Mixed Reality (Holography)-Guided Minimally Invasive Cardiac Surgery—A Novel Comparative Feasibility Study †
Abstract
1. Introduction
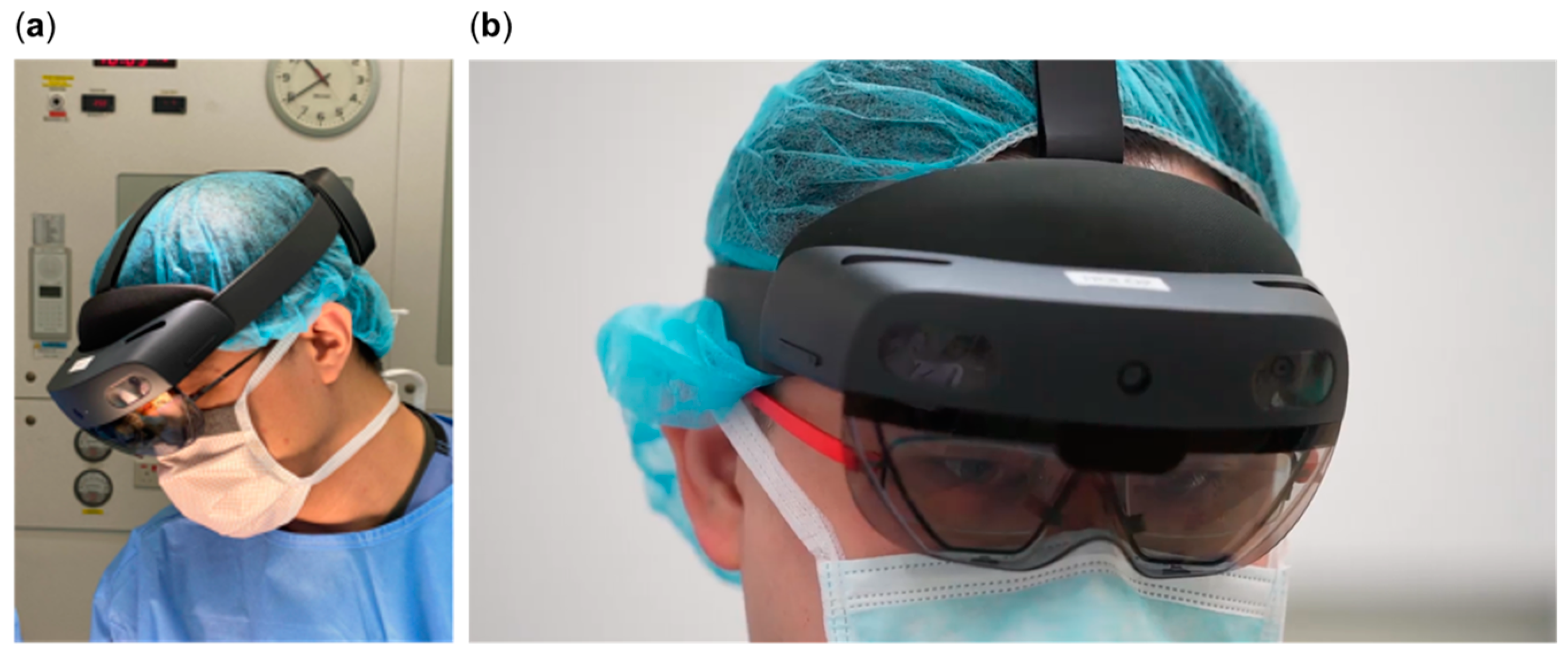
2. Materials and Methods
2.1. Computed Tomography Angiography (CTA)
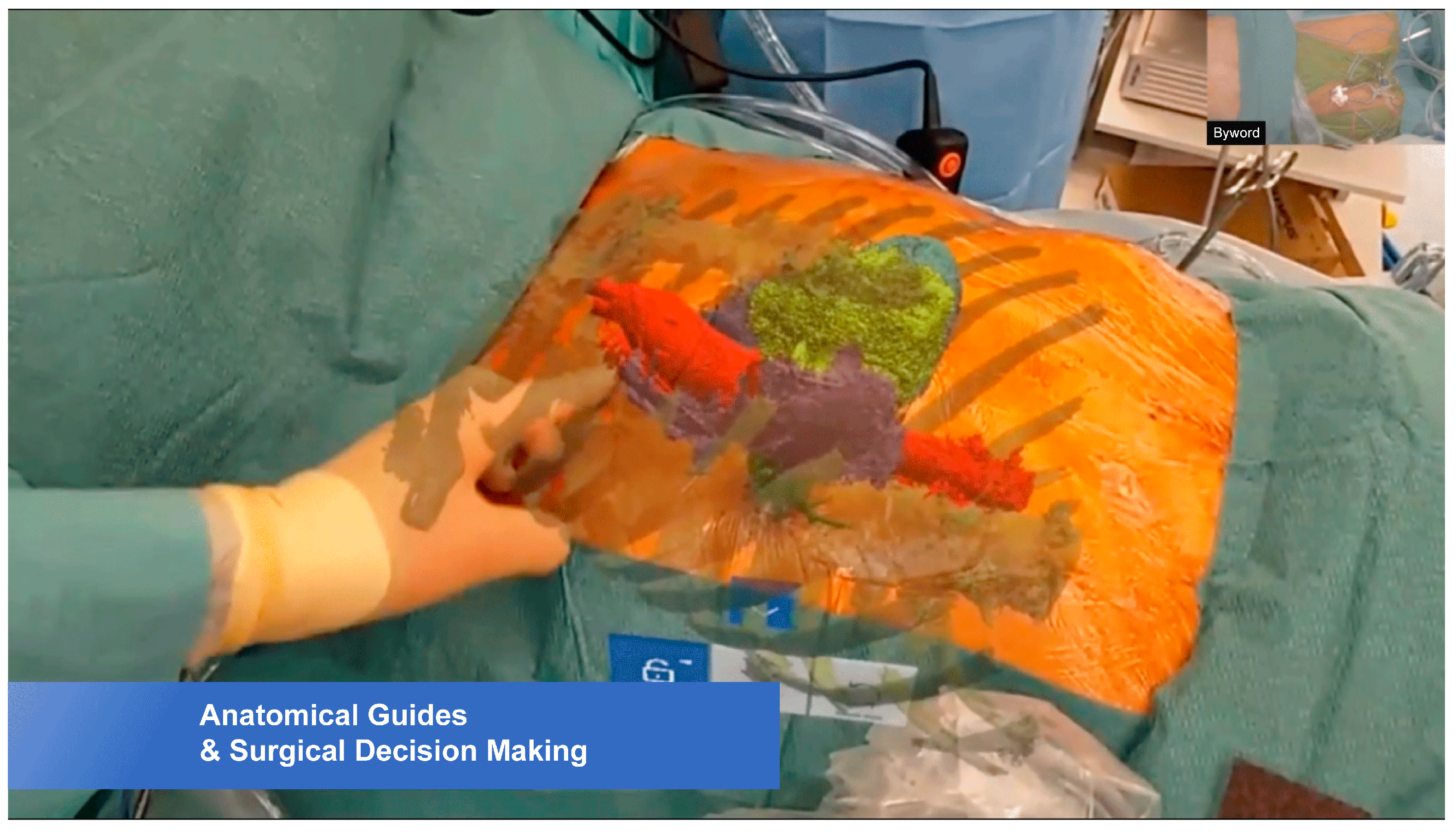
2.2. Hologram Creation
2.3. Surgical Procedures
3. Results
4. Discussion
4.1. Benefits
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kofidis, T. Minimally Invasive Cardiac Surgery. A Practical Guide; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Kiraly, L.; Aye, W.M.; Subbian, S.K.; Kofidis, T.; Al Hakami, A. Minimal incision and less invasive techniques in congenital cardiac surgery: A narrative review. Pediatr. Med. 2024, 7, 14. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, X.; Li, T.; Luo, C.; Yang, L. Mixed-reality hologram for diagnosis and surgical planning of double outlet right ventricle: A pilot study. Clin. Radiol. 2021, 76, 237. [Google Scholar] [CrossRef] [PubMed]
- Brun, H.; Bugge, R.A.; Suther, L.K.; Birkeland, S.; Kumar, R.; Pelanis, E.; Elle, O.J. Mixed reality holograms for heart surgery planning: First user experience in congenital heart disease. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 883–888. [Google Scholar] [CrossRef]
- Liu, S.; Xie, M.; Zhang, Z.; Wu, X.; Gao, F.; Lu, L.; Zhang, J.; Xie, Y.; Yang, F.; Ye, Z. A 3 D Hologram with Mixed Reality Technique to improve Understanding of Pulmonary Lesions Caused by COVID-19; Randomised Control Trial. Med. Internet Res. 2021, 23, e24081. [Google Scholar] [CrossRef]
- Karmonik, C.; Boone, T.B.; Khavari, R. Workflow for visualization of neuroimaging data with an augmented reality device. J. Digit. Imaging 2018, 31, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Rudy, H.L.; Lefkowitz, A.; Weimer, K.A.; Marks, S.M.; Stern, C.S.; Garfein, E.S. Mixed reality with HoloLens: Where virtual reality meets augmented reality in the operating room. Plast. Reconstr. Surg. 2017, 140, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.G. What Is the Microsoft HoloLens? Develop Microsoft HoloLens Apps Now; Springer: Berlin/Heidelberg, Germany, 2016; pp. 3–7. [Google Scholar]
- Cai, E.Z.; Gao, Y.; Ngiam, K.Y.; Lim, T.C. Mixed Reality Intraoperative Navigation in Craniomaxillofacial Surgery. Plast. Reconstr. Surg. 2021, 148, 686e–688e. [Google Scholar] [CrossRef] [PubMed]
- Scherl, C.; Stratemeier, J.; Rotter, N.; Hesser, J.; Schönberg, S.O.; Servais, J.J.; Männle, D.; Lammert, A. Augmented Reality with HoloLens in Parotid Tumour Surgery: A Prospective Feasibility Study. ORL J. Otorhinolaryngol. Relat. Spec. 2021, 83, 439–448. [Google Scholar] [CrossRef]
- Deib, G.; Johnson, A.; Unberath, M.; Yu, K.; Andress, S.; Qian, L.; Osgood, G.; Navab, N.; Hui, F.; Gailloud, P. Image guided percutaneous spine procedures using an optical see-through head mounted display: Proof of concept and rational. J. Neurointerv. Surg. 2018, 10, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Aye, W.M.M.; Kiraly, L.; Kumar, S.S.; Kasivishvanaath, A.; Gao, Y.; Kofidis, T. Mixed Reality (Holography)-Guided Minimally Invasive Cardiac Surgery—A First-in-Man Comparative Feasibility Study. In Proceedings of the 30th Annual Meeting of the Asian Society for Cardiovascular and Thoracic Surgery (ASCVTS 2022)—Plenary 2, C-PL2-7, Nara, Japan, 25 March 2022. [Google Scholar]
- Redman, J.D.; Wolton, W.P.; Shuttleworth, E. Use of holography to make truly three-dimensional X-ray images. Nature 1968, 220, 58–60. [Google Scholar] [CrossRef]
- Carbone, M.; Piazza, R.; Condino, S. Commercially Available Head- Mounted Displays Are Unsuitable for Augmented Reality Surgical Guidance: A Call for Focused Research for Surgical Applications. Surg. Innov. 2020, 27, 254–255. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.; Hadzijusufovic, E.; Hansen, C.; Paschold, M.; Lang, H.; Kneist, W. Head-mounted mixed-reality technology during robotic-assisted transanal total mesorectal excision. Dis. Colon. Rectum. 2019, 62, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Ameri, G.; Baxter, J.S.; Bainbridge, D.; Peters, T.M.; Chen, E.C. Mixed reality ultrasound guidance system: A case study in system development and cautionary tale. Int. J. Comput. Assist. Radiol. Surg. 2018, 13, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.; Figueiredo, E.G.; Rabelo, N.N.; Teixeira, M.J.; Zanon, N. Development and evaluation of a new pediatric mixed-reality model for neurosurgical training. J. Neurosurg. Pediatr. 2019, 2, 423–432. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Wang, N.; Zhang, W.; Li, D.; Zhang, L.; Qu, X.; Cheng, W.; Xu, Y.; Chen, W.; et al. A wearable mixed-reality holographic computer for guiding external ventricular draining insertion at the bedside. J. Neurosurg. 2018, 10, 1599–1606. [Google Scholar]
- Sauer, I.M.; Queisner, M.; Tang, P.; Moosburner, S.; Hoepfner, O.; Horner, R.; Lohmann, R.; Pratschke, J. Mixed reality in visceral surgery development of a suitable workflow and evaluation of intraoperative use-cases. Ann. Surg. 2017, 266, 706–712. [Google Scholar] [CrossRef]
- Pratt, P.; Ives, M.; Lawton, G. Through the HoloLens looking glass: Augmented reality for extremity reconstruction surgery using 3D vascular models with perforating vessels. Eur. Radiol. Exp. 2018, 2, 2. [Google Scholar] [CrossRef]
- Elmi-Terander, A.; Nachabe, R.; Skulason, H.; Pedersen, K.; Söderman, M.; Racadio, J.; Babic, D.; Gerdhem, P.; Edström, E. Feasibility and accuracy of thoracolumbar minimally invasive pedicle screw placement with augmented reality navigation technology. Spine 2018, 43, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Butler, E.; Li, K.; Lu, A.; Ji, S.; Zhang, S. Large-scale exploration of neuronal morphologies using deep learning and augmented reality. Neuroinformatics 2018, 16, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Fiori, C.; Checcucci, E.; Amparore, D.; Bertolo, R. Augmented reality robot-assist- ed radical prostatectomy: Preliminary experience. Urology 2018, 115, 184. [Google Scholar] [CrossRef]
- Bong, J.H.; Song, H.J.; Oh, Y.; Park, N.; Kim, H.; Park, S. Endoscopic navigation system with extended field of view using augmented reality technology. Int. J. Med. Robot. 2018, 14, e1886. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.Z.; Feng, X.B.; Shao, Z.W.; Xie, M.; Xu, S.; Wu, X.H.; Ye, Z.W. Application and Prospect of Mixed Reality Technology in Medical Field. Curr. Med. Sci. 2019, 39, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wainman, B.; Pukas, G.; Wolak, L.; Mohanraj, S.; Lamb, J.; Norman, G.R. The Critical Role of Stereopsis in Virtual and Mixed Reality Learning Environments. Anat. Sci. Educ. 2020, 13, 401–412. [Google Scholar] [CrossRef]
- Hilt, A.D.; Kapllani, K.M.; Hierck, B.P.; Kemp, A.C.; Albayrak, A.; Melles, M.; Schalij, M.J.; Scherptong, R.W. Perspectives of Patients and Professionals on Information and Education After Myocardial Infarction With Insight for Mixed Reality Implementation: Cross-Sectional Interview Study. JMIR Hum. Factors 2020, 7, e17147. [Google Scholar] [CrossRef] [PubMed]
- Lau, I.; Gupta, A.; Sun, Z. Clinical Valve of Virtual Reality versus 3D Printing in Congenital Heart Disease. Biomolecules 2021, 11, 884. [Google Scholar] [CrossRef]
- Jang, J.; Tschabrunn, C.M.; Barkagan, M.; Anter, E.; Menze, B.; Nezafat, R. Three-dimensional holographic visualization of high-resolution myocardial scar on HoloLens. PLoS ONE 2018, 13, e0205188. [Google Scholar] [CrossRef] [PubMed]
- Incekara, F.; Smits, M.; Dirven, C.; Vincent, A. Clinical feasibility of a wearable mixed-reality device in neurosurgery. World Neurosurg. 2018, 118, e422–e427. [Google Scholar] [CrossRef] [PubMed]
- Kersten-Oertel, M.; Jannin, P.; Collins, D.L. The State of the Art of Visualization in Mixed Reality Image Guided Surgery. Comput. Med. Imaging Graph. 2013, 37, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, R.; Yu, J.; Xu, S.; Yang, C.; Yang, S.; Shao, Z.; Ye, Z. Mixed Reality Technology Launches in Orthopedic Surgery for Comprehensive Preoperative Management of Complicated Cervical Fractures. Surg. Innov. 2018, 25, 421–422. [Google Scholar] [CrossRef]
- El Shallaly, G.; Cuschieri, A. Optimum view distance for laparoscopic surgery. Surg. Endosc. 2006, 20, 1879–1882. [Google Scholar] [CrossRef]
- Kirshbom, P.M.; Myung, R.J.; Simsic, J.M.; Kramer, Z.B.; Leong, T.; Kogon, B.E.; Kanter, K.R. One Thousand repeat sternotomies for congenital cardiac surgery: Risk factors for re-entry injury. Ann. Thorac. Surg. 2009, 88, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, S.; Stephens, E.H.; Dearani, J.A. High-Risk Reoperative Sternotomy-How We Do It, How We Teach It. World J. Pediatr. Congenit. Heart Surg. 2020, 11, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Valente, T.; Bocchini, G.; Rossi, G.; Sica, G.; Davison, H.; Scaglione, M. MDCT prior to median re-sternotomy in cardiovascular surgery: Our experiences, infrequent findings and the crucial role of radiological report. Br. J. Radiol. 2019, 92, 20170980. [Google Scholar] [CrossRef] [PubMed]
- Henaine, R.; Yoshimura, N.; Di Filippo, S.; Ninet, J. Pulmonary valve replacement in repaired tetralogy of Fallot by left thoracotomy avoid ascending aorta injury. J. Thorac. Cardiovasc. Surg. 2011, 141, 590–592. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, R.P.; Pelanis, E.; Bugge, R.; Brun, H.; Palomar, R.; Aghayan, D.L.; Fretland, Å.A.; Edwin, B.; Elle, O.J. Use of Mixed Reality for Surgery Planning: Assessment and Development Workflow. J. Biomed. Inform. 2020, 112, 100077. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aye, W.M.M.; Kiraly, L.; Kumar, S.S.; Kasivishvanaath, A.; Gao, Y.; Kofidis, T. Mixed Reality (Holography)-Guided Minimally Invasive Cardiac Surgery—A Novel Comparative Feasibility Study. J. Cardiovasc. Dev. Dis. 2025, 12, 49. https://doi.org/10.3390/jcdd12020049
Aye WMM, Kiraly L, Kumar SS, Kasivishvanaath A, Gao Y, Kofidis T. Mixed Reality (Holography)-Guided Minimally Invasive Cardiac Surgery—A Novel Comparative Feasibility Study. Journal of Cardiovascular Development and Disease. 2025; 12(2):49. https://doi.org/10.3390/jcdd12020049
Chicago/Turabian StyleAye, Winn Maung Maung, Laszlo Kiraly, Senthil S. Kumar, Ayyadarshan Kasivishvanaath, Yujia Gao, and Theodoros Kofidis. 2025. "Mixed Reality (Holography)-Guided Minimally Invasive Cardiac Surgery—A Novel Comparative Feasibility Study" Journal of Cardiovascular Development and Disease 12, no. 2: 49. https://doi.org/10.3390/jcdd12020049
APA StyleAye, W. M. M., Kiraly, L., Kumar, S. S., Kasivishvanaath, A., Gao, Y., & Kofidis, T. (2025). Mixed Reality (Holography)-Guided Minimally Invasive Cardiac Surgery—A Novel Comparative Feasibility Study. Journal of Cardiovascular Development and Disease, 12(2), 49. https://doi.org/10.3390/jcdd12020049





