Late Complications After Aortic Coarctation Repair
Abstract
1. Introduction
2. Surgical Approaches for CoA
2.1. Open Surgical Repair: Techniques and Considerations
2.2. Endovascular Treatment of Aortic Coarctation: Evolving Role and Techniques
3. Late Complications After CoA Repair: Post-Aortic Coarctation Aneurysms (pCoAA)
3.1. Epidemiology
3.2. Pathophysiology and Morphological Spectrum
3.3. Surveillance: Imaging and Follow-Up Protocols
3.4. Clinical Implications and Indications for Intervention
3.5. Therapeutic Strategies: Open Versus Endovascular Interventions
3.5.1. Open Surgical Repair
3.5.2. Endovascular Repair (TEVAR)
3.6. Comparative Early and Long-Term Outcomes
4. Late Complications After CoA Repair: Re-Coarctation
4.1. Epidemiology
- Young age and low weight at repair: neonates and low-birth-weight infants have smaller vessels, increasing the risk of restenosis [48].
- Aortic arch hypoplasia: associated hypoplasia, especially of the transverse arch, contributes to persistent or progressive gradients post-repair [49].
- Surgical technique: end-to-end anastomosis and subclavian flap repairs have different restenosis rates; the latter is associated with higher re-coarctation risks if not properly sized [49].
- Residual pressure gradients: a persistent gradient >10 mmHg immediately after surgery is a predictor of recurrence [50].
- Genetic syndromes: Turner syndrome, bicuspid aortic valve, and other connective tissue disorders can predispose patients to vascular abnormalities and restenosis.
4.2. Pathophysiology and Morphological Spectrum
4.3. Clinical Presentation and Surveillance Imaging
- Echocardiography: first-line for evaluating aortic arch gradients and ventricular function. A Doppler gradient >20 mmHg suggests significant obstruction [53]
- MRI/CT angiography: provide detailed anatomical assessment, especially of the transverse arch and collateral vessels
- Cardiac catheterization: gold standard for gradient measurement and anatomical delineation. Peak-to-peak systolic gradient ≥20 mmHg is commonly used to justify intervention [47]
4.4. Therapeutic Strategies: Open Versus Endovascular Interventions
4.4.1. Balloon Angioplasty
4.4.2. Stent Placement
4.4.3. Surgical Re-Intervention
4.5. Early and Long-Term Outcomes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CoA | Aortic coarctation |
| pCoAA | Post-aortic coarctation aneurysm |
| BAV | Bicuspid aortic valve |
| CTA | Computerized tomography angiography |
| MRI | Magnetic Resonance Imaging |
| OSR | Open surgical repair |
| TEVAR | Thoracic endovascular aortic repair |
References
- Salciccioli, K.B.; Zachariah, J.P. Coarctation of the Aorta: Modern Paradigms Across the Lifespan. Hypertension 2023, 80, 1970–1979. [Google Scholar] [CrossRef]
- O’Brien, P.; Marshall, A.C. Coarctation of the Aorta. Circulation 2015, 131, e363–e365. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Burkhart, H.M.; Connolly, H.M.; Dearani, J.A.; Cetta, F.; Li, Z.; Oliver, W.C.; Warnes, C.A.; Schaff, H.V. Coarctation of the Aorta. J. Am. Coll. Cardiol. 2013, 62, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Abbruzzese, P.A.; Aidala, E. Aortic coarctation: An overview. J. Cardiovasc. Med. 2007, 8, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Aggarwal, S.; Jenkins, P.; Kharabish, A.; Anwer, S.; Cullington, D.; Jones, J.; Dua, J.; Papaioannou, V.; Ashrafi, R.; et al. Coarctation of the Aorta: Diagnosis and Management. Diagnostics 2023, 13, 2189. [Google Scholar] [CrossRef]
- Heider, A.; Gordon, D.; Coleman, D.M.; Eliason, J.L.; Ganesh, S.K.; Stanley, J.C. Histologic and morphologic character of pediatric abdominal aortic developmental coarctation and hypoplasia. J. Vasc. Surg. 2022, 76, 556–563.e4. [Google Scholar] [CrossRef]
- Lande, A. Takayasu’s arteritis and congenital coarctation of the descending thoracic and abdominal aorta: A critical review. Am. J. Roentgenol. 1976, 127, 227–233. [Google Scholar] [CrossRef]
- Calhoun, T.R.; Thumwood, R.G.; Tennyson, K.B.; Wright, R.M.; Kitten, C.M.; Windham, P.A. Coarctation of the abdominal aorta. Tex. Heart Inst. J. 1983, 10, 269–273. [Google Scholar]
- Obata, S.; Mukai, S.; Morimoto, H.; Hiraoka, T.; Uchida, H.; Yamane, Y. Successful Ascending Aorta-Abdominal Aorta Bypass Graft through the Left Thoracic Cavity in a Patient with Atypical Coarctation. Ann. Vasc. Dis. 2013, 6, 670–673. [Google Scholar] [CrossRef]
- Rajagopalan, B.K.; Jose, R.; Kader, N.P.; Varma, P.K. Coarctation of the aorta aneurysm with aberrant right subclavian artery and single carotid artery: Surgical and perfusion strategies. J. Thorac. Cardiovasc. Surg. 2019, 157, e17–e19. [Google Scholar] [CrossRef]
- Buckley, A.D.; Han Um, K.Y.; Ganame, J.I.; Salehian, O.; Karbassi, A. Prevalence of Intracranial Aneurysms in Patients With Coarctation of the Aorta. JACC Adv. 2023, 2, 100394. [Google Scholar] [CrossRef]
- Duijnhouwer, A.; Hoven, A.v.D.; Merkx, R.; Schokking, M.; van Kimmenade, R.; Kempers, M.; van Dijk, A.; de Boer, M.-J.; Roos-Hesselink, J. Differences in Aortopathy in Patients with a Bicuspid Aortic Valve with or without Aortic Coarctation. J. Clin. Med. 2020, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Isselbacher, E.M.; Preventza, O.; Black, J.H., 3rd; Augoustides, J.G.; Beck, A.W.; Bolen, M.A.; Braverman, A.C.; Bray, B.E.; Brown-Zimmerman, M.M.; Chen, E.P.; et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2022, 146, e334–e482. [Google Scholar] [PubMed]
- Kenny, D.; Hijazi, Z.M. Coarctation of the aorta: From fetal life to adulthood. Cardiol. J. 2011, 19, 487–495. [Google Scholar] [CrossRef]
- Sinning, C.; Zengin, E.; Kozlik-Feldmann, R.; Blankenberg, S.; Rickers, C.; von Kodolitsch, Y.; Girdauskas, E. Bicuspid aortic valve and aortic coarctation in congenital heart disease are important aspects of treatment with a focus on aortic vasculopathy. Cardiovasc. Diagn. Ther. 2018, 8, 780–788. [Google Scholar] [CrossRef]
- Warnes, C.A. Bicuspid aortic valve and coarctation: Two villains part of a diffuse problem. Heart 2003, 89, 965–966. [Google Scholar] [CrossRef]
- Hysko, K.; Bertram, H.; Bobylev, D.; Horke, A.; Hansmann, G. Advances in the Treatment of Neonatal Coarctation of the Aorta. Pediatrics 2025, 155, e2024067434. [Google Scholar] [CrossRef]
- Campbell, M. Natural history of coarctation of the aorta. Heart 1970, 32, 633–640. [Google Scholar] [CrossRef]
- Venkatesh, V.; Frishman, W.H.; Aronow, W.S. Coarctation of the Aorta: Review of Current Literature. Cardiol. Rev. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Toro-Salazar, O.H.; Steinberger, J.; Thomas, W.; Rocchini, A.P.; Carpenter, B.; Moller, J.H. Long-term follow-up of patients after coarctation of the aorta repair. Am. J. Cardiol. 2002, 89, 541–547. [Google Scholar] [CrossRef]
- Hu, Z.P.; Wang, Z.W.; Dai, X.F.; Zhan, B.T.; Ren, W.; Li, L.C.; Zhang, H.; Ren, Z.L. Outcomes of Surgical versus Balloon Angioplasty Treatment for Native Coarctation of the Aorta: A Meta-Analysis. Ann. Vasc. Surg. 2014, 28, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Frankel, W.C.; Roselli, E.E. Strategies for Complex Reoperative Aortic Arch Reconstruction in Patients With Congenital Heart Disease. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2023, 26, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Vasile, C.M.; Laforest, G.; Bulescu, C.; Jalal, Z.; Thambo, J.B.; Iriart, X. From Crafoord’s End-to-End Anastomosis Approach to Percutaneous Interventions: Coarctation of the Aorta Management Strategies and Reinterventions. J. Clin. Med. 2023, 12, 7350. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Masaki, N.; Sai, S. Combined procedure using a double flap as a surgical option for coarctation of the aorta with delayed diagnosis. Gen. Thorac. Cardiovasc. Surg. 2025, 73, 66–69. [Google Scholar] [CrossRef]
- Meloro, B.; Gigioli, J.; Kovach, R.; Domer, G. Vertebrobasilar insufficiency after subclavian flap aortoplasty for aortic coarctation. J. Vasc. Surg. Cases Innov. Tech. 2024, 10, 101409. [Google Scholar] [CrossRef]
- Beckmann, E.; Jassar, A.S. Coarctation repair—Redo challenges in the adults: What to do? J. Vis. Surg. 2018, 4, 76. [Google Scholar] [CrossRef]
- Price, J.D.; LaPar, D.J. The Challenges of Redo Aortic Coarctation Repair in Adults. Curr. Cardiol. Rep. 2019, 21, 99. [Google Scholar] [CrossRef]
- Ahmadi, A.; Mansourian, M.; Sabri, M.R.; Ghaderian, M.; Karimi, R.; Roustazadeh, R. Follow-up outcomes and effectiveness of stent implantation for aortic coarctation: A systematic review and meta-analysis. Curr. Probl. Cardiol. 2024, 49, 102513. [Google Scholar] [CrossRef]
- Melissano, G.; Canaud, L.; Pacini, D.; Bilman, V.; Erben, Y.; Oo, A.Y.; Riambau, V.; Pedro, L.M.; Oderich, G.S.; Estrera, A.L.; et al. Surgical and endovascular treatment of late postcoarctation repair aortic aneurysms: Results from an international multicenter study. J. Vasc. Surg. 2022, 76, 1449–1457.e4. [Google Scholar] [CrossRef]
- Nana, P.; Spanos, K.; Brodis, A.; Kouvelos, G.; Rickers, C.; Kozlik-Feldmann, R.; Giannoukas, A.; Kölbel, T. A Systematic Review and Meta-analysis on Stenting for Aortic Coarctation Management in Adults. J. Endovasc. Ther. 2025, 32, 548–557. [Google Scholar] [CrossRef]
- Righini, P.; Mazzaccaro, D.; Galligani, M.; Giannetta, M.; Secchi, F.; Carminati, M.; Nano, G. Solving Intraoperative Complications During Endovascular Repair of Late Contained Ruptured Aortic Pseudoaneurysm After Surgical De-coarctation: Case Report and Systematic Review of Literature. J. Endovasc. Ther. 2025, 32, 290–302. [Google Scholar] [CrossRef]
- Cramer, J.W.; Ginde, S.; Bartz, P.J.; Tweddell, J.S.; Litwin, S.B.; Earing, M.G. Aortic Aneurysms Remain a Significant Source of Morbidity and Mortality After Use of Dacron® Patch Aortoplasty to Repair Coarctation of the Aorta: Results from a Single Center. Pediatr. Cardiol. 2013, 34, 296–301. [Google Scholar] [CrossRef]
- Warnes, C.A.; Williams, R.G.; Bashore, T.M.; Child, J.S.; Connolly, H.M.; Dearani, J.A.; Del Nido, P.; Fasules, J.W.; Graham, T.P., Jr.; Hijazi, Z.M. ACC/AHA 2008 Guidelines for the Management of Adults With Congenital Heart Disease. J. Am. Coll. Cardiol. 2008, 52, e143–e263. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.W.J.; Mulder, B.J.M. Late complications in patients after repair of aortic coarctation: Implications for management. Int. J. Cardiol. 2005, 101, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Paç, M. Long-term outcomes after surgical repair of the coarctation of the aorta beyond the infancy period. Turk. J. Thorac. Cardiovasc. Surg. 2017, 25, 558–564. [Google Scholar] [CrossRef]
- Del Nido, P.J.; Williams, W.G.; Wilson, G.J.; Coles, J.G.; Moes, C.A.; Hosokawa, Y.; McLaughlin, P.R.; Fowler, R.S.; Izukawa, T.; Rowe, R.D. Synthetic patch angioplasty for repair of coarctation of the aorta: Experience with aneurysm formation. Circulation 1986, 74, I32–I36. [Google Scholar]
- von Kodolitsch, Y.; Aydin, M.A.; Koschyk, D.H.; Loose, R.; Schalwat, I.; Karck, M.; Cremer, J.; Haverich, A.; Berger, J.; Meinertz, T.; et al. Predictors of aneurysmal formation after surgical correction of aortic coarctation. J. Am. Coll. Cardiol. 2002, 39, 617–624. [Google Scholar] [CrossRef]
- Shi, D.; Zhang, M.; Zhang, Y.; Shi, Y.; Liu, X.; Wu, X.; Yang, Z. The Pathophysiological Role of Vascular Smooth Muscle Cells in Abdominal Aortic Aneurysm. Cells 2025, 14, 1009. [Google Scholar] [CrossRef]
- Bouchart, F.; Dubar, A.; Tabley, A.; Litzler, P.Y.; Haas-Hubscher, C.; Redonnet, M.; Bessou, J.P.; Soyer, R. Coarctation of the aorta in adults: Surgical results and long-term follow-up. Ann. Thorac. Surg. 2000, 70, 1483–1488. [Google Scholar] [CrossRef]
- Cohen, M.; Fuster, V.; Steele, P.M.; Driscoll, D.; McGoon, D.C. Coarctation of the aorta. Long-term follow-up and prediction of outcome after surgical correction. Circulation 1989, 80, 840–845. [Google Scholar] [CrossRef]
- Høimyr, H.; Christensen, T.D.; Emmertsen, K.; Johnsen, S.P.; Riis, A.; Hansen, O.K.; Hjortdal, V.E. Surgical repair of coarctation of the aorta: Up to 40 years of follow-up. Eur. J. Cardio-Thorac. Surg. 2006, 30, 910–916. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Zwinderman, A.H.; Bogers, A.J.; Rohmer, J.; Huysmans, H.A. More than thirty-five years of coarctation repair. An unexpected high relapse rate. J. Thorac. Cardiovasc. Surg. 1994, 107, 87–95. [Google Scholar] [CrossRef]
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.; et al. Editor’s Choice—Management of Descending Thoracic Aorta Diseases. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, E.; Loschi, D.; Favia, N.; Santoro, A.; Chiesa, R.; Melissano, G. Spinal Cord Ischemia in Open and Endovascular Aortic Repair. AORTA 2021, 10, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Idrees, J.; Arafat, A.; Svensson, L.G.; Clair, D.; Roselli, E.E. Hybrid repair of aortic aneurysm in patients with previous coarctation. J. Thorac. Cardiovasc. Surg. 2014, 148, 60–64. [Google Scholar] [CrossRef]
- Lehnert, A.; Villemain, O.; Gaudin, R.; Méot, M.; Raisky, O.; Bonnet, D. Risk factors of mortality and recoarctation after coarctation repair in infancy. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 469–475. [Google Scholar] [CrossRef]
- Dodge-Khatami, A.; Backer, C.L.; Mavroudis, C. Risk Factors for Recoarctation and Results of Reoperation: A 40-Year Review. J. Card. Surg. 2010, 15, 369–377. [Google Scholar] [CrossRef]
- Suradi, H.; Hijazi, Z.M. Current management of coarctation of the aorta. Glob. Cardiol. Sci. Pract. 2015, 2015, 44. [Google Scholar] [CrossRef]
- Zhao, Z.; Pan, Z.; Wu, C.; Tian, J.; Qin, J.; Zhang, Y.; Jin, X. Risk factors for recurrence after surgical repair of coarctation of the aorta in children: A single-center experience based on 51 children. Front. Cardiovasc. Med. 2023, 10, 1144755. [Google Scholar] [CrossRef]
- Torok, R.D. Coarctation of the aorta: Management from infancy to adulthood. World J. Cardiol. 2015, 7, 765. [Google Scholar] [CrossRef]
- Russell, G.A.; Berry, P.J.; Watterson, K.; Dhasmana, J.P.; Wisheart, J.D. Patterns of ductal tissue in coarctation of the aorta in the first three months of life. J. Thorac. Cardiovasc. Surg. 1991, 102, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.B.; Lantin-Hermoso, M.R.; Daniels, C.J.; Jaquiss, R.; Landis, B.J.; Marino, B.S.; Rathod, R.H.; Vincent, R.N.; Keller, B.B.; Villafane, J. Isolated Coarctation of the Aorta: Current Concepts and Perspectives. Front. Cardiovasc. Med. 2022, 9, 817866. [Google Scholar] [CrossRef]
- Galzerano, D.; Pergola, V.; Eltayeb, A.; Ludovica, F.; Arbili, L.; Tashkandi, L.; Di Michele, S.; Barchitta, A.; Parato, M.V.; Di Salvo, G. Echocardiography in Simple Congenital Heart Diseases: Guiding Adult Patient Management. J. Cardiovasc. Echogr. 2023, 33, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.G.; Sullivan, I.D.; Wren, C. Treatment of recoarctation: Balloon dilation angioplasty. J. Am. Coll. Cardiol. 1989, 14, 413–419. [Google Scholar] [CrossRef] [PubMed]
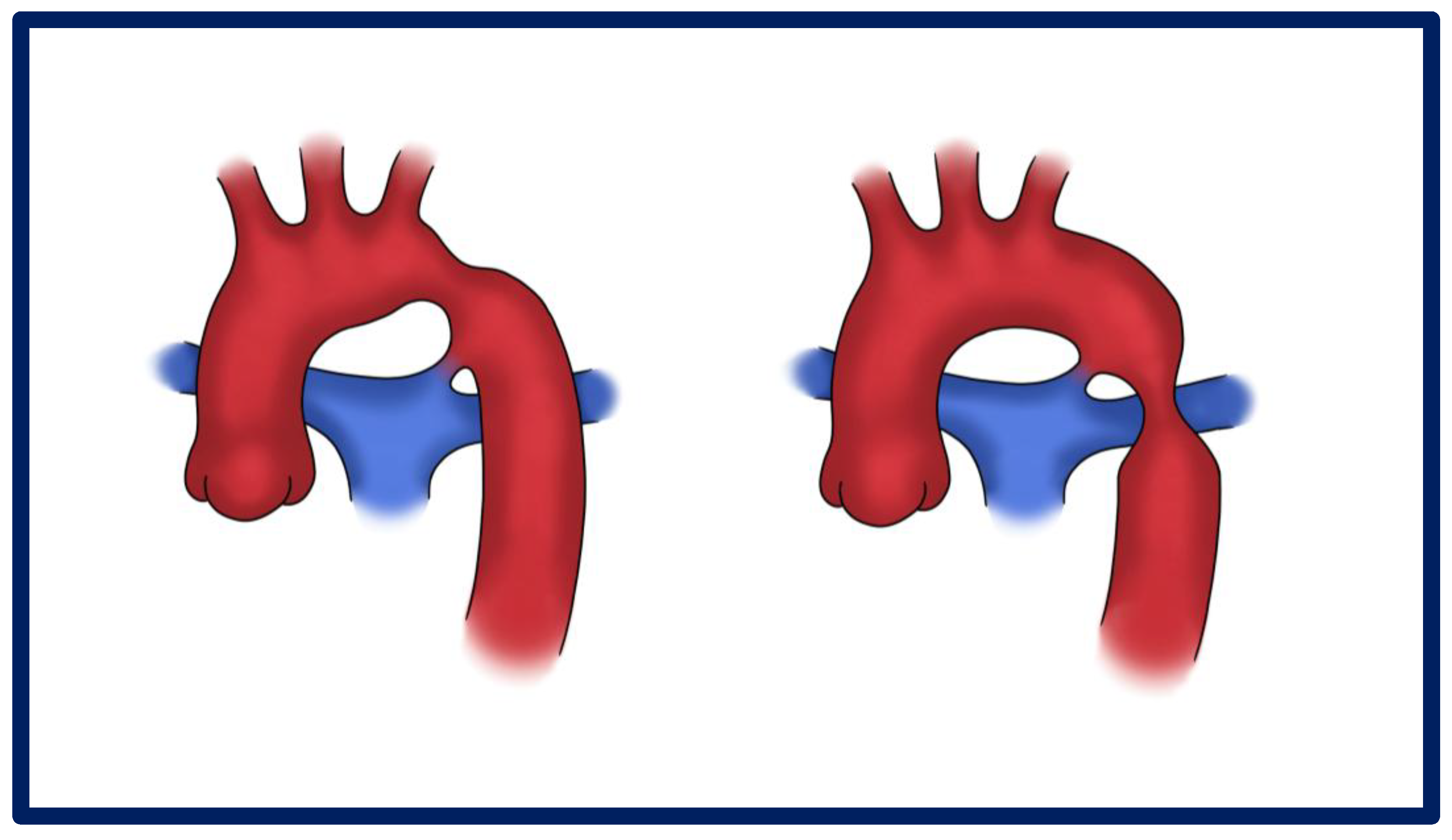

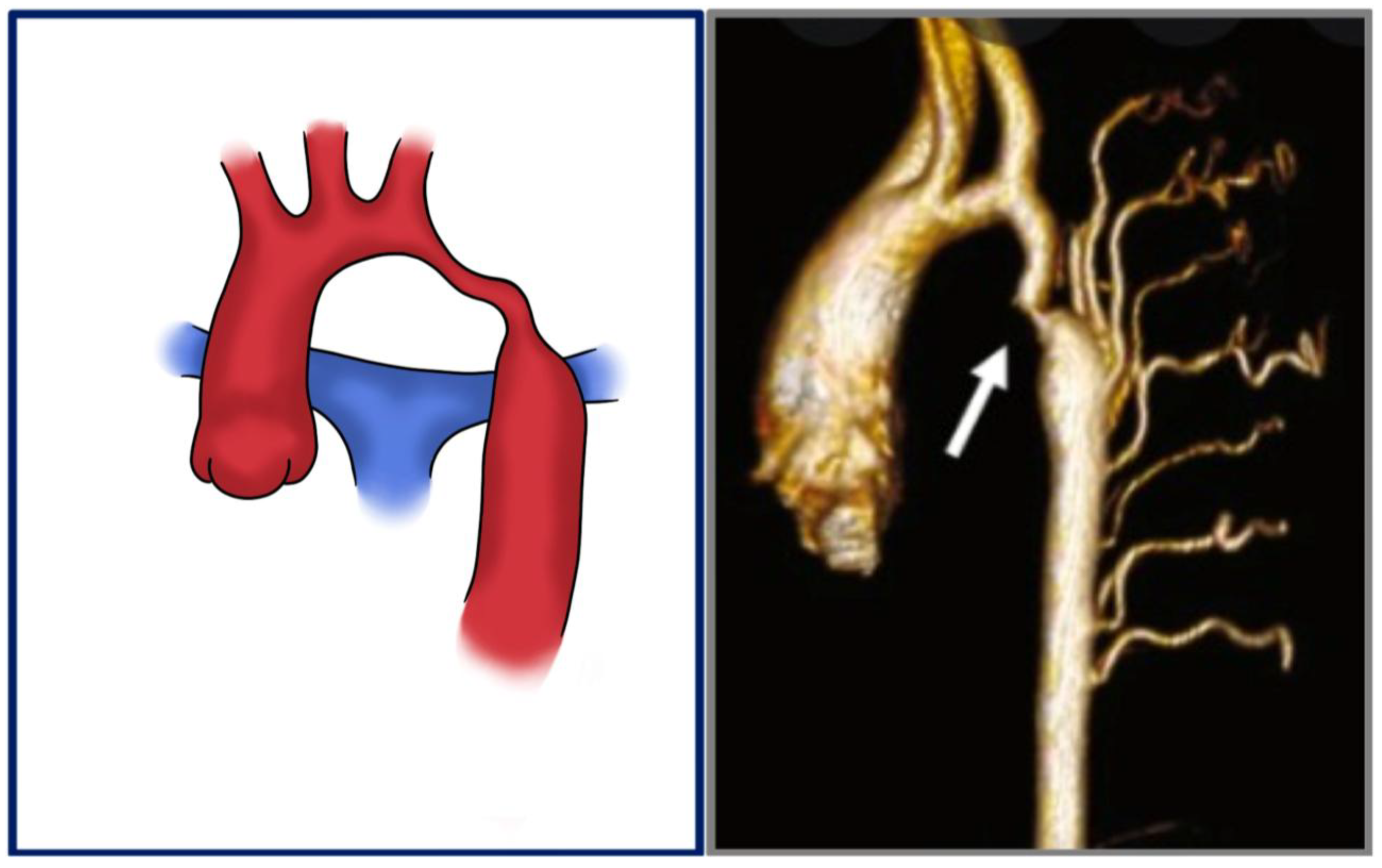
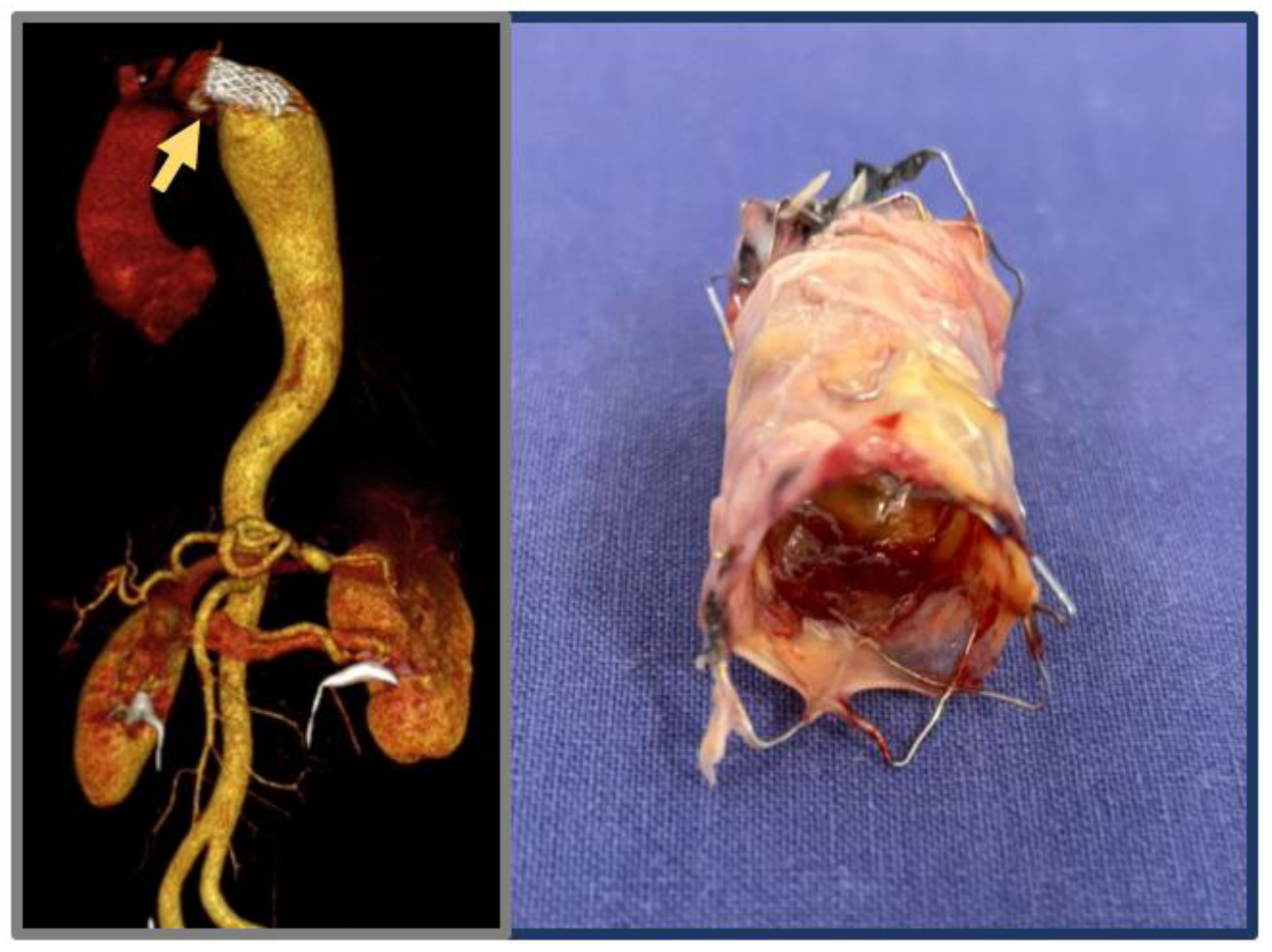
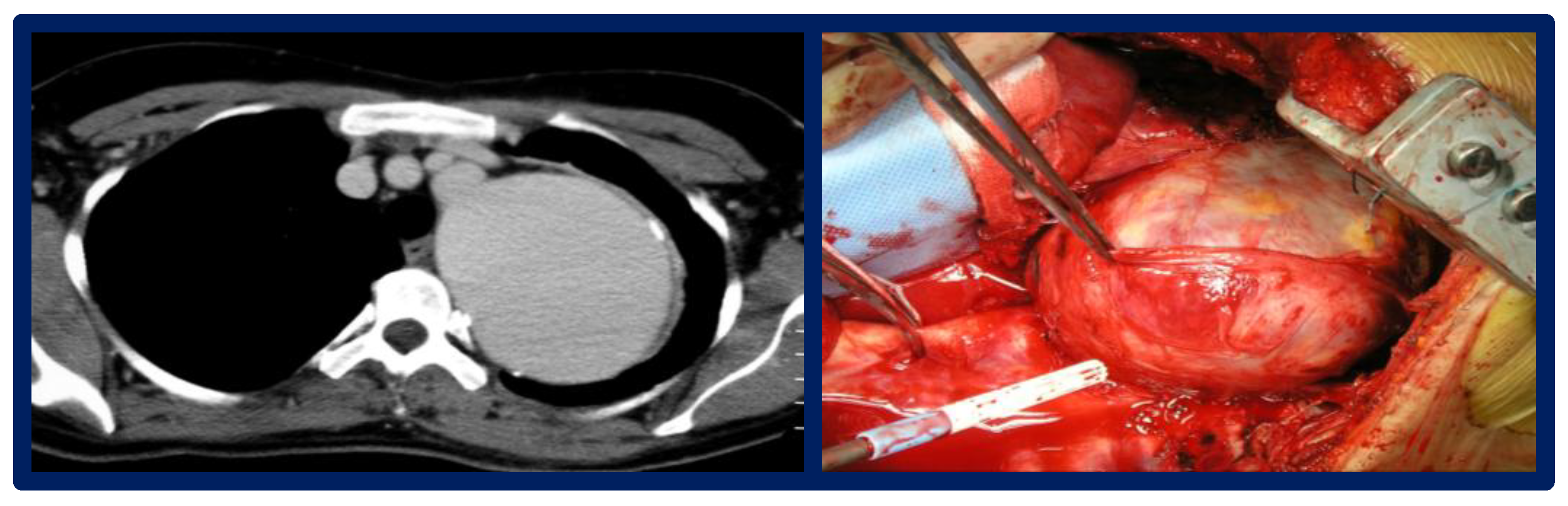
| Technique | PROs, CONs | In-Hospital Mortality | Reintervention |
|---|---|---|---|
| Open Surgical Repair |
| Up to 3.6% | / |
| Endovascular Repair |
| Up to 2.2% | 4–15% |
| Comparative Outcomes |
| Up to 2.7% | / |
| Treatment | Indications/Preferred Patients | Advantages | Limitations/ Complications | Long-Term Considerations |
|---|---|---|---|---|
| Balloon Angioplasty | Infants and small children; discrete narrowing without arch hypoplasia | Minimally invasive, effective for focal stenosis | Restenosis risk up to 30%; aneurysm formation | May require repeat procedures; careful imaging follow-up |
| Stent Placement | Older children (>25–30 kg), adolescents, adults | Durable lumen expansion; low recurrence; covered stents reduce aneurysm risk | Stent fracture, migration, re-narrowing with growth; need for re-dilation during somatic growth | Lifelong imaging to assess stent integrity; possible re-intervention |
| Surgical Re-intervention | Complex arch anatomy, long-segment narrowing, failed percutaneous repair | Definitive anatomical repair; multiple surgical options (extended end-to-end anastomosis, patch, bypass) | Technical difficulties related to re-do surgery | Durable in complex cases; reoperation risk in the future if further degeneration occurs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, A.; De Lisio, F.; Fedorovna Bezborodova, A.; Chiesa, R.; Melissano, G. Late Complications After Aortic Coarctation Repair. J. Cardiovasc. Dev. Dis. 2025, 12, 450. https://doi.org/10.3390/jcdd12110450
Santoro A, De Lisio F, Fedorovna Bezborodova A, Chiesa R, Melissano G. Late Complications After Aortic Coarctation Repair. Journal of Cardiovascular Development and Disease. 2025; 12(11):450. https://doi.org/10.3390/jcdd12110450
Chicago/Turabian StyleSantoro, Annarita, Fiorenza De Lisio, Alexandra Fedorovna Bezborodova, Roberto Chiesa, and Germano Melissano. 2025. "Late Complications After Aortic Coarctation Repair" Journal of Cardiovascular Development and Disease 12, no. 11: 450. https://doi.org/10.3390/jcdd12110450
APA StyleSantoro, A., De Lisio, F., Fedorovna Bezborodova, A., Chiesa, R., & Melissano, G. (2025). Late Complications After Aortic Coarctation Repair. Journal of Cardiovascular Development and Disease, 12(11), 450. https://doi.org/10.3390/jcdd12110450







