Feeding Approach to Optimizing Nutrition in Infants with Congenital Heart Disease
Abstract
1. Introduction
2. Proposed Hemodynamic-Focused, Risk-Stratified Feeding Approach
2.1. CHD Categories
- Systemic hypoperfusion: Reduced blood flow to systemic organs limits oxygen and nutrient delivery. This is often caused by structural left-sided obstructions or imbalances caused by systemic blood flow diverting to pulmonary circulation.
- Global hypoxia: less oxygen is delivered to tissues due to right-sided obstructions, right-to-left shunting, or mixing of oxygenated and deoxygenated blood.
- Pulmonary overcirculation: Excessive pulmonary blood flow caused by left-to-right shunts at the levels of atria, ventricles, or great vessels. This can lead to lung congestion and breathing difficulties.
2.2. CHD Severity
2.3. Dynamic Changes over Time
2.4. Co-Morbidities
3. Monitoring
3.1. Monitoring Systemic Hypoperfusion and Hypoxia
3.2. Monitoring Pulmonary Overcirculation
4. Enteral Nutrition Feeding Strategies
4.1. Overview
4.2. Trophic and Limited Feeds—For Infants at Risk for Hypoperfusion and Hypoxia
4.3. Feeding Strategies for Infants with Pulmonary Overcirculation
5. Parenteral Nutrition (PN) Strategies
6. The Overlooked Importance of Micronutrients for Cardiovascular Health
6.1. Zinc
6.2. Thiamine (Vitamin B1)
6.3. Vitamin A
6.4. Magnesium
6.5. Calcium
7. Nutrition-Focused Physical Exam and Growth Assessment
8. Standardized Feeding Protocols
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ross, F.; Latham, G.; Joffe, D.; Richards, M.; Geiduschek, J.; Eisses, M.; Thompson, D.; Radman, M. Preoperative malnutrition is associated with increased mortality and adverse outcomes after pediatric cardiac surgery. Cardiol. Young 2017, 27, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo, S.L.; McCulley, M.E.; Khemani, R.G.; Jeffries, H.E.; Thomas, D.W.; Peregrine, J.; Wells, W.J.; Starnes, V.A.; Moromisato, D.Y. Reducing the incidence of necrotizing enterocolitis in neonates with hypoplastic left heart syndrome with the introduction of an enteral feed protocol. Pediatr. Crit. Care Med. 2010, 11, 373–377. [Google Scholar] [CrossRef]
- McElhinney, D.B.; Hedrick, H.L.; Bush, D.M.; Pereira, G.R.; Stafford, P.W.; Gaynor, J.W.; Spray, T.L.; Wernovsky, G. Necrotizing enterocolitis in neonates with congenital heart disease: Risk factors and outcomes. Pediatrics 2000, 106, 1080–1087. [Google Scholar] [CrossRef]
- Miller, M.; Vaidya, R.; Rastogi, D.; Bhutada, A.; Rastogi, S. From parenteral to enteral nutrition: A nutrition-based approach for evaluating postnatal growth failure in preterm infants. JPEN J. Parenter. Enteral Nutr. 2014, 38, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Donda, K.; Bhutada, A.; Rastogi, D.; Rastogi, S. Transitioning preterm infants from parenteral nutrition: A comparison of 2 protocols. JPEN J. Parenter. Enteral Nutr. 2017, 41, 1371–1379. [Google Scholar] [CrossRef]
- Kataria-Hale, J.; Roddy, D.J.; Cognata, A.; Hochevar, P.; Zender, J.; Sheaks, P.; Osborne, S.; Tucker, K.; Hurst, N.; Hagan, J.; et al. A preoperative standardized feeding protocol improves human milk use in infants with complex congenital heart disease. J. Perinatol. 2021, 41, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Centeno-Malfaz, F.; Moráis-López, A.; Caro-Barri, A.; Peña-Quintana, L.; Gil-Villanueva, N.; Redecillas-Ferreiro, S.; Marcos-Alonso, S.; Ros-Arnal, I.; Tejero, M.Á.; Sánchez, C.S.; et al. Nutrition in congenital heart disease: Consensus document. An. Pediatr. (Engl. Ed.) 2023, 98, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Salvatori, G.; De Rose, D.U.; Massolo, A.C.; Patel, N.; Capolupo, I.; Giliberti, P.; Evangelisti, M.; Parisi, P.; Toscano, A.; Dotta, A.; et al. Current Strategies to Optimize Nutrition and Growth in Newborns and Infants with Congenital Heart Disease: A Narrative Review. J. Clin. Med. 2022, 11, 1841. [Google Scholar] [CrossRef]
- Maskatia, S.A.; Kwiatkowski, D.; Bhombal, S.; Davis, A.S.; McElhinney, D.B.; Tacy, T.A.; Algaze, C.; Blumenfeld, Y.; Quirin, A.; Punn, R. A fetal risk stratification pathway for neonatal aortic coarctation reduces medical exposure. J. Pediatr. 2021, 237, 102–108.e3. [Google Scholar] [CrossRef]
- Chan, B.; Gordon, S.; Yang, M.; Weekes, J.; Dance, L. Abdominal ultrasound assists the diagnosis and management of necrotizing enterocolitis. Adv. Neonatal Care 2021, 21, 365–370. [Google Scholar] [CrossRef]
- Gan, Y.; Ying, J.; Qiu, X.; You, S.; Zhang, T.; Ruan, T.; Zhou, R.; Ye, Y.; Yue, Y.; Zhang, L.; et al. Value of near-infrared spectroscopy in evaluating the risk of neonatal necrotizing enterocolitis: A systematic review and meta-analysis. Early Hum. Dev. 2024, 195, 106083. [Google Scholar] [CrossRef]
- DeWitt, A.G.; Charpie, J.R.; Donohue, J.E.; Yu, S.; Owens, G.E. Splanchnic near-infrared spectroscopy and risk of necrotizing enterocolitis after neonatal heart surgery. Pediatr. Cardiol. 2014, 35, 1286–1294. [Google Scholar] [CrossRef]
- Zong, H.F.; Guo, G.; Liu, J.; Bao, L.L.; Yang, C.Z. Using lung ultrasound to evaluate pulmonary water content. Pediatr. Pulmonol. 2020, 55, 729–739. [Google Scholar] [CrossRef]
- Kaya, B.; Dilli, D.; Sarikaya, Y.; Akduman, H.; Citli, R.; Orun, U.A.; Tasar, M.; Zenciroglu, A. Lung ultrasound in the evaluation of pulmonary edema in newborns with critical congenital heart disease. Pediatr Neonatol. 2024, 65, 532–538. [Google Scholar] [CrossRef]
- Khairy, P.; Ionescu-Ittu, R.; Mackie, A.S.; Abrahamowicz, M.; Pilote, L.; Marelli, A.J. Changing mortality in congenital heart disease. J. Am. Coll. Cardiol. 2010, 56, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.T.; McMahon, C.J.; James, A. Necrotizing enterocolitis in children with congenital heart disease: A literature review. Pediatr. Cardiol. 2021, 42, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Burge, K.Y.; Gunasekaran, A.; Makoni, M.M.; Mir, A.M.; Burkhart, H.M.; Chaaban, H. Clinical characteristics and potential pathogenesis of cardiac necrotizing enterocolitis in neonates with congenital heart disease: A narrative review. J. Clin. Med. 2022, 11, 3987. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.C.; Hornik, C.P.; Cotten, C.M.; Clark, R.H.; Hill, K.D.; Smith, P.B.; Lenfestey, R.W. Necrotizing enterocolitis in infants with ductal-dependent congenital heart disease. Am. J. Perinatol. 2014, 32, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.I.; Kim, J.H.; Fogg, K.; Goldshtrom, N.; Graham, E.M.; Kataria-Hale, J.; Osborne, S.W.; Figueroa, M. Nutritional considerations for the neonate with congenital heart disease. Pediatrics 2022, 150 (Suppl. 2), e2022056415G. [Google Scholar] [CrossRef]
- Willis, L.; Thureen, P.; Kaufman, J.; Wymore, E.; Skillman, H.; da Cruz, E. Enteral feeding in prostaglandin-dependent neonates: Is it a safe practice? J. Pediatr. 2008, 153, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Havranek, T.; Johanboeke, P.; Madramootoo, C.; Carver, J.D. Umbilical artery catheters do not affect intestinal blood flow responses to minimal enteral feedings. J. Perinatol. 2007, 27, 375–379. [Google Scholar] [CrossRef]
- Mankouski, A.; Bahr, T.M.; Braski, K.L.; Lewis, K.W.; Baserga, M.C. Cerebral and Splanchnic Tissue Oxygenation Are Significantly Affected in Premature infants with Ductal-Dependent Congenital Heart Disease. J. Pediatr. Clin. Pract. 2024, 14, 200126. [Google Scholar] [CrossRef] [PubMed]
- Tume, L.N.; Valla, F.V.; Joosten, K.; Jotterand Chaparro, C.; Latten, L.; Marino, L.V.; Macleod, I.; Moullet, C.; Pathan, N.; Rooze, S.; et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med. 2020, 46, 411–425. [Google Scholar] [CrossRef]
- Cognata, A.; Kataria-Hale, J.; Griffiths, P.; Maskatia, S.; Rios, D.; O’Donnell, A.; Roddy, D.J.; Mehollin-Ray, A.; Hagan, J.; Placencia, J.; et al. Human milk muse in the perioperative period is associated with a lower risk for necrotizing enterocolitis in neonates with complex congenital heart disease. J. Pediatr. 2019, 215, 11–16. [Google Scholar] [CrossRef]
- Faienza, M.F.; D’Amato, E.; Natale, M.P.; Grano, M.; Chiarito, M.; Brunetti, G.; D’Amato, G. Metabolic bone disease of prematurity: Diagnosis and management. Front. Pediatr. 2019, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Neonatal Parenteral Nutrition; NICE Guideline, No. 154; National Institute for Health and Care Excellence (NICE): London, UK, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555677/ (accessed on 25 November 2024).
- Alemmari, A.; Miller, G.G.; Bertolo, R.F.; Dinesh, C.; Brunton, J.A.; Arnold, C.J.; Zello, G.A. Reduced aluminum contamination decreases parenteral nutrition-associated liver injury. J. Pediatr. Surg. 2012, 47, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Belza, C.; Wales, P.W.; Bouchard, S.; de Silva, N.; Avitzur, Y.; Wales, P.W. An observational study of Smoflipid vs. Intralipid on the evolution of intestinal failure-associated liver disease in infants with intestinal failure. JPEN J. Parenter. Enteral Nutr. 2020, 44, 688–696. [Google Scholar] [CrossRef]
- Rastegar, M.; Teshnizi, S.; Khaleghinia, M. Vitamin D and zinc deficiency in children with congenital heart defects. J. Comp. Pediatr. 2024, 15. [Google Scholar] [CrossRef]
- Sadoh, W.E.; Sadoh, A.E. Serum zinc values in children with congenital heart disease. Afr. Health Sci. 2013, 13, 601–606. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abu-Hamdan, D.K.; Desai, H.; Sondheimer, J.; Felicetta, J.; Mahajan, S.; McDonald, F. Taste acuity and zinc metabolism in captopril-treated hypertensive male patients. Am. J. Hypertens. 1988, 1 Pt 3, 303S–308S. [Google Scholar] [CrossRef]
- Reyes, A.J.; Leary, W.P.; Lockett, C.J.; Alcocer, L. Diuretics and zinc. S. Afr. Med. J. 1982, 62, 373–375. [Google Scholar] [PubMed]
- Leary, W.P.; Reyes, A.J.; Wynne, R.D.; van der Byl, K. Renal excretory actions of furosemide, of hydrochlorothiazide and of the vasodilator flosequinan in healthy subjects. J. Int. Med. Res. 1990, 18, 120–141. [Google Scholar] [CrossRef]
- Terrin, G.; Boscarino, G.; Di Chiara, M.; Iacobelli, S.; Faccioli, F.; Greco, C.; Onestà, E.; Sabatini, G.; Pietravalle, A.; Oliva, S.; et al. Nutritional intake influences zinc levels in preterm newborns: An observational study. Nutrients 2020, 12, 529. [Google Scholar] [CrossRef] [PubMed]
- Marreiro, D.D.; Cruz, K.J.; Morais, J.B.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R. Zinc and oxidative stress: Current mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Ysphaneendramallimoggala; Biswas, M.; Anburaj, S.E.; Iqbal, F.; Suryakanth, V.B.; Lewis, L.E.S. Thiamine: An indispensable regulator of pediatric neuro-cardiovascular health and diseases. Eur. J. Pediatr. 2024, 183, 4597–4610. [Google Scholar] [CrossRef] [PubMed]
- Tanné, C.; Nguyen, J.; Blondé, R. Shoshin beriberi and thiamine-responsive right heart failure: A case report in Mayotte. Arch. Pediatr. 2022, 29, 624–625. [Google Scholar] [CrossRef]
- Hasan, S.A.; Shajira, E.S. Lactic acidosis due to thiamine deficiency in a preterm infant associated with inadequate parenteral nutrition. Am. J. Case Rep. 2023, 24, e939008. [Google Scholar] [CrossRef]
- Leigh, R.S.; Kaynak, B.L. Vitamin A as a transcriptional regulator of cardiovascular disease. Hearts 2020, 1, 13. [Google Scholar] [CrossRef]
- Perl, E.; Waxman, J.S. Retinoic Acid Signaling and Heart Development. Sub-Cell. Biochem. 2020, 95, 119–149. [Google Scholar] [CrossRef]
- Cammisotto, V.; Nocella, C.; Bartimoccia, S.; Sanguigni, V.; Francomano, D.; Sciarretta, S.; Pastori, D.; Peruzzi, M.; Cavarretta, E.; D’Amico, A.; et al. The Role of Antioxidants Supplementation in Clinical Practice: Focus on Cardiovascular Risk Factors. Antioxidants 2021, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.; Jandial, A.; Sandal, R.; Khadwal, A.; Malhotra, P. Night blindness, Bitot’s spot and vitamin A deficiency. QJM 2019, 112, 225. [Google Scholar] [CrossRef]
- Caspi, J.; Coles, J.G.; Benson, L.N.; Herman, S.L.; Augustine, J.; Wilson, G.J. Dose-related effects of magnesium on myocardial function in the neonate. Hypertension 1994, 23, 174–178. [Google Scholar] [CrossRef]
- Fritzen, R.; Davies, A.; Veenhuizen, M.; Campbell, M.; Pitt, S.J.; Ajjan, R.A.; Stewart, A.J. Magnesium deficiency and cardiometabolic disease. Nutrients 2023, 15, 2355. [Google Scholar] [CrossRef]
- Gomella, T.; Cunningham, M.; Eyal, F.G.; Tuttle, D.J. (Eds.) Magnesium Disorders (Hypomagnesemia, Hypermagnesemia). In Neonatology: Management, Procedures, On-Call Problems, Diseases, and Drugs, 7th ed.; McGraw-Hill Education: New York, NY, USA, 2013; Available online: https://accesspediatrics.mhmedical.com/content.aspx?bookid=1303§ionid=79658865 (accessed on 25 November 2024).
- Fiorentini, D.; Cappadone, C.; Farruggia, G.; Prata, C. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Nutrients 2021, 13, 1136. [Google Scholar] [CrossRef]
- Pitzer Mutchler, A.; Huynh, L.; Patel, R.; Lam, T.; Bain, D.; Jamison, S.; Kirabo, A.; Ray, E.C. The role of dietary magnesium deficiency in inflammatory hypertension. Front. Physiol. 2023, 14, 1167904. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S.; Fukuda, N. Regulation of myocardial contraction as revealed by intracellular Ca2+ measurements using aequorin. J. Physiol. Sci. 2024, 74, 12. [Google Scholar] [CrossRef] [PubMed]
- Maiya, S.; Sullivan, I.; Allgrove, J.; Yates, R.; Malone, M.; Brain, C.; Archer, N.; Mok, Q.; Daubeney, P.; Tulloh, R.; et al. Hypocalcaemia and vitamin D deficiency: An important, but preventable, cause of life-threatening infant heart failure. Heart 2008, 94, 581–584. [Google Scholar] [CrossRef]
- Ibrahim, M.; El Raichani, N.; Otis, A.S.; Lavoie, J.C. Parenteral Cysteine Supplementation in Preterm Infants: One Size Does Not Fit All. Biomedicines 2024, 12, 63. [Google Scholar] [CrossRef]
- DeTallo, C. (Ed.) The Practitioner’s Guide to Nutrition-Focused Physical Exam of Infants, Children, and Adolescents: An Illustrated Handbook; Academy of Nutrition and Dietetics: Chicago, IL, USA, 2019. [Google Scholar]
- O’Neal Maynord, P.; Johnson, M.; Xu, M.; Slaughter, J.C.; Killen, S.A.S. A Multi-Interventional Nutrition Program for Newborns with Congenital Heart Disease. J. Pediatr. 2021, 228, 66–73.e2. [Google Scholar] [CrossRef] [PubMed]
- Furlong-Dillard, J.; Neary, A.; Marietta, J.; Jones, C.; Jeffers, G.; Gakenheimer, L.; Puchalski, M.; Eckauser, A.; Delgado-Corcoran, C. Evaluating the impact of a feeding protocol in neonates before and after biventricular cardiac surgery. Pediatr. Qual. Saf. 2018, 3, e080. [Google Scholar] [CrossRef] [PubMed]
- Slicker, J.; Sables-Baus, S.; Lambert, L.M.; Peterson, L.E.; Woodard, F.K.; Ocampo, E.C.; National Pediatric Cardiology-Quality Improvement Collaborative Feeding Work Group, National Pediatric Cardiology-Quality Improvement Collaborative Feeding Work Group. Perioperative feeding approaches in single ventricle infants: A survey of 46 centers. Congenit. Heart Dis. 2016, 11, 707–715. [Google Scholar] [CrossRef]
- Simsic, J.M.; Carpenito, K.R.; Kirchner, K.; Peters, S.; Miller-Tate, H.; Joy, B.; Galantowicz, M. Reducing variation in feeding newborns with congenital heart disease. Congenit. Heart Dis. 2017, 12, 275–281. [Google Scholar] [CrossRef]
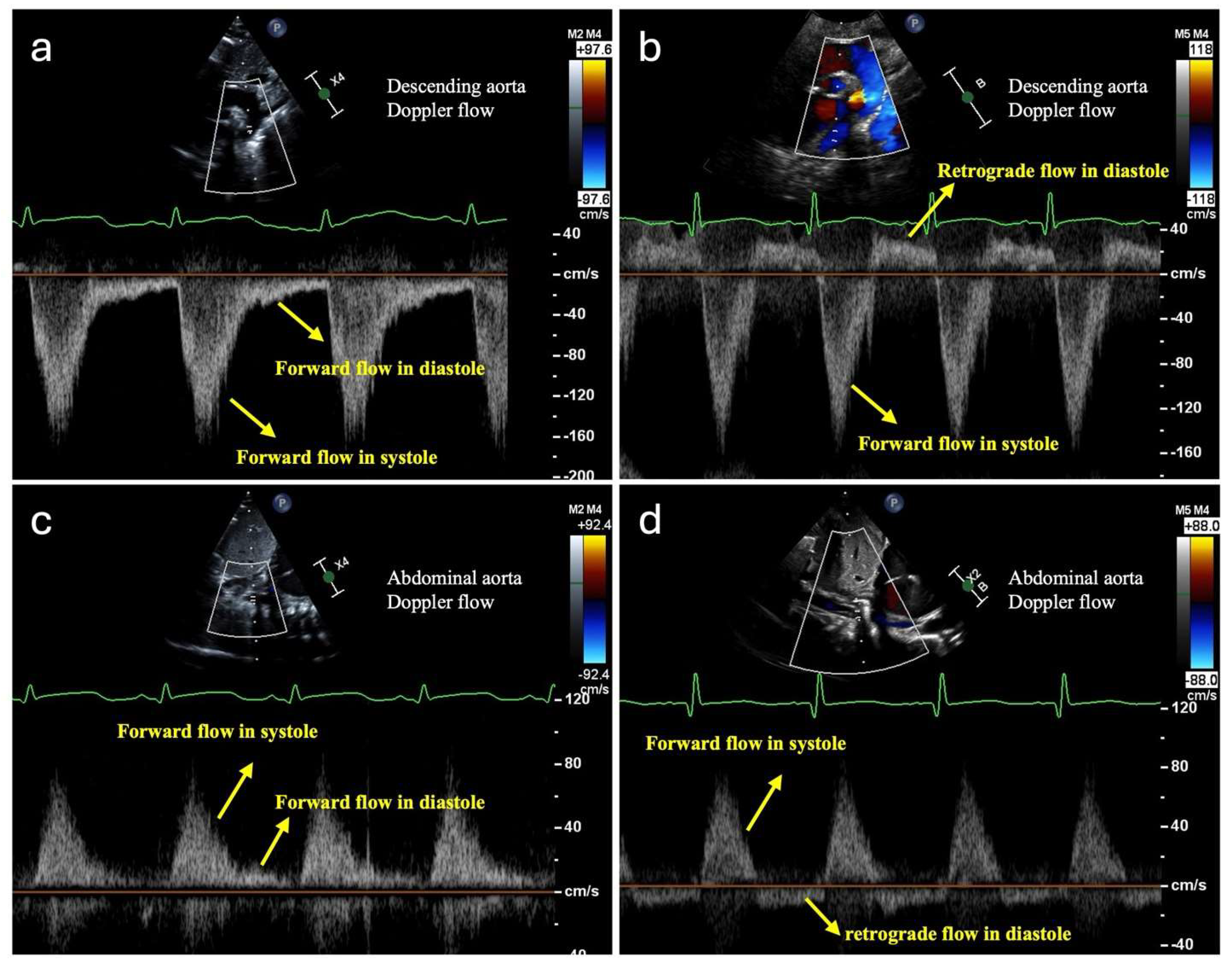
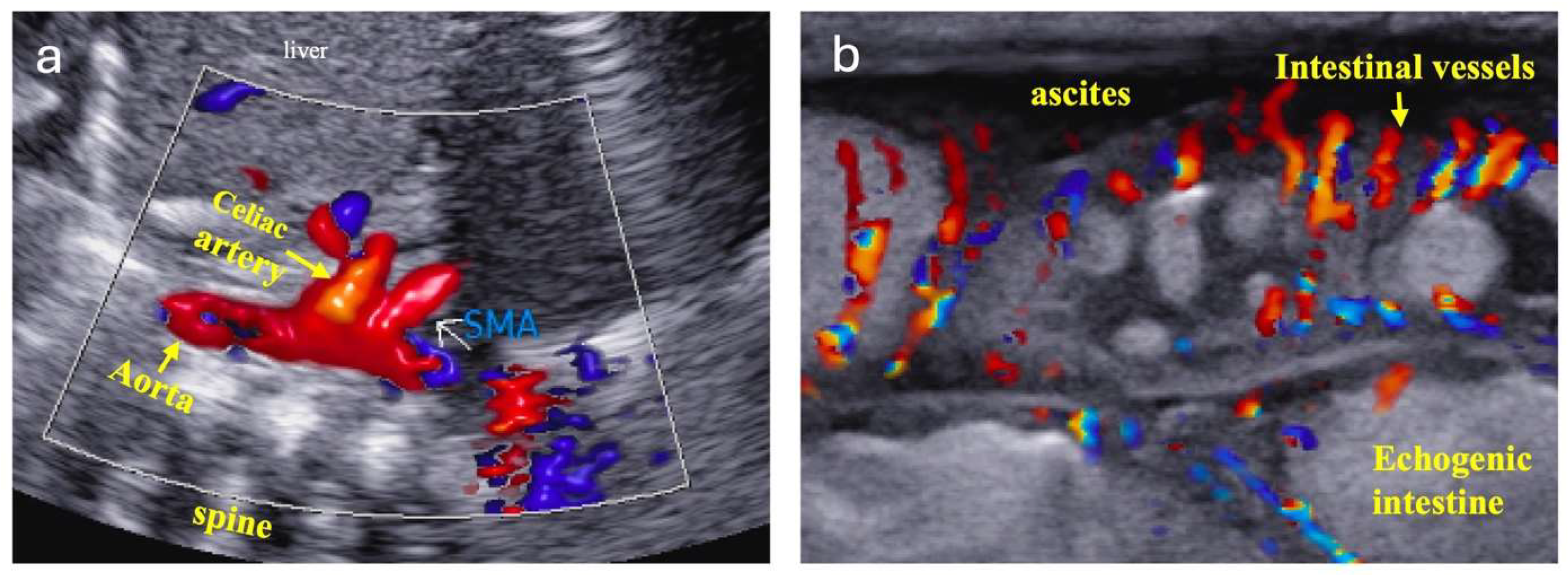
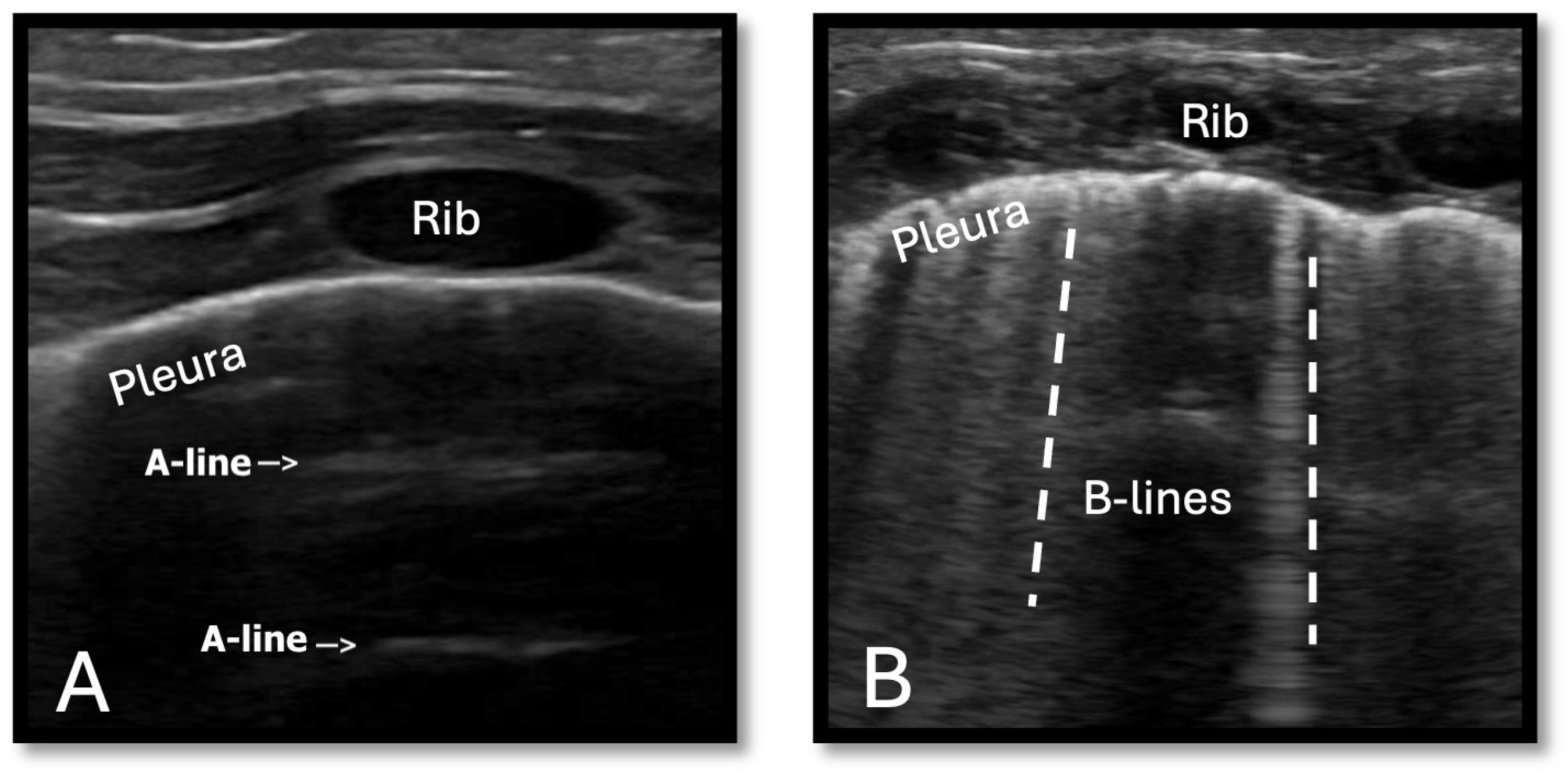


| Systemic Hypoperfusion | Global Hypoxia | Pulmonary Overcirculation | |
|---|---|---|---|
| Causes |
|
|
|
| Examples of Cardiac lesions |
|
|
|
| Hemodynamic and end-organ effects |
|
|
|
| Signs and symptoms |
|
|
|
| ECHO parameters |
|
|
|
| External monitoring |
|
|
|
| Feeding considerations |
|
|
|
| Low Suspicion | Moderate Suspicion | High Suspicion | |
|---|---|---|---|
| Prenatal Echocardiography findings |
|
|
|
| Care plan and feeding strategy | Routine newborn care:
| Cautious approach until CoA is ruled out:
| Immediate CoA management:
|
| Signs of Malnutrition in Nutrition-Focused Physical Exams (NFPE) [51] | |
|---|---|
| General |
|
| Face |
|
| Upper Body |
|
| Upper Back |
|
| Midaxillary Line |
|
| Lower Body |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, B.; Woodbury, A.; Hazelwood, L.; Singh, Y. Feeding Approach to Optimizing Nutrition in Infants with Congenital Heart Disease. J. Cardiovasc. Dev. Dis. 2025, 12, 38. https://doi.org/10.3390/jcdd12020038
Chan B, Woodbury A, Hazelwood L, Singh Y. Feeding Approach to Optimizing Nutrition in Infants with Congenital Heart Disease. Journal of Cardiovascular Development and Disease. 2025; 12(2):38. https://doi.org/10.3390/jcdd12020038
Chicago/Turabian StyleChan, Belinda, Anne Woodbury, Libbi Hazelwood, and Yogen Singh. 2025. "Feeding Approach to Optimizing Nutrition in Infants with Congenital Heart Disease" Journal of Cardiovascular Development and Disease 12, no. 2: 38. https://doi.org/10.3390/jcdd12020038
APA StyleChan, B., Woodbury, A., Hazelwood, L., & Singh, Y. (2025). Feeding Approach to Optimizing Nutrition in Infants with Congenital Heart Disease. Journal of Cardiovascular Development and Disease, 12(2), 38. https://doi.org/10.3390/jcdd12020038






