Effect of Antihypertensive Losartan on Ca2+ Mobilization in the Aorta of Middle-Aged Spontaneously Hypertensive Female Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Losartan Treatment
2.3. Sample Preparation
2.4. Functional Studies
2.4.1. Concentration–Response Curve
2.4.2. Time–Force Curves
2.4.3. Ca2+ Protocol
2.5. Ca2+ Assay Kit
2.6. Echocardiography
2.7. Atomic Force Microscopy
2.8. Paraffin Embedding
2.9. Collagen Detection
2.10. Elastin
2.11. Data Analysis
3. Results
3.1. Losartan Treatment Improves the Animal Profile in Hypertensive Females
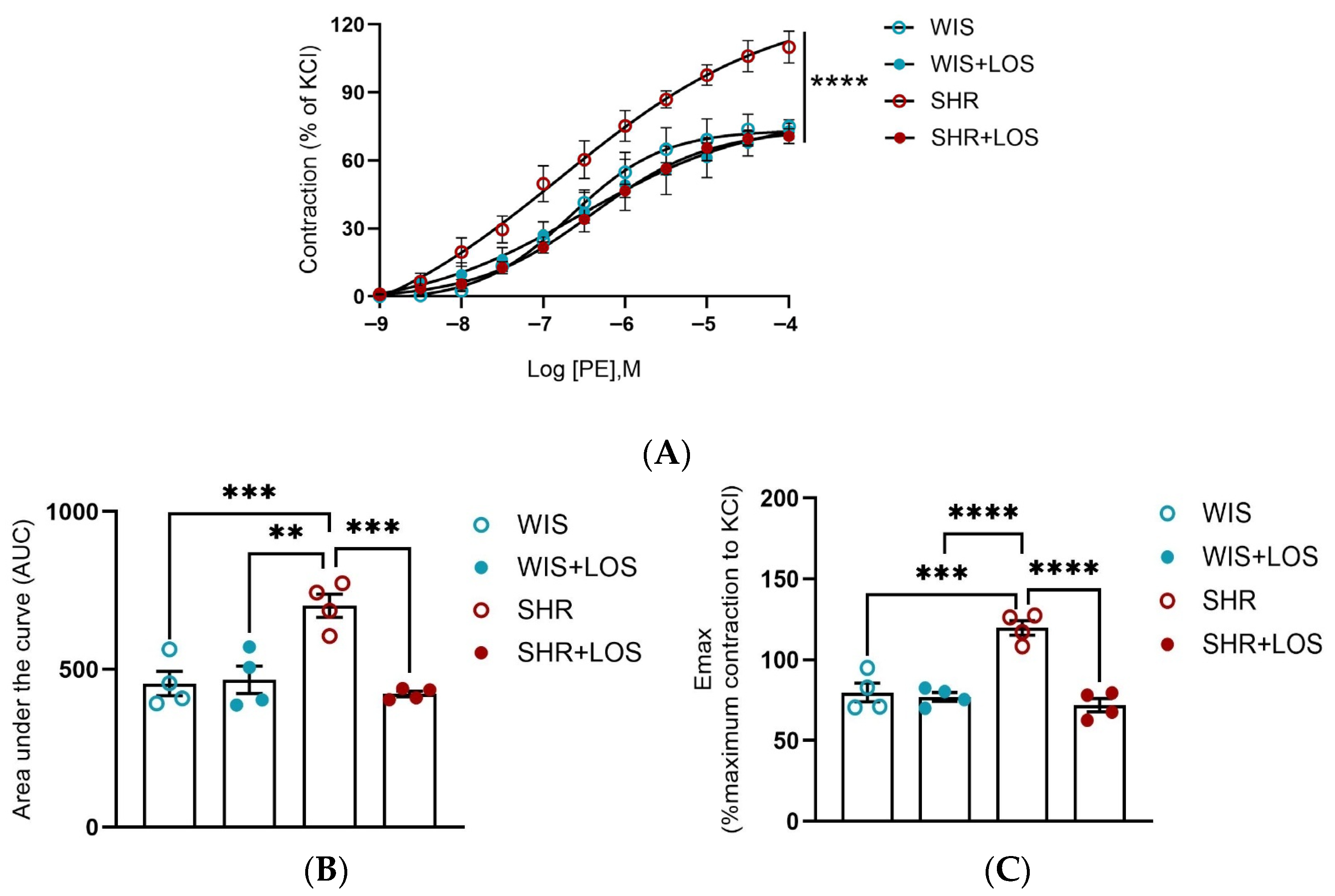
3.2. Hypercontractility to PE in the Aorta of Middle-Aged Hypertensive Female Rats Was Restored by Losartan Treatment
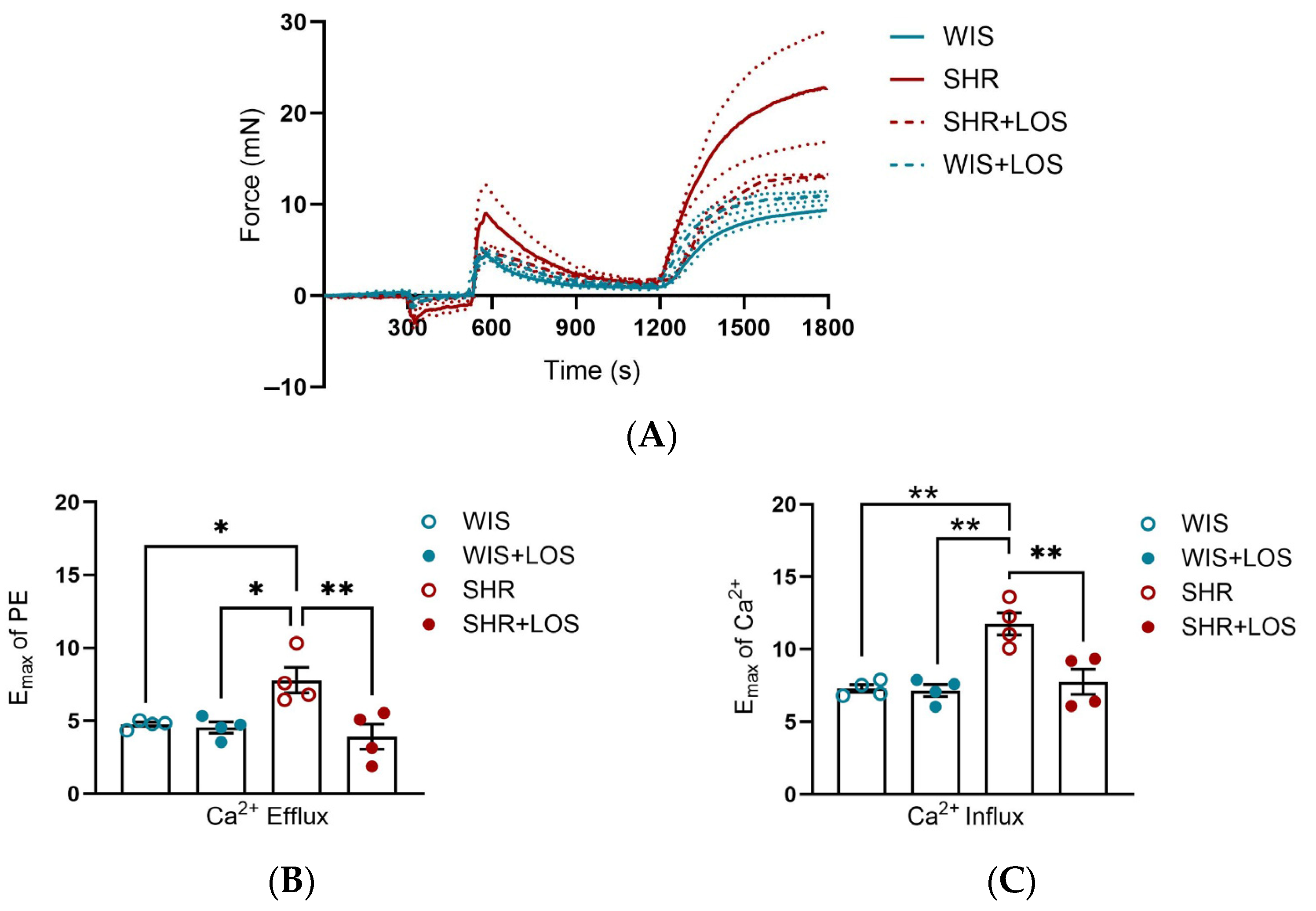
3.3. Losartan Treatment Attenuates Ca2+ Mishandling in the Aorta of Middle-Aged Hypertensive Females
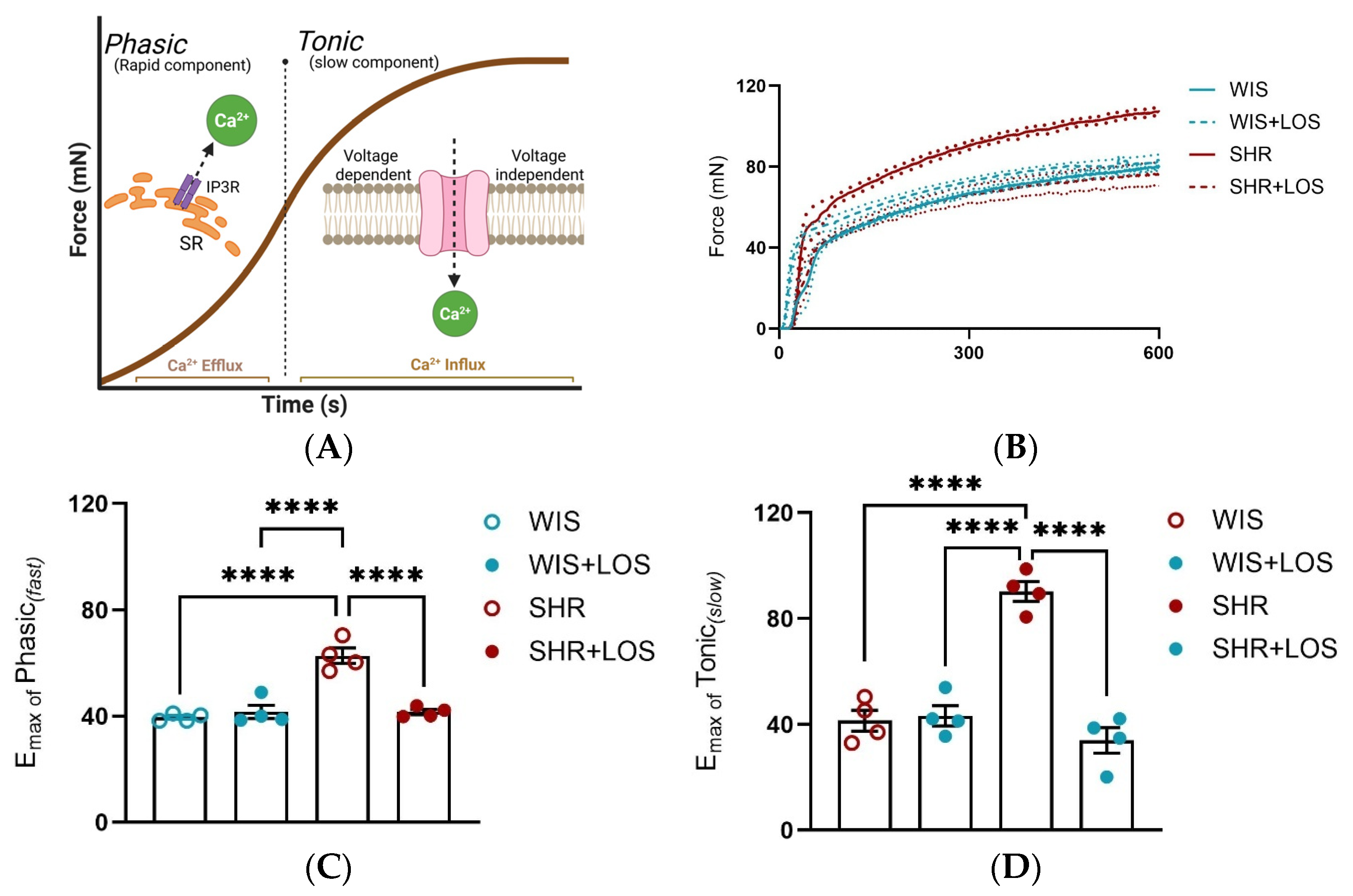
3.4. Losartan Ameliorates the Phasic and Tonic Components of the Contraction Curve in the Hypertensive Aorta
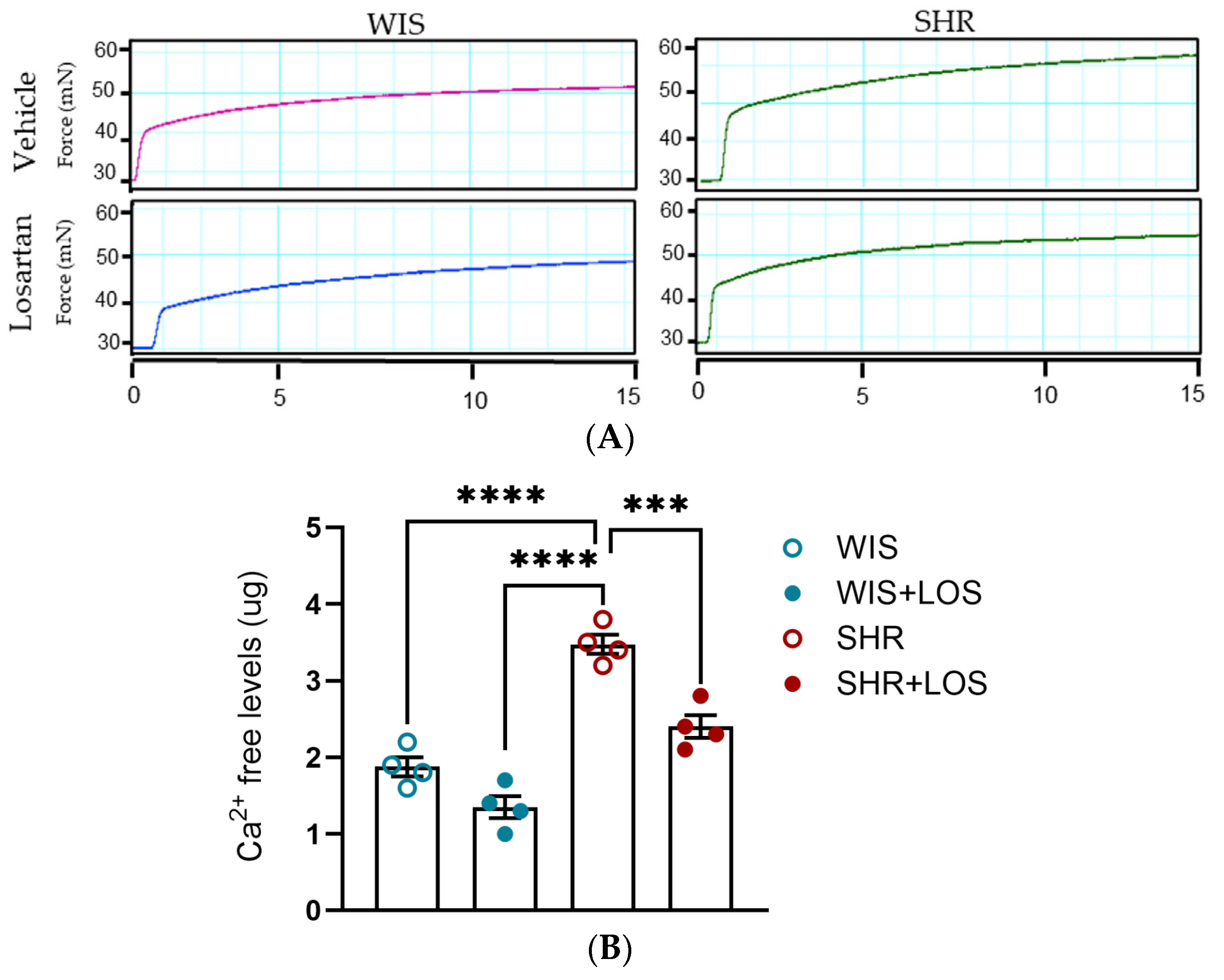
3.5. Losartan Reduced Free Intracellular Ca2+ Levels in the Hypertensive Aorta of Middle-Aged Females
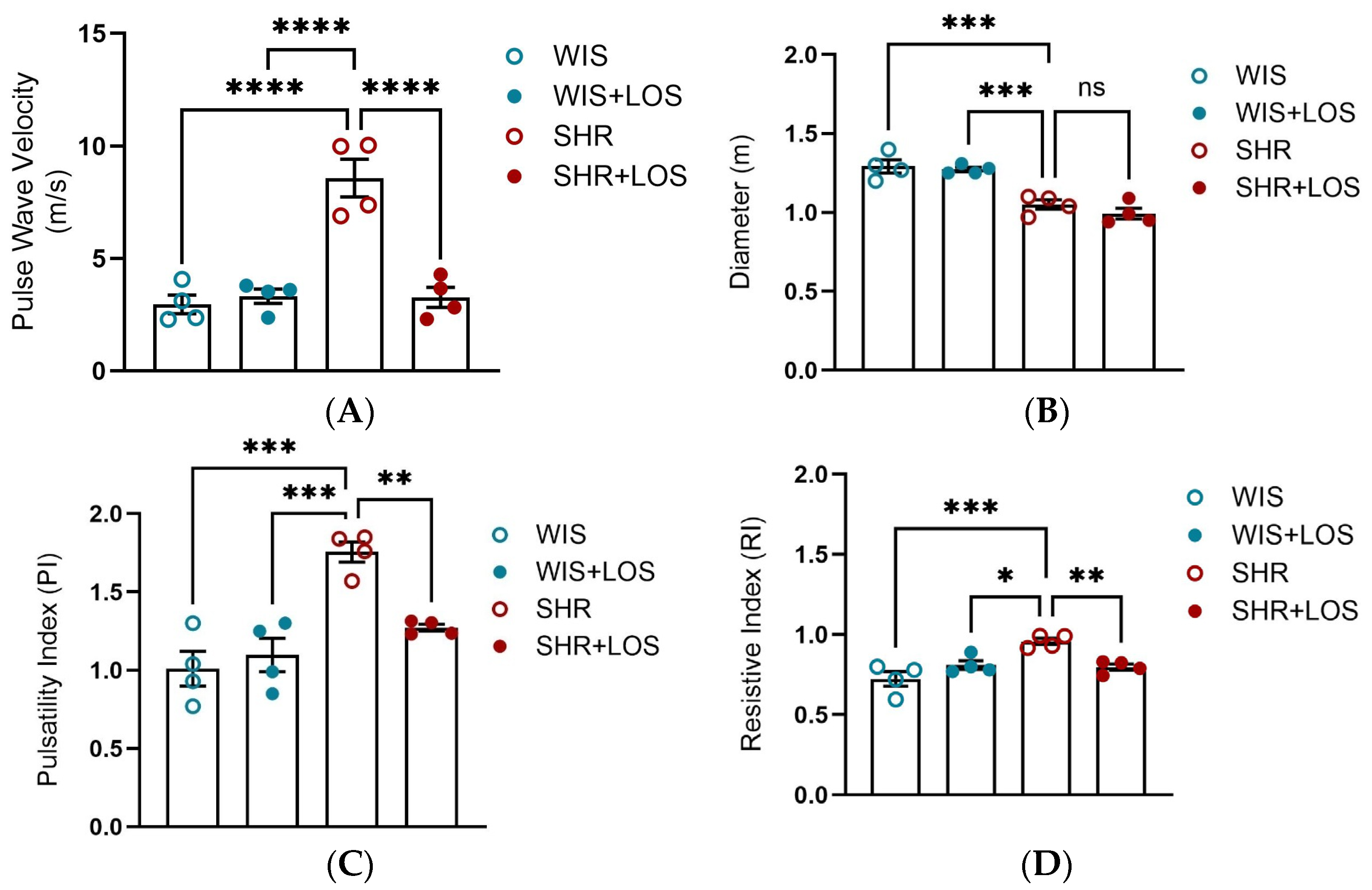
3.6. Losartan Restored Pulse Wave Velocity and Hemodynamic Parameters in the Aorta of Middle-Aged SHR Females
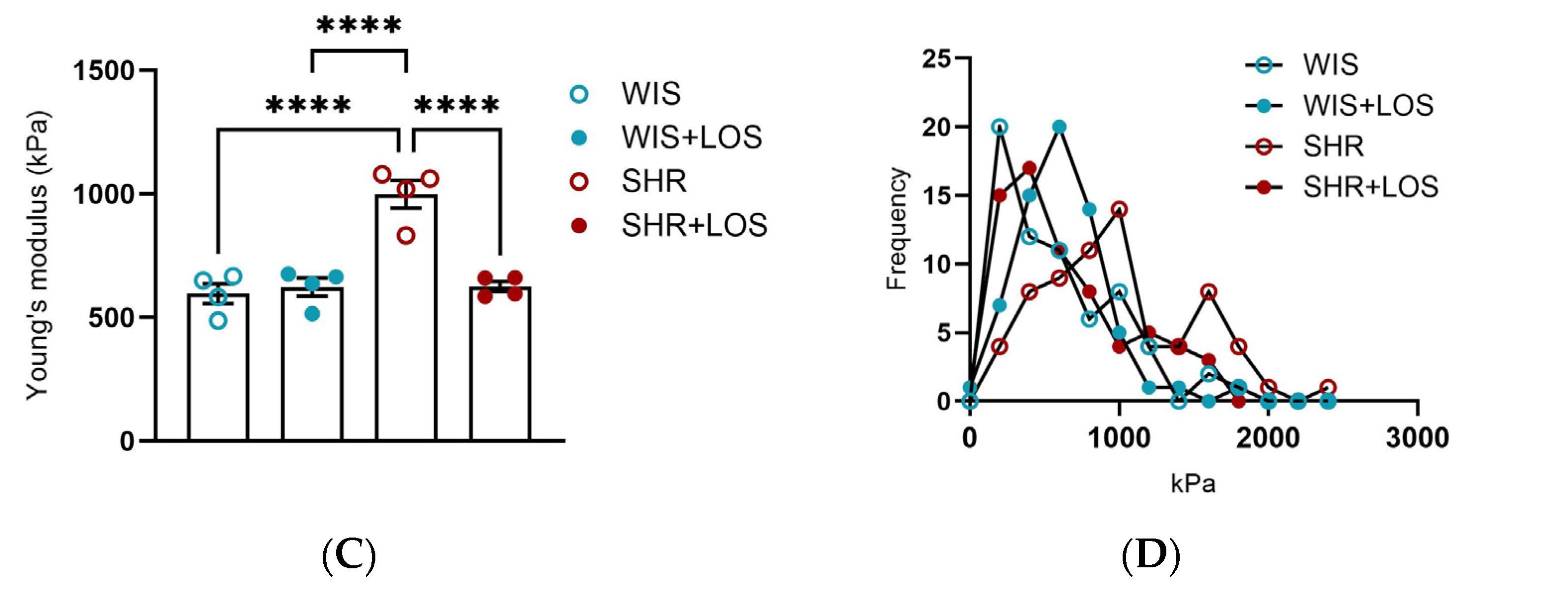
3.7. Losartan Prevented Augmented Stiffness in the Aorta of Middle-Aged SHR Females
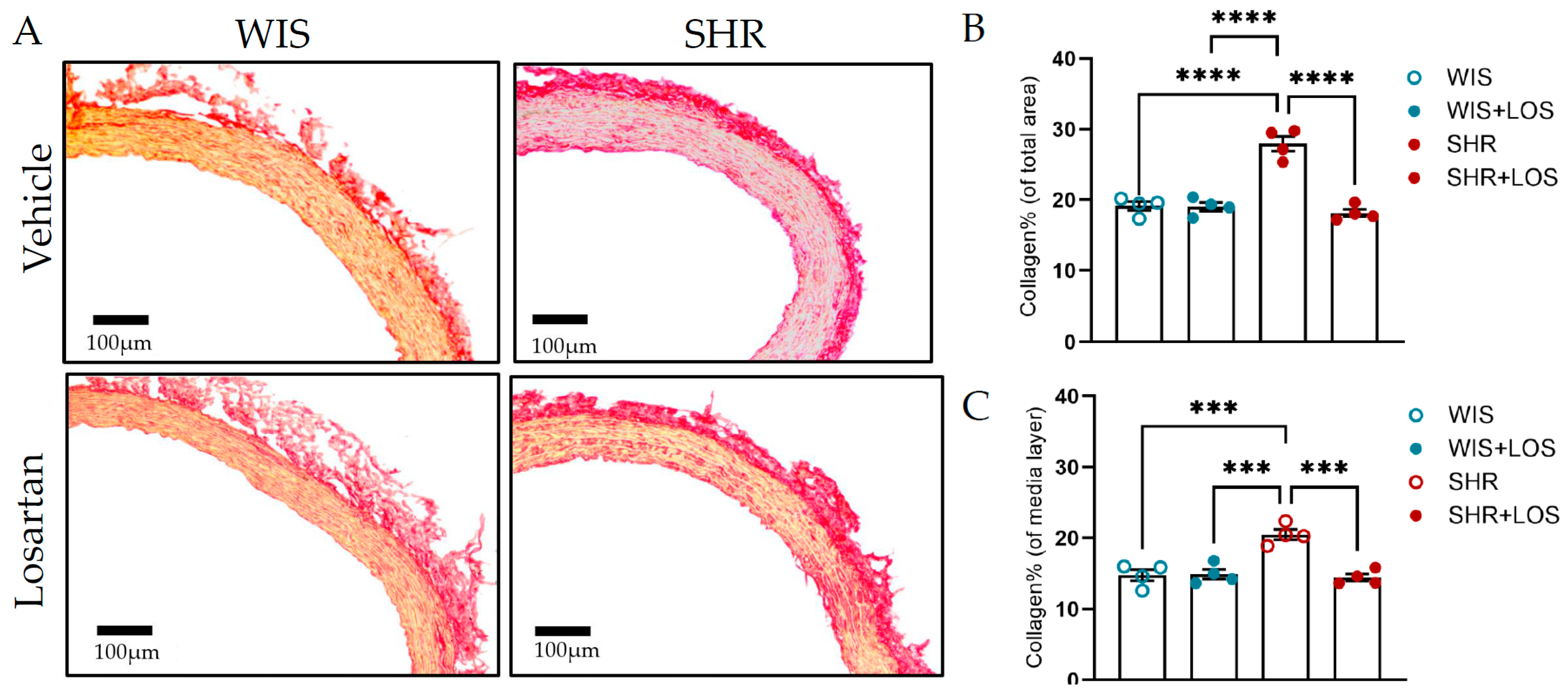
3.8. Losartan Treatment Restores Collagen and Elastin Content in the Aorta of Middle-Aged SHR Females
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VSMCs | Vascular Smooth Muscle Cells |
| CVD | Cardiovascular disease |
| MAP | Mean Arterial Pressure |
| AngII | Angiotensin II |
| ARB | Angiotensin II Receptor Blocker |
| SHR | Spontaneously Hypertensive Rats |
| WIS | Wistar |
| AT1R | Angiotensin I Receptor Blocker |
| PE | Phenylephrine |
| PWV | Pulse Wave Velocity |
| AFM | Atomic Force Microscopy |
| PI | Pulsatility Index |
| RI | Resistive Index |
References
- Mills, K.T.; Bundy, J.D.; Kelly, T.N.; Reed, J.E.; Kearney, P.M.; Reynolds, K.; Chen, J.; He, J. Global Disparities of Hypertension Prevalence and Control: A Systematic Analysis of Population-Based Studies From 90 Countries. Circulation 2016, 134, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.M.; Jamal, S.F. Essential Hypertension. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: http://www.ncbi.nlm.nih.gov/books/NBK539859/ (accessed on 20 October 2025).
- Kario, K.; Okura, A.; Hoshide, S.; Mogi, M. The WHO Global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2024, 47, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Bairey Merz, C.N. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, K.L.; Ozemek, C.; Kohrt, W.M.; Blatchford, P.J.; Moreau, K.L. Vascular dysfunction across the stages of the menopausal transition is associated with menopausal symptoms and quality of life. Menopause 2018, 25, 1011–1019. [Google Scholar] [CrossRef]
- Kwan, C.Y. Dysfunction of calcium handling by smooth muscle in hypertension. Can. J. Physiol. Pharmacol. 1985, 63, 366–374. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Ehrlich, B.E. Signaling in muscle contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023. [Google Scholar] [CrossRef]
- Fransen, P.; Van Hove, C.E.; Leloup, A.J.; Martinet, W.; De Meyer, G.R.; Lemmens, K.; Bult, H.; Schrijvers, D.M. Dissecting out the complex Ca2+-mediated phenylephrine-induced contractions of mouse aortic segments. PLoS ONE 2015, 10, e0121634. [Google Scholar] [CrossRef]
- Dai, C.; Khalil, R.A. Calcium Signaling Dynamics in Vascular Cells and Their Dysregulation in Vascular Disease. Biomolecules 2025, 15, 892. [Google Scholar] [CrossRef]
- Harraz, O.F.; Jensen, L.J. Vascular calcium signalling and ageing. J. Physiol. 2021, 599, 5361–5377. [Google Scholar] [CrossRef]
- Crews, J.K.; Murphy, J.G.; Khalil, R.A. Gender differences in Ca2+ entry mechanisms of vasoconstriction in Wistar-Kyoto and spontaneously hypertensive rats. Hypertension 1999, 34 Pt 2, 931–936. [Google Scholar] [CrossRef]
- Murphy, J.G.; Khalil, R.A. Gender-specific reduction in contractility and [Ca2+] in vascular smooth muscle cells of female rat. Am. J. Physiol. Physiol. 2000, 278, C834–C844. [Google Scholar] [CrossRef] [PubMed]
- Silva-Antonialli, M.; Fortes, Z.; Carvalho, M.; Scivoletto, R.; Nigro, D. Sexual dimorphism in the response of thoracic aorta from SHRs to losartan. Gen. Pharmacol. Vasc. Syst. 2000, 34, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Os, I.; Franco, V.; Kjeldsen, S.E.; Manhem, K.; Devereux, R.B.; Gerdts, E.; Hille, D.A.; Lyle, P.A.; Okin, P.M.; Dahlöf, B.; et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: Results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension 2008, 51, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, S.H.; Wagner, C.; Kurtz, A.; Maeda, N.; Coffman, T.; Arendshorst, W.J. Angiotensin AT1B receptor mediates calcium signaling in vascular smooth muscle cells of AT1A receptor-deficient mice. Hypertension 1998, 31, 1171–1177. [Google Scholar] [CrossRef]
- Samain, E.; Bouillier, H.; Perret, C.; Safar, M.; Dagher, G. ANG II-induced Ca2+ increase in smooth muscle cells from SHR is regulated by actin and microtubule networks. Am. J. Physiol. Circ. Physiol. 1999, 277, H834–H841. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Writing Committee Members; Jones, D.W.; Ferdinand, K.C.; Taler, S.J.; Johnson, H.M.; Shimbo, D.; Abdalla, M.; Altieri, M.M.; Bansal, N.; Bello, N.A.; et al. 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2025, 152, e114–e218. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension: Developed by the task force on the management of elevated blood pressure and hypertension of the European Society of Cardiology (ESC) and endorsed by the European Society of Endocrinology (ESE) and the European Stroke Organisation (ESO). Eur. Heart J. 2024, 45, 3912–4018. Available online: https://academic.oup.com/eurheartj/article/45/38/3912/7741010 (accessed on 24 October 2025).
- Soltis, E.E. Alterations in vascular structure and function after short-term losartan treatment in spontaneously hypertensive rats. J. Pharmacol. Exp. Ther. 1993, 266, 642–646. [Google Scholar] [CrossRef]
- Maeso, R.; Rodrigo, E.; Muñoz-García, R.; Navarro-Cid, J.; Ruilope, L.M.; Cachofeiro, V.; Lahera, V. Chronic treatment with losartan ameliorates vascular dysfunction induced by aging in spontaneously hypertensive rats. J. Hypertens. 1998, 16, 665–672. [Google Scholar] [CrossRef]
- Cerbai, E.; Crucitti, A.; Sartiani, L.; De Paoli, P.; Pino, R.; Rodriguez, M.L.; Gensini, G.; Mugelli, A. Long-term treatment of spontaneously hypertensive rats with losartan and electrophysiological remodeling of cardiac myocytes. Cardiovasc. Res. 2000, 45, 388–396. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Priviero, F.; Tostes, R.C.; Webb, R.C.; Nunes, K.P. Dissecting the interaction between HSP70 and vascular contraction: Role of Ca2+ handling mechanisms. Sci. Rep. 2021, 11, 1420. [Google Scholar] [CrossRef]
- Lee, L.; Cui, J.Z.; Cua, M.; Esfandiarei, M.; Sheng, X.; Chui, W.A.; Xu, M.H.; Sarunic, M.V.; Beg, M.F.; van Breemen, C.; et al. Aortic and Cardiac Structure and Function Using High-Resolution Echocardiography and Optical Coherence Tomography in a Mouse Model of Marfan Syndrome. PLoS ONE 2016, 11, e0164778. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xie, M.; Qiu, H. The Progress of Advanced Ultrasonography in Assessing Aortic Stiffness and the Application Discrepancy between Humans and Rodents. Diagnostics 2021, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Bradley, T.J.; Potts, J.E.; Potts, M.T.; DeSouza, A.M.; Sandor, G.G. Echocardiographic Doppler assessment of the biophysical properties of the aorta in pediatric patients with the Marfan syndrome. Am. J. Cardiol. 2005, 96, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Safar, M.E.; Smulyan, H. Hypertension in women. Am. J. Hypertens. 2004, 17, 82–87. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Hudson, M.; Rahme, E.; Behlouli, H.; Sheppard, R.; Pilote, L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure—A population study. Eur. J. Heart Fail. 2007, 9, 602–609. [Google Scholar] [CrossRef]
- Yamori, Y. Pathogenesis of spontaneous hypertension as a model for essential hypertension. Jpn. Circ. J. 1977, 41, 259–266. [Google Scholar] [CrossRef]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2019, 73, E87–E120. [Google Scholar] [CrossRef]
- Safar, M.; Chamiot-Clerc, P.; Dagher, G.; Renaud, J.F. Pulse pressure, endothelium function, and arterial stiffness in spontaneously hypertensive rats. Hypertension 2001, 38, 1416–1421. [Google Scholar] [CrossRef] [PubMed]
- Nyborg, N.C.; Bevan, J.A. Increased alpha-adrenergic receptor affinity in resistance vessels from hypertensive rats. Hypertension 1988, 11 Pt 2, 635–638. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.A.; Priviero, F.; Webb, R.C.; Nunes, K.P. Increased eHSP70-to-iHSP70 ratio disrupts vascular responses to calcium and activates the TLR4-MD2 complex in type 1 diabetes. Life Sci. 2022, 310, 121079. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.A.; Nunes, K.P. An additional physiological role for HSP70: Assistance of vascular reactivity. Life Sci. 2020, 256, 117986. [Google Scholar] [CrossRef]
- Touyz, R.M.; Schiffrin, E.L. Role of calcium influx and intracellular calcium stores in angiotensin II-mediated calcium hyper-responsiveness in smooth muscle from spontaneously hypertensive rats. J. Hypertens. 1997, 15 Pt 1, 1431–1439. [Google Scholar] [CrossRef]
- Nelson, L.D.; Mashburn, N.A.; Bell, P.D. Altered sodium-calcium exchange in afferent arterioles of the spontaneously hypertensive rat. Kidney Int. 1996, 50, 1889–1896. [Google Scholar] [CrossRef]
- Taniguchi, S.; Furukawa, K.-I.; Sasamura, S.; Ohizumi, Y.; Seya, K.; Motomura, S. Gene expression and functional activity of sodium/calcium exchanger enhanced in vascular smooth muscle cells of spontaneously hypertensive rats. J. Cardiovasc. Pharmacol. 2004, 43, 629–637. [Google Scholar] [CrossRef]
- Liu, D.; Yang, D.; He, H.; Chen, X.; Cao, T.; Feng, X.; Ma, L.; Luo, Z.; Wang, L.; Yan, Z.; et al. Increased transient receptor potential canonical type 3 channels in vasculature from hypertensive rats. Hypertension 2009, 53, 70–76. [Google Scholar] [CrossRef]
- Wu, B.; Su, C.; Wu, J.; He, J.; Pan, J. Effects of losartan on intracellular free calcium concentration of aortic smooth muscle cells in spontaneously hypertensive rat. Guangdong Med. J. 2002, 23, 681. [Google Scholar]
- Ko, Y.; Görg, A.; Appenheimer, M.; Wieczorek, A.J.; Düsing, R.; Vetter, H.; Sachinidis, A. Losartan inhibits the angiotensin II-induced stimulation of the phosphoinositide signalling system in vascular smooth muscle cells. Eur. J. Pharmacol. Mol. Pharmacol. 1992, 227, 215–219. [Google Scholar] [CrossRef]
- d’Uscio, L.V.; Shaw, S.; Barton, M.; Lüscher, T.F. Losartan but not verapamil inhibits angiotensin II-induced tissue endothelin-1 increase: Role of blood pressure and endothelial function. Hypertension 1998, 31, 1305–1310. [Google Scholar] [CrossRef]
- de Oliveira, A.A.; Mendoza, V.O.; Priviero, F.; Webb, R.C.; Nunes, K.P. Age-Related Decline in Vascular Responses to Phenylephrine Is Associated with Reduced Levels of HSP70. Biomolecules 2022, 12, 1125. [Google Scholar] [CrossRef]
- Maeso, R.; Navarro-Cid, J.; Muñoz-García, R.; Rodrigo, E.; Ruilope, L.M.; Lahera, V.; Cachofeiro, V. Losartan reduces phenylephrine constrictor response in aortic rings from spontaneously hypertensive rats. Role of nitric oxide and angiotensin II type 2 receptors. Hypertension 1996, 28, 967–972. [Google Scholar] [CrossRef] [PubMed]
- de PRodrigues, S.F.; dos Santos, R.A.; Silva-Antonialli, M.M.; Scavone, C.; Nigro, D.; Carvalho, M.H.; de Cássia Tostes, R.; Fortes, Z.B. Differential effect of losartan in female and male spontaneously hypertensive rats. Life Sci. 2006, 78, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, R.; Webb, R.; Singh, D.; Ashley, T.; Brock, T. Calcium fluxes, calcium binding, and adenosine cyclic 3′,5′-monophosphate-dependent protein kinase activity in the aorta of spontaneously hypertensive and Kyoto Wistar normotensive rats. Mol. Pharmacol. 1978, 14, 468–477. [Google Scholar] [CrossRef] [PubMed]
- Nabika, T.; Velletri, P.; Beaven, M.; Endo, J.; Lovenberg, W. Vasopressin-induced calcium increases in smooth muscle cells from spontaneously hypertensive rats. Life Sci. 1985, 37, 579–584. [Google Scholar] [CrossRef]
- Et-Taouil, K.; Safar, M.; Plante, G.E. Mechanisms and consequences of large artery rigidity. Can. J. Physiol. Pharmacol. 2003, 81, 205–211. [Google Scholar] [CrossRef]
- Cai, Z.; Gong, Z.; Li, Z.; Li, L.; Kong, W. Vascular Extracellular Matrix Remodeling and Hypertension. Antioxid. Redox Signal. 2021, 34, 765–783. [Google Scholar] [CrossRef]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial Stiffness and Cardiovascular Risk in Hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef]
- Brown, I.A.M.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension. Arter. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef]
- Safar, M.E.; Levy, B.I.; Struijker-Boudier, H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation 2003, 107, 2864–2869. [Google Scholar] [CrossRef]
- Morgan, E.E.; Casabianca, A.B.; Khouri, S.J.; Kalinoski, A.L.N. In vivo assessment of arterial stiffness in the isoflurane anesthetized spontaneously hypertensive rat. Cardiovasc. Ultrasound 2014, 12, 37. [Google Scholar] [CrossRef]
- Lindesay, G.; Ragonnet, C.; Chimenti, S.; Villeneuve, N.; Vayssettes-Courchay, C. Age and hypertension strongly induce aortic stiffening in rats at basal and matched blood pressure levels. Physiol. Rep. 2016, 4, e12805. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, S.; Zhou, X.; Zhao, Y.; Dong, B.; Shen, J.; Yang, K.; Li, L.; Zhu, D. Effects of Long-Term Intervention with Losartan, Aspirin and Atorvastatin on Vascular Remodeling in Juvenile Spontaneously Hypertensive Rats. Molecules 2023, 28, 1844. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-S.; Sharifi, A.M.; Schiffrin, E.L. Effect of AT1 angiotensin-receptor blockade on structure and function of small arteries in SHR. J. Cardiovasc. Pharmacol. 1997, 30, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Majalan, N.; Nafisi, R.; Moghadasi-Mousavi, S. Effect of losartan on Doppler sonography indices in kidney transplant patients: A randomized clinical trial. Vasc. Health Risk Manag. 2009, 5, 97–100. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.A.; Priviero, F.; Delgado, A.; Dong, P.; Mendoza, V.O.; Gu, L.; Webb, R.C.; Nunes, K.P. Connecting Aortic Stiffness to Vascular Contraction: Does Sex Matter? Int. J. Mol. Sci. 2022, 23, 11314. [Google Scholar] [CrossRef]
- Deyl, Z.; Jelínek, J.; Macek, K.; Chaldakov, G.; Vankov, V. Collagen and elastin synthesis in the aorta of spontaneously hypertensive rats. J. Vasc. Res. 1987, 24, 313–320. [Google Scholar] [CrossRef]
- Keeley, F.W.; Alatawi, A. Response of aortic elastin synthesis and accumulation to developing hypertension and the inhibitory effect of colchicine on this response. Lab. Investig. J. Tech. Methods Pathol. 1991, 64, 499–507. [Google Scholar]
- Keeley, F.W.; Johnson, D.J. The effect of developing hypertension on the synthesis and accumulation of elastin in the aorta of the rat. Biochem. Cell Biol. 1986, 64, 38–43. [Google Scholar] [CrossRef]
- Ito, H.; Kwan, C.; Daniel, E. Elastin and elastase-like enzyme change in aorta of rat with malignant hypertension. Exp. Mol. Pathol. 1987, 47, 26–36. [Google Scholar] [CrossRef]
- Arribas, S.M.; Hinek, A.; González, M.C. Elastic fibres and vascular structure in hypertension. Pharmacol. Ther. 2006, 111, 771–791. [Google Scholar] [CrossRef]
- Wang, K.; Meng, X.; Guo, Z. Elastin Structure, Synthesis, Regulatory Mechanism and Relationship With Cardiovascular Diseases. Front. Cell Dev. Biol. 2021, 9, 596702. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastogi, S.; Liaw, J.; Zhai, Y.; Karpova, T.; Gu, L.; Nunes, K. Effect of Antihypertensive Losartan on Ca2+ Mobilization in the Aorta of Middle-Aged Spontaneously Hypertensive Female Rats. J. Cardiovasc. Dev. Dis. 2025, 12, 441. https://doi.org/10.3390/jcdd12110441
Rastogi S, Liaw J, Zhai Y, Karpova T, Gu L, Nunes K. Effect of Antihypertensive Losartan on Ca2+ Mobilization in the Aorta of Middle-Aged Spontaneously Hypertensive Female Rats. Journal of Cardiovascular Development and Disease. 2025; 12(11):441. https://doi.org/10.3390/jcdd12110441
Chicago/Turabian StyleRastogi, Swasti, Jessica Liaw, Yingnan Zhai, Tatiana Karpova, Linxia Gu, and Kenia Nunes. 2025. "Effect of Antihypertensive Losartan on Ca2+ Mobilization in the Aorta of Middle-Aged Spontaneously Hypertensive Female Rats" Journal of Cardiovascular Development and Disease 12, no. 11: 441. https://doi.org/10.3390/jcdd12110441
APA StyleRastogi, S., Liaw, J., Zhai, Y., Karpova, T., Gu, L., & Nunes, K. (2025). Effect of Antihypertensive Losartan on Ca2+ Mobilization in the Aorta of Middle-Aged Spontaneously Hypertensive Female Rats. Journal of Cardiovascular Development and Disease, 12(11), 441. https://doi.org/10.3390/jcdd12110441







