.6 ± 2.5 | 5.2 ± 2.2 | 7.6 | 83 |
| P2 | 13.3 ± 2.4 | 3.9 ± 4.2 | 8.5 | 90 |
| L2 | 11.2 ± 2.8 | 2.2 ± 3.5 | 5.6 | 90 |
| A2 | 10.3 ± 2.2 | 2.3 ± 2.7 | 5.9 | 90 |
| S3 | 3.7 ± 1.5 | 1.6 ± 2.0 | 2.2 | 70 |
| I3 | 5.2 ± 1.8 | 0.8 ± 1.7 | 3 | 80 |
| L3 | 4.9 ± 1.6 | 0.2 ± 2.5 | 2.9 | 90 |
| A3 | 3.2 ± 1.7 | 0.8 ± 1.4 | 2.5 | 83 |
| Ap | 4.2 ± 0.8 | 4.1 ± 1.4 | 2.6 | 93.3 |
Table 6.
Comparison of segmental strain. AS—anterior septum, S—septum, I—inferior segments, P—posterior segments, L—lateral segments, A—anterior segments, Ap—apex, 1—basal segments, 2—mid-ventricular segments, and 3—apical segments.
Table 6.
Comparison of segmental strain. AS—anterior septum, S—septum, I—inferior segments, P—posterior segments, L—lateral segments, A—anterior segments, Ap—apex, 1—basal segments, 2—mid-ventricular segments, and 3—apical segments.
| Segment | Strain (%) Healthy Subjects | Strain (%) LV Dysfunction | Lower Limit of Normal (5th percentile), % | Abnormal Rate of Strain in LV Dysfunction Patients (%) | Strain < 10% | Strain < 12% | Strain < 14% |
|---|
| AS1 | −16.8 ± 2.0 | −9.2 ± 5.1 | −12.9 | 80 | 56.7 | 76.7 | 86.7 |
| S1 | −14.7 ± 2.4 | −10.5 ± 3.2 | −9.9 | 33.3 | 33.3 | 66.7 | 80 |
| I1 | −18.3 ± 2.3 | −13.2 ± 5.1 | −13.7 | 53.3 | 23.3 | 36.7 | 53.3 |
| P1 | −17.1 ± 3.8 | −14.4 ± 5.9 | −9.5 | 13.3 | 13.3 | 36.7 | 40 |
| L1 | −16.6 ± 3.4 | −11.4 ± 5.2 | −9.8 | 30 | 30 | 40 | 60 |
| A1 | −17.4 ± 2.7 | −10.5 ± 5.0 | −12.0 | 50 | 36.7 | 50 | 60 |
| AS2 | −19.8 ± 2.0 | −7.5 ± 4.5 | −15.8 | 93.3 | 83.3 | 90 | 90 |
| S2 | −18.4 ± 2.5 | −9.0 ± 4.6 | −13.4 | 86.7 | 46.7 | 73.3 | 86.7 |
| I2 | −20.2 ± 2.2 | −11.6 ± 5.0 | −15.8 | 76.7 | 33.3 | 50 | 60 |
| P2 | −18.1 ± 2.8 | −11.0 ± 5.1 | −12.5 | 60 | 33.3 | 40 | 73.3 |
| L2 | −18.1 ± 2.6 | −7.2 ± 4.5 | −12.9 | 93.3 | 73.3 | 93.3 | 93.3 |
| A2 | −19.4 ± 2.1 | −6.8 ± 4.4 | −15.2 | 96.7 | 76.7 | 90 | 96.7 |
| S3 | −22.9 ± 2.9 | −3.4 ± 4.9 | −17.1 | 100 | 80 | 90 | 100 |
| I3 | −22.0 ± 2.9 | −4.3 ± 5.2 | −16.2 | 100 | 83.3 | 90 | 96.7 |
| L3 | −20.6 ± 3.0 | −3.4 ± 4.8 | −14.6 | 100 | 93.3 | 96.7 | 100 |
| A3 | −21.0 ± 2.8 | −1.3 ± 4.2 | −15.4 | 100 | 96.7 | 100 | 100 |
| A | −21.3 ± 2.8 | −3.0 ± 4.4 | −15.7 | 100 | 90 | 96.7 | 100 |
Table 7.
Comparison of manual GLS values. GLSm—GLS measured manually using the method suggested in this work; GLSm(r)—GLS measured manually according to the reference; and GLS—GLS measured using the EchoPAC software.
Table 7.
Comparison of manual GLS values. GLSm—GLS measured manually using the method suggested in this work; GLSm(r)—GLS measured manually according to the reference; and GLS—GLS measured using the EchoPAC software.
| GLS | Strain (%) Healthy Subjects | Strain (%) LV Dysfunction | Lower Limit of Normal (5th Percentile), % | Abnormal Rate of GLS in LV Dysfunction Patients (%) | Strain < 16% |
|---|
| GLSm | −18.5 ± 1.8 | −8.9 ± 2.1 | −14.9 | 100 | 100 |
| GLSm(r) | −18.3 ± 1.8 | −8.9 ± 2.0 | −14.7 | 100 | 100 |
| GLS | −18.8 ± 1.6 | −8.8 ± 2.0 | −15.6 | 100 | 100 |