High-Risk Plaque Characteristics in Patients with Suspected Stable Coronary Artery Disease and Impaired Glucose Tolerance: A Coronary Computed Tomography Angiography Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
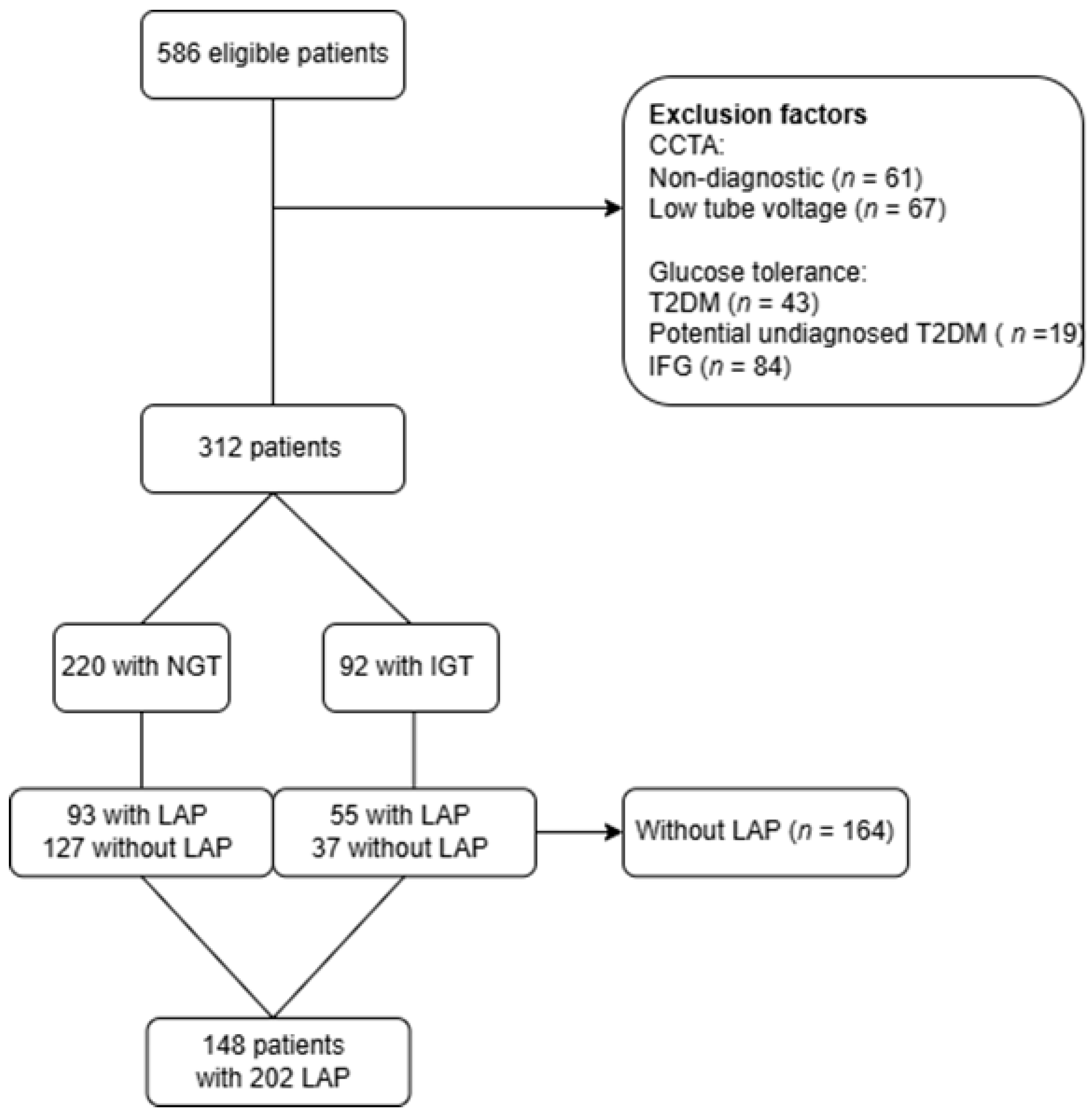
2.2. Study Population
2.3. Clinical and Biochemical Assessments
2.4. Glucose Tolerance Testing and Stratification
2.5. CCTA Acquisition
2.6. Quantitative CCTA Analysis
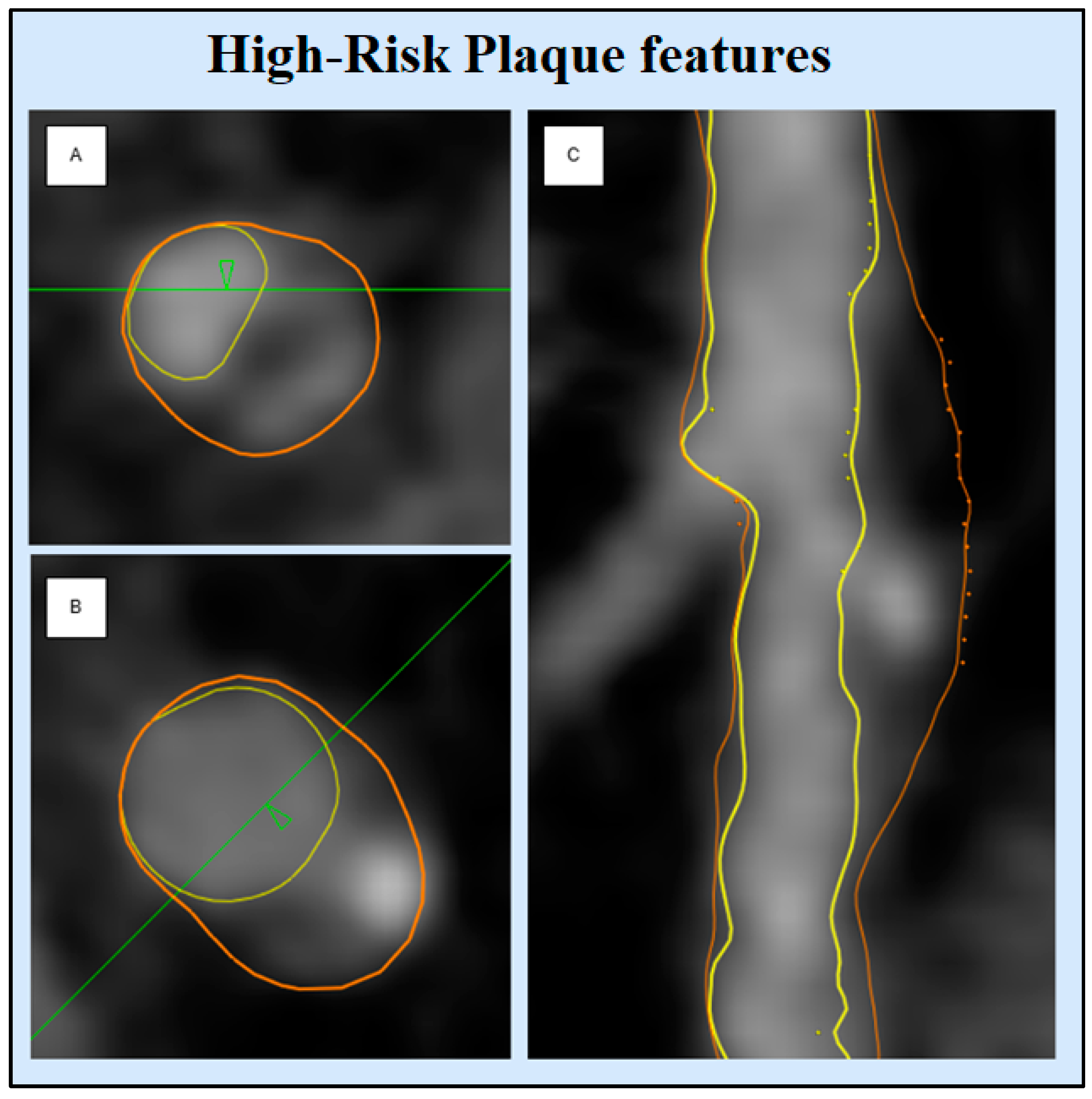
High-Risk Plaque Characteristics
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics
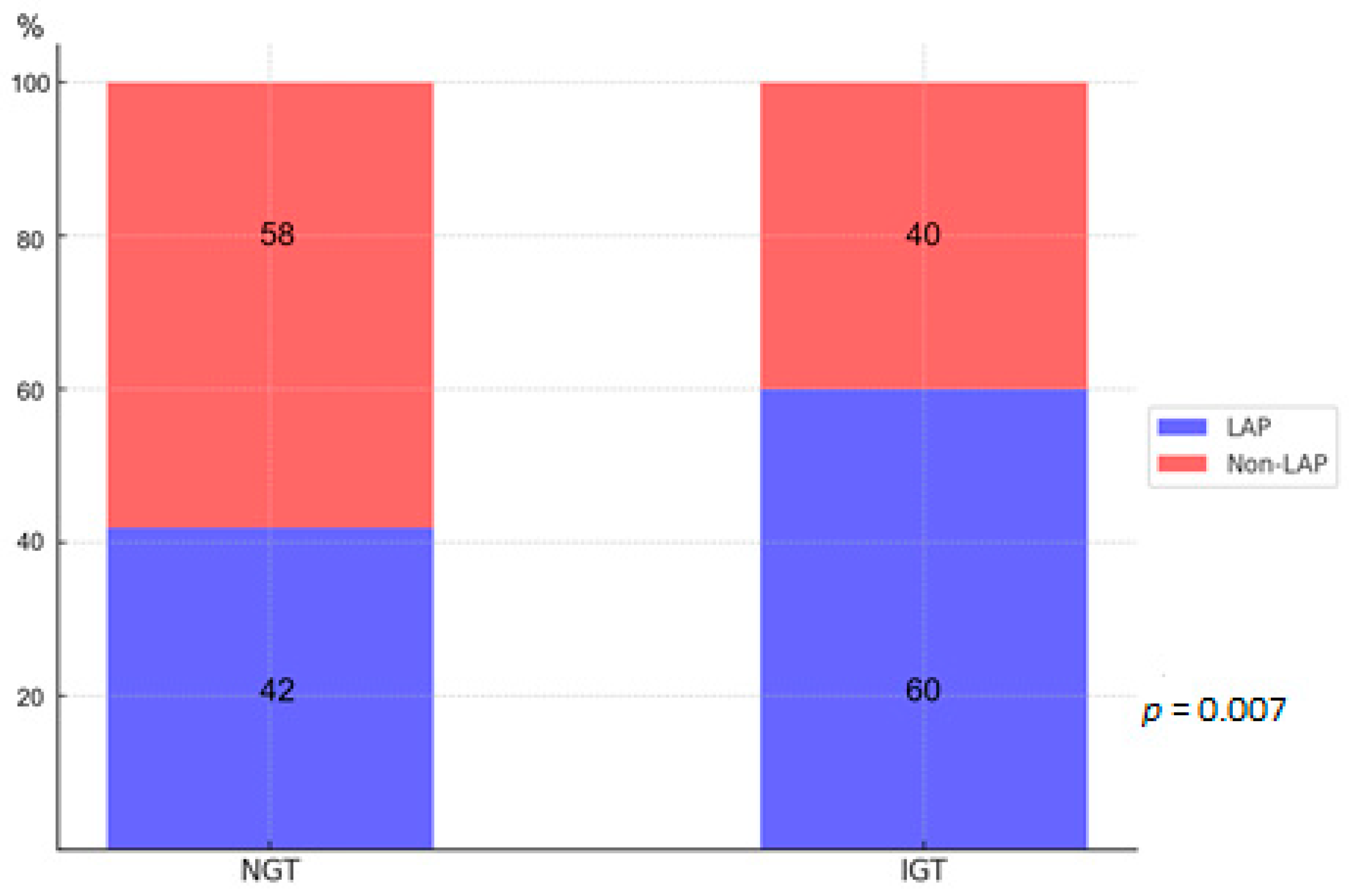
3.2. CCTA Findings
3.3. Univariate and Multivariate Analysis
4. Discussion
4.1. Prediabetes in a Clinical Context
4.2. IGT vs. IFG: Different Paths to CVD?
4.3. Plaque Burden in Prediabetes
4.4. High-Risk Plaque Features in Prediabetes
4.5. Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norhammar, A.; Tenerz, A.; Nilsson, G.; Hamsten, A.; Efendíc, S.; Rydén, L.; Malmberg, K. Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: A prospective study. Lancet 2002, 359, 2140–2144. [Google Scholar] [CrossRef]
- Ferrannini, G.; De Bacquer, D.; De Backer, G.; Kotseva, K.; Mellbin, L.; Wood, D.; Rydén, L. Screening for Glucose Perturbations and Risk Factor Management in Dysglycemic Patients With Coronary Artery Disease-A Persistent Challenge in Need of Substantial Improvement: A Report From ESC EORP EUROASPIRE V. Diabetes Care 2020, 43, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, G.; Tuomilehto, J.; De Backer, G.; Kotseva, K.; Mellbin, L.; Schnell, O.; Wood, D.; De Bacquer, D.; Rydén, L. Dysglycaemia screening and its prognostic impact in patients with coronary artery disease: Experiences from the EUROASPIRE IV and V cohort studies. Lancet Diabetes Endocrinol. 2024, 12, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; He, Y.; Wang, J.; Yu, L.; Gong, Q.; Chen, Y.; An, Y.; He, S.; Li, G.; Zhang, B. Influence of impaired glucose tolerance alone and combined with metabolic syndrome on long-term risk of cardiovascular events and mortality. J. Diabetes 2024, 16, e13598. [Google Scholar] [CrossRef]
- Suzuki, K.; Takano, H.; Kubota, Y.; Inui, K.; Nakamura, S.; Tokita, Y.; Kato, K.; Asai, K.; Shimizu, W. Plaque Characteristics in Coronary Artery Disease Patients with Impaired Glucose Tolerance. PLoS ONE 2016, 11, e0167645. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Kitagawa, T.; Ohashi, N.; Utsunomiya, H.; Kunita, E.; Oka, T.; Urabe, Y.; Tsushima, H.; Awai, K.; Kihara, Y. Noncalcified atherosclerotic lesions with vulnerable characteristics detected by coronary CT angiography and future coronary events. J. Cardiovasc. Comput. Tomogr. 2013, 7, 192–199. [Google Scholar]
- Thomsen, C.; Abdulla, J. Characteristics of high-risk coronary plaques identified by computed tomographic angiography and associated prognosis: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 120–129. [Google Scholar] [PubMed]
- Motoyama, S.; Sarai, M.; Narula, J.; Ozaki, Y. Coronary CT angiography and high-risk plaque morphology. Cardiovasc. Interv. Ther. 2013, 28, 1–8. [Google Scholar] [CrossRef]
- Bittner, D.O.; Mayrhofer, T.; Puchner, S.B.; Lu, M.T.; Maurovich-Horvat, P.; Ghemigian, K.; Kitslaar, P.H.; Broersen, A.; Bamberg, F.; Truong, Q.A.; et al. Coronary Computed Tomography Angiography–Specific Definitions of High-Risk Plaque Features Improve Detection of Acute Coronary Syndrome. Circ. Cardiovasc. Imaging 2018, 11, e007657. [Google Scholar] [CrossRef] [PubMed]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients With Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Puchner, S.B.; Liu, T.; Mayrhofer, T.; Truong, Q.A.; Lee, H.; Fleg, J.L.; Nagurney, J.T.; Udelson, J.E.; Hoffmann, U.; Ferencik, M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef]
- Nerlekar, N.; Ha, F.J.; Cheshire, C.; Rashid, H.; Cameron, J.D.; Wong, D.T.; Seneviratne, S.; Brown, A.J. Computed Tomographic Coronary Angiography–Derived Plaque Characteristics Predict Major Adverse Cardiovascular Events. Circ. Cardiovasc. Imaging 2018, 11, e006973. [Google Scholar] [CrossRef] [PubMed]
- Nakazato, R.; Otake, H.; Konishi, A.; Iwasaki, M.; Koo, B.K.; Fukuya, H.; Shinke, T.; Hirata, K.; Leipsic, J.; Berman, D.S.; et al. Atherosclerotic plaque characterization by CT angiography for identification of high-risk coronary artery lesions: A comparison to optical coherence tomography. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Leipsic, J.A.; Indraratna, P.; Gulsin, G.; Khasanova, E.; Tzimas, G.; Lin, F.Y.; Shaw, L.J.; Lee, S.E.; Andreini, D.; et al. Association of Tube Voltage With Plaque Composition on Coronary CT Angiography: Results From PARADIGM Registry. JACC Cardiovasc. Imaging 2021, 14, 2429–2440. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43 (Suppl. S1), S14–S31. [Google Scholar] [CrossRef]
- Anand, S.S.; Dagenais, G.R.; Mohan, V.; Diaz, R.; Probstfield, J.; Freeman, R.; Shaw, J.; Lanas, F.; Avezum, A.; Budaj, A.; et al. Glucose levels are associated with cardiovascular disease and death in an international cohort of normal glycaemic and dysglycaemic men and women: The EpiDREAM cohort study. Eur. J. Prev. Cardiol. 2012, 19, 755–764. [Google Scholar] [CrossRef]
- Williams, M.C.; Kwiecinski, J.; Doris, M.; McElhinney, P.; D’Souza, M.S.; Cadet, S.; Adamson, P.D.; Moss, A.J.; Alam, S.; Hunter, A.; et al. Low-Attenuation Noncalcified Plaque on Coronary Computed Tomography Angiography Predicts Myocardial Infarction: Results From the Multicenter SCOT-HEART Trial (Scottish Computed Tomography of the HEART). Circulation 2020, 141, 1452–1462. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Conte, E.; Annoni, A.; Pontone, G.; Mushtaq, S.; Guglielmo, M.; Baggiano, A.; Volpato, V.; Agalbato, C.; Bonomi, A.; Veglia, F.; et al. Evaluation of coronary plaque characteristics with coronary computed tomography angiography in patients with non-obstructive coronary artery disease: A long-term follow-up study. Eur. Heart J. Cardiovasc. Imaging 2016, 18, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Yahyavi, S.K.; Snorgaard, O.; Knop, F.K.; Schou, M.; Lee, C.; Selmer, C.; Gislason, G.; Torp-Pedersen, C.; Blomberg Jensen, M.; Nissen Bonde, A. Prediabetes Defined by First Measured HbA(1c) Predicts Higher Cardiovascular Risk Compared With HbA(1c) in the Diabetes Range: A Cohort Study of Nationwide Registries. Diabetes Care 2021, 44, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes. Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar]
- Marx, N.; Rydén, L.; Federici, M.; Marx-Schütt, K.; Verket, M.; Müller-Wieland, D.; Gerstein, H.C.; Chan, J.; Cosentino, F.; Holman, R.R.; et al. Great debate: Pre-diabetes is not an evidence-based treatment target for cardiovascular risk reduction. Eur. Heart J. 2024, 45, 5117–5126. [Google Scholar] [CrossRef] [PubMed]
- Glucose tolerance and mortality: Comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet 1999, 354, 617–621.
- Gurudevan, S.; Garg, P.; Malik, S.; Khattar, R.; Saremi, F.; Hecht, H.; DeMaria, A.; Narula, J. Impaired fasting glucose is associated with increased severity of subclinical coronary artery disease compared to patients with diabetes and normal fasting glucose: Evaluation by coronary computed tomographic angiography. BMJ Open 2016, 6, e005148. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Pyörälä, K.; Pyörälä, M.; Nissinen, A.; Lindström, J.; Tilvis, R.; Tuomilehto, J. Two-hour glucose is a better risk predictor for incident coronary heart disease and cardiovascular mortality than fasting glucose. Eur. Heart J. 2002, 23, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, O.; Takano, M.; Yamamoto, M.; Shirakabe, A.; Kimata, N.; Inami, T.; Kobayashi, N.; Munakata, R.; Murakami, D.; Inami, S.; et al. Impact of prediabetic status on coronary atherosclerosis: A multivessel angioscopic study. Diabetes Care 2013, 36, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Sassa, S.; Shimada, K.; Yoshida, K.; Tanaka, H.; Jissho, S.; Yoshikawa, J. Comparison of 64-slice multi-detector computed tomography coronary angiography between asymptomatic, type 2 diabetes mellitus and impaired glucose tolerance patients. J. Cardiol. 2008, 52, 133–139. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsson, J.; Auscher, S.; Shamoun, A.; Pararajasingam, G.; Heinsen, L.J.; Andersen, T.R.; Lindholt, J.S.; Diederichsen, A.C.P.; Lambrechtsen, J.; Egstrup, K. Insulin resistance is associated with high-risk coronary artery plaque composition in asymptomatic men between 65 and 75 years and no diabetes: A DANCAVAS cross-sectional sub-study. Atherosclerosis 2023, 385, 117328. [Google Scholar] [CrossRef] [PubMed]
- Neglia, D.; Caselli, C.; Maffei, E.; Cademartiri, F.; Meloni, A.; Bossone, E.; Saba, L.; Lee, S.E.; Sung, J.M.; Andreini, D.; et al. Rapid Plaque Progression Is Independently Associated With Hyperglycemia and Low HDL Cholesterol in Patients With Stable Coronary Artery Disease: A PARADIGM Study. Cardiovasc. Imaging 2024, 17, e016481. [Google Scholar] [CrossRef]
- Farhan, S.; Redfors, B.; Maehara, A.; McAndrew, T.; Ben-Yehuda, O.; De Bruyne, B.; Mehran, R.; Giustino, G.; Kirtane, A.J.; Serruys, P.W.; et al. Impact of Pre-Diabetes on Coronary Plaque Composition and Clinical Outcome in Patients With Acute Coronary Syndromes: An Analysis From the PROSPECT Study. JACC Cardiovasc. Imaging 2019, 12, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Maurovich-Horvat, P.; Mayrhofer, T.; Puchner, S.B.; Lu, M.T.; Ghemigian, K.; Kitslaar, P.H.; Broersen, A.; Pursnani, A.; Hoffmann, U.; et al. Quantitative coronary plaque analysis predicts high-risk plaque morphology on coronary computed tomography angiography: Results from the ROMICAT II trial. Int. J. Cardiovasc. Imaging 2018, 34, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sheng, Z.; Liu, C.; Zhou, P.; Li, J.; Chen, R.; Song, L.; Zhao, H.; Yan, H. Association between Admission Hyperglycemia and Culprit Lesion Characteristics in Nondiabetic Patients with Acute Myocardial Infarction: An Intravascular Optical Coherence Tomography Study. J. Diabetes Res. 2020, 2020, 1763567. [Google Scholar] [CrossRef]
- Patel, K.V.; Budoff, M.J.; Valero-Elizondo, J.; Lahan, S.; Ali, S.S.; Taha, M.B.; Blaha, M.J.; Blankstein, R.; Shapiro, M.D.; Pandey, A.; et al. Coronary Atherosclerosis Across the Glycemic Spectrum Among Asymptomatic Adults: The Miami Heart Study at Baptist Health South Florida. Circ. Cardiovasc. Imaging 2023, 16, e015314. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Crainiceanu, C.M.; Brancati, F.L.; Coresh, J. Short-term variability in measures of glycemia and implications for the classification of diabetes. Arch. Intern. Med. 2007, 167, 1545–1551. [Google Scholar] [CrossRef]

| NGT n = 93 | IGT n = 55 | p-Value | |
|---|---|---|---|
| Age, years, n (SD) | 64.7 (8.8) | 61.0 (9.4) | 0.02 |
| Sex, male, n (%) | 62 (67%) | 42 (76%) | 0.2 |
| BMI, kg/m2 (SD) | 27.4 (6.0) | 28.9 (4.3) | 0.1 |
| Systolic BP, mmHg (SD) | 144 (25) | 150 (22) | 0.2 |
| Diastolic BP, mmHg (SD) | 80 (13) | 83 (12) | 0.2 |
| Never smoker, n (%) | 44 (47%) | 20 (36%) | 0.7 |
| Former smoker, n (%) | 38 (41%) | 25 (45%) | 0.1 |
| Active smoker, n (%) | 11 (12%) | 9 (16%) | 0.4 |
| Family history of CVD, n (%) | 29 (31%) | 22 (40%) | 0.3 |
| Fasting glucose, mmol/L (SD) | 5.6 (0.3) | 6.1 (0.5) | <0.001 |
| 120 min glucose, mmol/L (SD) | 5.7 (1.1) | 8.7 (1.2) | <0.001 |
| HbA1c, mmol/mol (SD) | 35 (4) | 37 (3) | 0.03 |
| Pre-diabetic HbA1c, n (%) | 34 (37%) | 18 (33%) | 0.7 |
| Total cholesterol, mmol/L (SD) | 5.0 (1.1) | 4.6 (1.2) | 0.06 |
| HDL, mmol/L (SD) | 1.5 (0.4) | 1.3 (0.3) | <0.001 |
| LDL, mmol/L (SD) | 2.9 (1.0) | 2.6 (1.1) | 0.2 |
| Triglycerides, mmol/L (SD) | 1.3 (0.8) | 1.8 (0.9) | 0.003 |
| CRP, mg/L (SD) | 2.1 (1.9) | 3.1 (2.8) | 0.02 |
| Antihypertensive medication, n (%) | 49 (53%) | 32 (60%) | 0.2 |
| Statins, n (%) | 26 (28%) | 26 (47%) | 0.02 |
| Medical history | |||
| AMI | 2 (2%) | 2(4%) | 0.6 |
| PCI or CABG | 1 (1%) | 1 (2%) | 0.7 |
| Stroke | 3 (3%) | 2(4%) | 0.8 |
| Heart failure | 1(1%) | 0 | 0.6 |
| Total CACS, (IQR) | 73 (4–233) | 76 (1–214) | 0.5 |
| Inflammatory disease | 6(6%) | 4(7%) | 0.8 |
| LAP lesions: | |||
| NCP, n (%) | 36 (30%) | 30 (38%) | 0.2 |
| Mixed mostly fibrous, n (%) | 72 (59%) | 42 (53%) | 0.4 |
| Mixed mostly calcified, n (%) | 12 (10%) | 8 (10%) | 0.4 |
| NGT n = 122 | IGT n = 80 | p-Value | |
|---|---|---|---|
| Plaque location | |||
| Left anterior descending artery | 70 (57%) | 42 (53%) | - |
| Circumflex artery | 18 (15%) | 5 (6%) | - |
| Right coronary artery | 34 (28%) | 33 (41%) | - |
| Plaque metrics | |||
| Lesion length, mm | 17.5 (5.4) | 17.4 (5.8) | 0.9 |
| TAV, mm3 | 155.6 (72.9) | 157.5 (76.0) | 0.9 |
| Degree stenosis % | 31%(18, 59) | 37(16, 62%) | 0.3 |
| Plaque volumes, mm3 | |||
| Calcified | 25.7 (37.7) | 22.1 (27.5) | 0.5 |
| Non-calcified | 109.8 (48.2) | 114.3 (61.4) | 0.6 |
| Low-attenuation | 12.6 (9.7) | 16.5 (12.5) | 0.01 |
| Plaque burdens, % | |||
| Calcified | 14.2 (14.2) | 12.8 (11.8) | 0.5 |
| Non-calcified | 72.3 (12.8) | 73.1 (10.5) | 0.7 |
| Low-attenuation | 8.623 (5.9) | 10.8 (6.8) | 0.02 |
| HRP features, n (%) | |||
| Positive remodeling | 21 (17%) | 15 (19%) | 0.8 |
| Spotty calcification | 28 (23%) | 26 (32%) | 0.1 |
| Napkin-ring sign | 5 (5%) | 10 (12%) | 0.02 |
| HRP ≥ 2 | 46 (38%) | 39 (49%) | 0.1 |
| HRP ≥ 3 | 8 (7%) | 10 (13%) | 0.1 |
| NGT vs. IGT | p-Value | NGT vs. IGT | p-Value | |
|---|---|---|---|---|
| Univariate | Multivariate | |||
| β | β | |||
| Plaque volumes, mm3 | ||||
| Calcified | 0.3 | 0.08 | 0.02 | 0.1 |
| Non-calcified | 4.4 | 0.5 | 0.04 | 0.6 |
| Low-attenuation | 3.7 | 0.02 | 2.9 | 0.06 |
| Plaque burdens, % | ||||
| Calcified | −0.1 | 0.7 | 0.3 | 0.3 |
| Non-calcified | 0.8 | 0.7 | 0.3 | 0.9 |
| Low-attenuation | 0.4 | 0.02 | 0.3 | 0.03 |
| HRP features, n | OR | OR | ||
| Positive remodeling | 1.1 | 0.8 | 1.04 | 0.9 |
| Spotty calcifications | 1.6 | 0.1 | 1.4 | 0.4 |
| Napkin-ring sign | 2.7 | 0.02 | 2.2 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersen, T.R.; Overgaard, K.S.; Heinsen, L.J.; Mohamed, R.A.; Madsen, F.S.; Precht, H.; Lambrechtsen, J.; Auscher, S.; Egstrup, K. High-Risk Plaque Characteristics in Patients with Suspected Stable Coronary Artery Disease and Impaired Glucose Tolerance: A Coronary Computed Tomography Angiography Study. J. Cardiovasc. Dev. Dis. 2025, 12, 37. https://doi.org/10.3390/jcdd12020037
Andersen TR, Overgaard KS, Heinsen LJ, Mohamed RA, Madsen FS, Precht H, Lambrechtsen J, Auscher S, Egstrup K. High-Risk Plaque Characteristics in Patients with Suspected Stable Coronary Artery Disease and Impaired Glucose Tolerance: A Coronary Computed Tomography Angiography Study. Journal of Cardiovascular Development and Disease. 2025; 12(2):37. https://doi.org/10.3390/jcdd12020037
Chicago/Turabian StyleAndersen, Thomas Rueskov, Katrine Schultz Overgaard, Laurits Juhl Heinsen, Roda Abdulkadir Mohamed, Freja Sønder Madsen, Helle Precht, Jess Lambrechtsen, Søren Auscher, and Kenneth Egstrup. 2025. "High-Risk Plaque Characteristics in Patients with Suspected Stable Coronary Artery Disease and Impaired Glucose Tolerance: A Coronary Computed Tomography Angiography Study" Journal of Cardiovascular Development and Disease 12, no. 2: 37. https://doi.org/10.3390/jcdd12020037
APA StyleAndersen, T. R., Overgaard, K. S., Heinsen, L. J., Mohamed, R. A., Madsen, F. S., Precht, H., Lambrechtsen, J., Auscher, S., & Egstrup, K. (2025). High-Risk Plaque Characteristics in Patients with Suspected Stable Coronary Artery Disease and Impaired Glucose Tolerance: A Coronary Computed Tomography Angiography Study. Journal of Cardiovascular Development and Disease, 12(2), 37. https://doi.org/10.3390/jcdd12020037






