Role of P2X7 Receptor on Hypoxia-Induced Vascular Endothelial Growth Factor Gene Expression in H9c2 Rat Cardiomyocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Kits
2.2. Cell Culture
2.3. Induction of Hypoxia
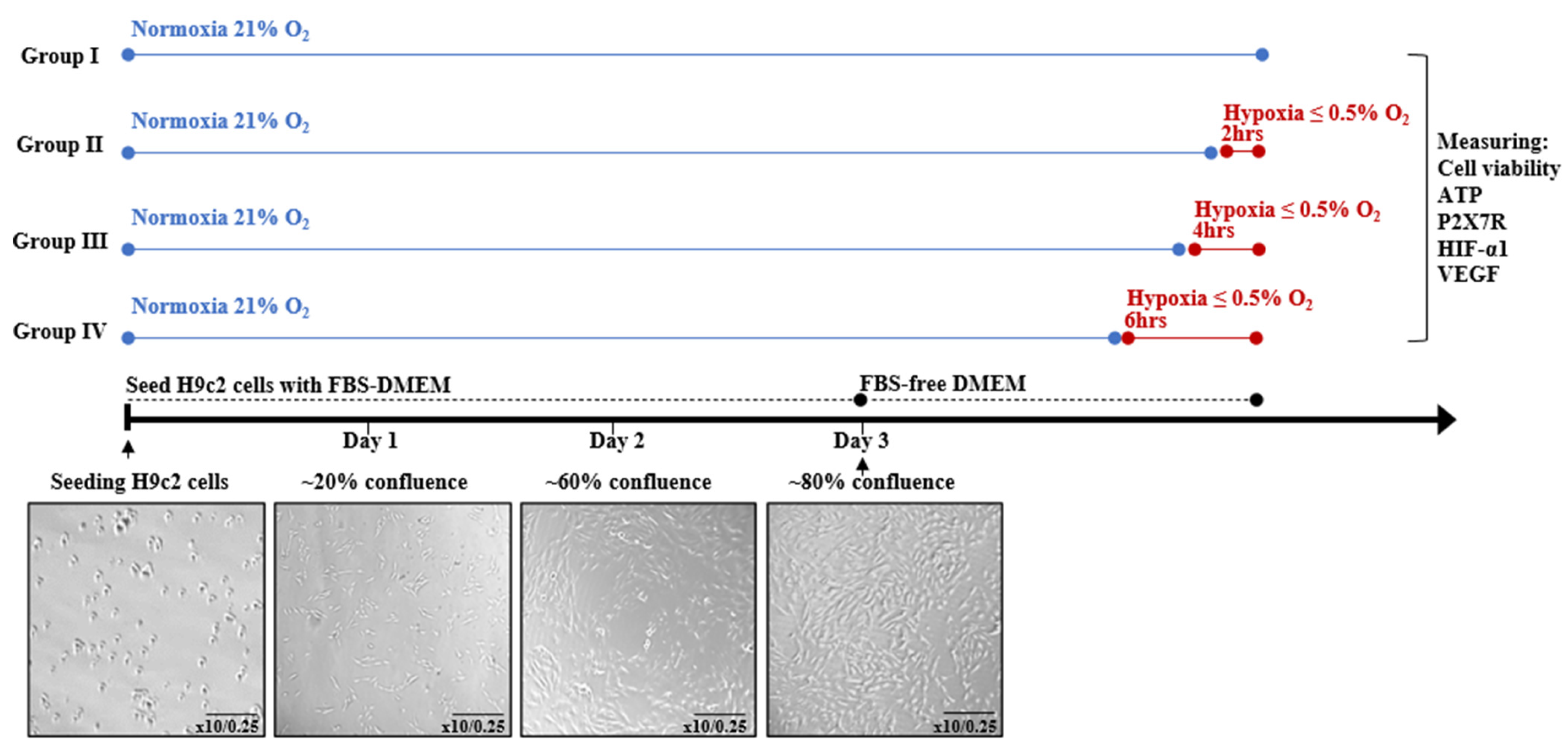
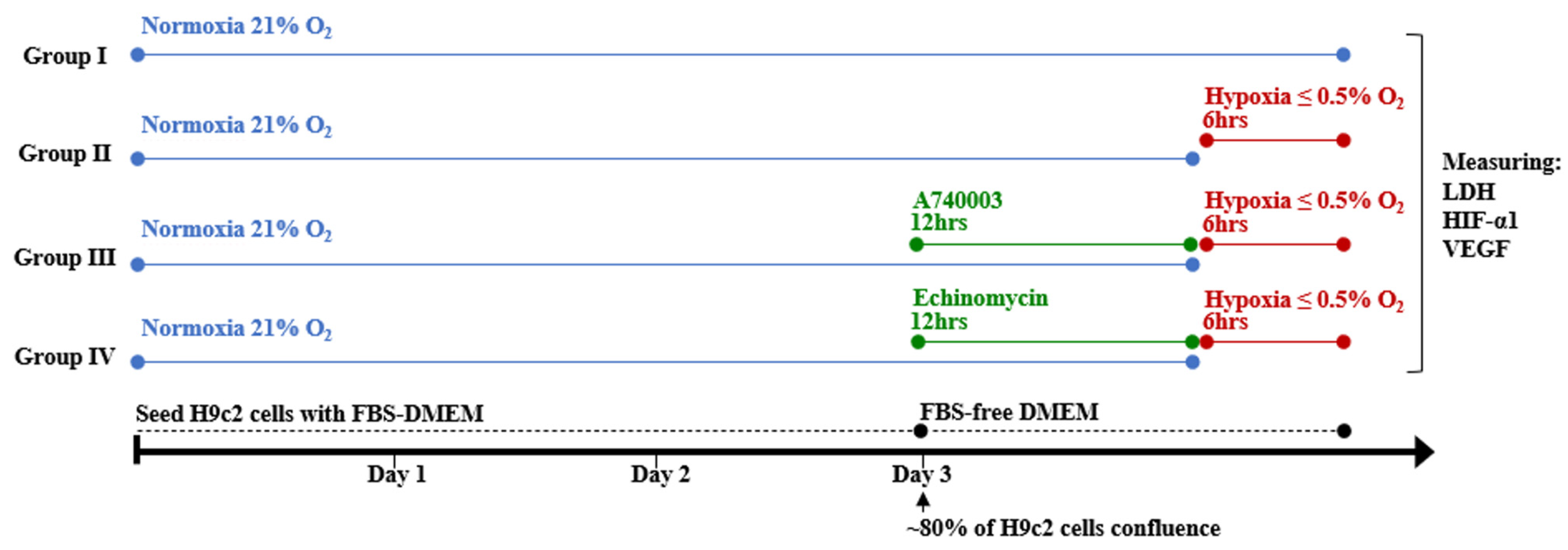
2.4. Experimental Design
2.5. Determination of Cell Viability
2.6. Determination of Cellular ATP
2.7. Determination of Lactate Dehydrogenase Cytotoxicity
2.8. Determination of Pro-Angiogenic Factor VEGF Concentration
2.9. Real-Time Polymerase Chain Reaction (RT-PCR) Analysis
2.10. Data Analysis
3. Results
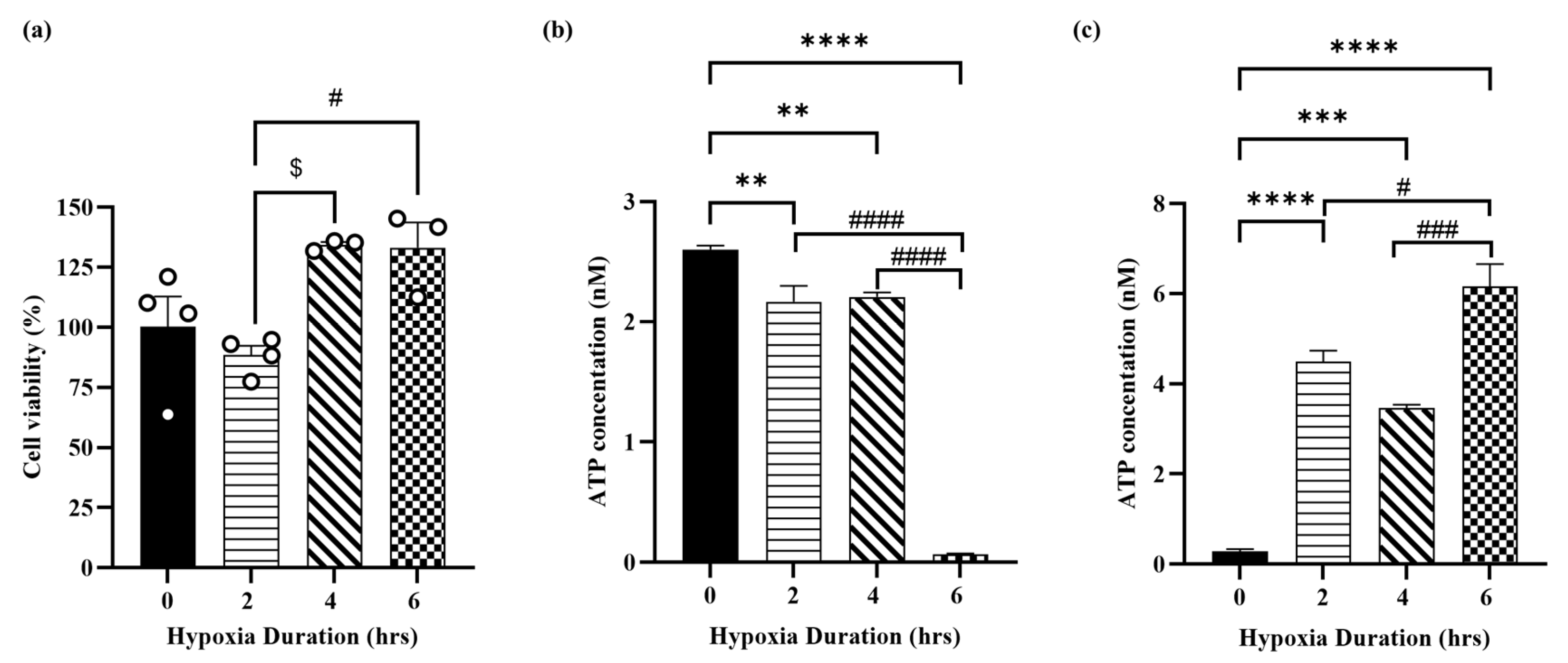
3.1. Hypoxia Induces Cellular Injury in H9c2 Cardiomyocytes
3.2. Hypoxia Induces Molecular Injury in H9c2 Cardiomyocytes
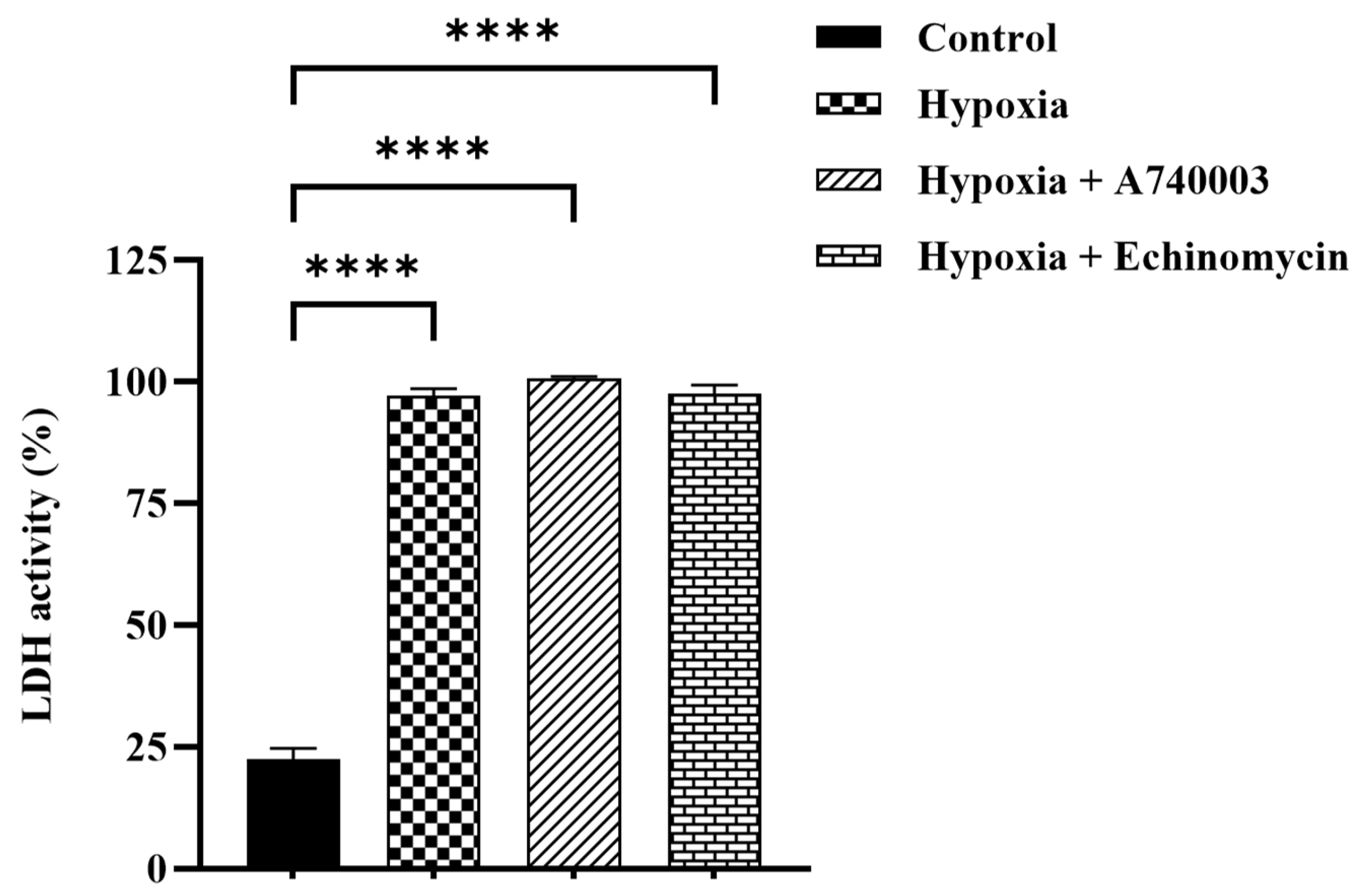
3.3. Effect of P2X7R Antagonist on Hypoxia-Induced Cytotoxicity in H9c2 Cells
3.4. P2X7R Antagonist Inhibits Hypoxia-Induced Pro-Angiogenic Factor Signaling in H9c2 Cells
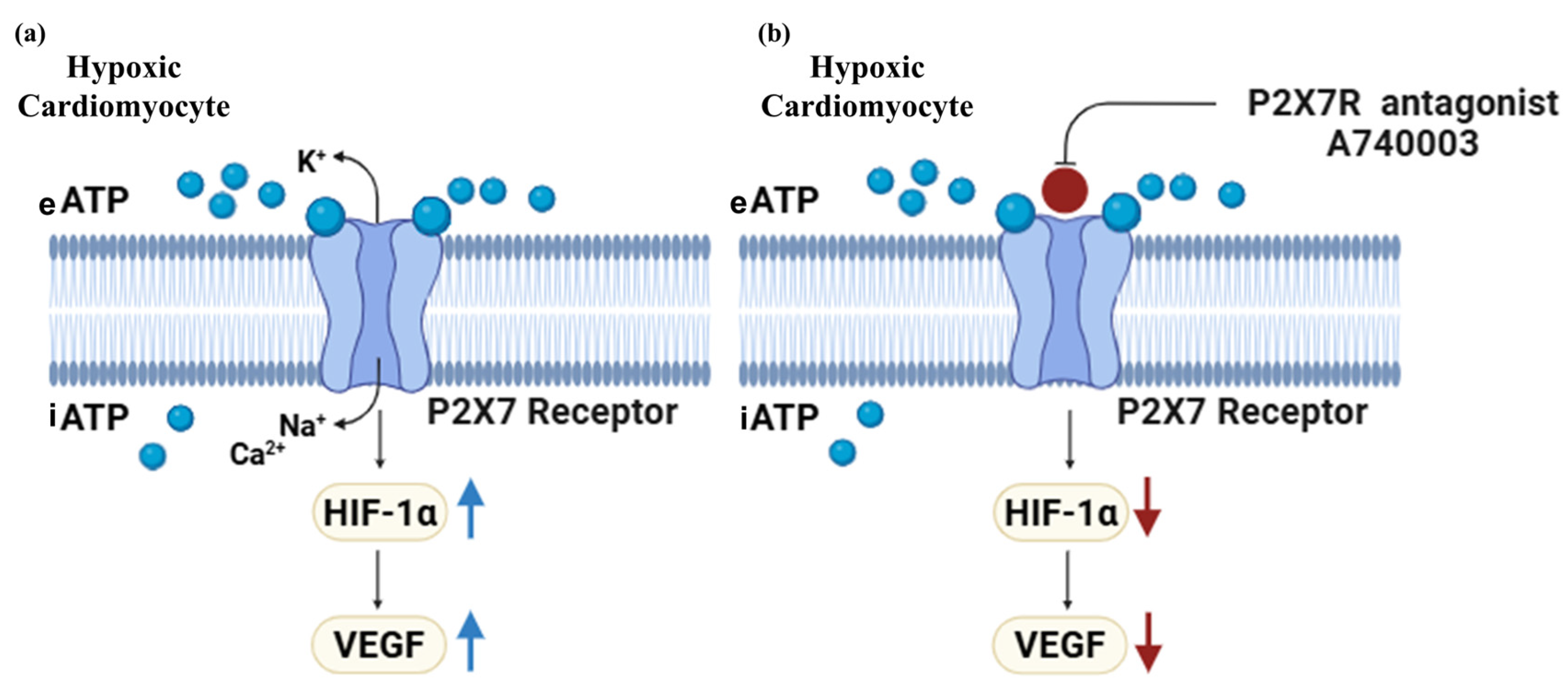
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CCK-8 | Cell Counting Kit-8 |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| HIF-1α | Hypoxia-inducible factor-1α |
| LAD | left anterior descending |
| LDH | Lactate Dehydrogenase |
| P2X7Rs | Purinergic P2X7 receptors |
| RT-PCR | Real-Time Polymerase Chain Reaction |
| SEM | Standard error of the mean |
| VEGF | Vascular endothelial growth factor |
References
- Jensen, R.V.; Hjortbak, M.V.; Bøtker, H.E. Ischemic heart disease: An update. Semin. Nucl. Med. 2020, 50, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Adachi, H.; Murata, M.; Tomono, J.; Oshima, S.; Kurabayashi, M. Importance of compensatory heart rate increase during myocardial ischemia to preserve appropriate oxygen kinetics. J. Cardiol. 2017, 70, 250–254. [Google Scholar] [CrossRef]
- Lekven, J.; Mjøs, O.D.; Kjekshus, J.K. Compensatory mechanisms during graded myocardial ischemia. Am. J. Cardiol. 1973, 31, 467–473. [Google Scholar] [CrossRef]
- Zimna, A.; Kurpisz, M. Hypoxia-inducible factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies. Biomed. Res. Int. 2015, 2015, 549412. [Google Scholar] [CrossRef]
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after acute myocardial infarction. Cardiovasc. Res. 2021, 117, 1257–1273. [Google Scholar] [CrossRef]
- Katagiri, M.; Yamada, S.; Katoh, M.; Ko, T.; Ito, M.; Komuro, I. Heart failure pathogenesis elucidation and new treatment method development. JMA J. 2022, 5, 399–406. [Google Scholar] [CrossRef]
- Cochain, C.; Channon, K.M.; Silvestre, J.-S. Angiogenesis in the infarcted myocardium. Antioxid. Redox Signal. 2013, 18, 1100–1113. [Google Scholar] [CrossRef]
- Bodin, P.; Burnstock, G. Purinergic signalling: ATP release. Neurochem. Res. 2001, 26, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.-H.; Roger, S.; Baldwin, S. Insights into the molecular mechanisms underlying mammalian P2X7 receptor functions and contributions in diseases, revealed by structural modeling and single nucleotide polymorphisms. Front. Pharmacol. 2013, 4, 55. [Google Scholar] [CrossRef]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP release by inflammatory cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef]
- Vessey, D.A.; Li, L.; Kelley, M. Pannexin-I/P2X 7 purinergic receptor channels mediate the release of cardioprotectants induced by ischemic pre-and postconditioning. J. Cardiovasc. Pharmacol. Ther. 2010, 15, 190–195. [Google Scholar] [CrossRef]
- Vessey, D.A.; Li, L.; Kelley, M. Ischemic preconditioning requires opening of pannexin-1/P2X7 channels not only during preconditioning but again after index ischemia at full reperfusion. Mol. Cell. Biochem. 2011, 351, 77–84. [Google Scholar] [CrossRef]
- Tu, G.; Zou, L.; Liu, S.; Wu, B.; Lv, Q.; Wang, S.; Xue, Y.; Zhang, C.; Yi, Z.; Zhang, X. Long noncoding NONRATT021972 siRNA normalized abnormal sympathetic activity mediated by the upregulation of P2X7 receptor in superior cervical ganglia after myocardial ischemia. Purinergic Signal. 2016, 12, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Zheng, A.-b.; Jin, J.; Cui, Y.; Zhang, N.; Che, Z.-p.; Wang, Y.; Zhan, J.; Tu, W.-j. Cardioprotective effects of genistin in rat myocardial ischemia-reperfusion injury studies by regulation of P2X7/NF-κB pathway. Evid.-Based Complement. Altern. Med. 2016, 2016, 5381290. [Google Scholar] [CrossRef]
- Wang, Y. Mitogen-activated protein kinases in heart development and diseases. Circulation 2007, 116, 1413–1423. [Google Scholar] [CrossRef]
- Matthiesen, S.; Jahnke, R.; Knittler, M.R. A straightforward hypoxic cell culture method suitable for standard incubators. Methods Protoc. 2021, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, H.; Li, W.; Wu, H.; Yang, Y. Highly-expressed P2X7 receptor promotes growth and metastasis of human HOS/MNNG osteosarcoma cells via PI3K/Akt/GSK3β/β-catenin and mTOR/HIF1α/VEGF signaling. Int. J. Cancer 2019, 145, 1068–1082. [Google Scholar] [CrossRef]
- Osuru, H.P.; Lavallee, M.; Thiele, R.H. Molecular and Cellular Response of the myocardium (H9C2 cells) towards hypoxia and HIF-1α inhibition. Front. Cardiovasc. Med. 2022, 9, 711421. [Google Scholar] [CrossRef] [PubMed]
- Gerasimovskaya, E.V.; Ahmad, S.; White, C.W.; Jones, P.L.; Carpenter, T.C.; Stenmark, K.R. Extracellular ATP is an autocrine/paracrine regulator of hypoxia-induced adventitial fibroblast growth: Signaling through extracellular signal-regulated kinase-1/2 and the Egr-1 transcription factor. J. Biol. Chem. 2002, 277, 44638–44650. [Google Scholar] [CrossRef]
- Ding, L.; Gong, C.; Zhao, J.; Liu, X.; Li, T.; Rao, S.; Wang, S.; Liu, Y.; Peng, S.; Xiao, W. Noncoding transcribed ultraconserved region (T-UCR) UC.48+ is a novel regulator of high-fat diet induced myocardial ischemia/reperfusion injury. J. Cell. Physiol. 2019, 234, 9849–9861. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-y.; Zou, C.; Liu, X.-f.; Yan, Y.-j.; Gu, S.-z.; Li, X. Vascular endothelial growth factor ameliorated palmitate-induced cardiomyocyte injury via JNK pathway. Vitr. Cell Dev. Biol. Anim. 2021, 57, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, H.; Liu, T.; Yang, Z.; Zhang, R.; Han, H. Co-cultured the MSCs and cardiomyocytes can promote the growth of cardiomyocytes. Cytotechnology 2018, 70, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Bonavita, F.; Stefanelli, C.; Giordano, E.; Columbaro, M.; Facchini, A.; Bonafè, F.; Caldarera, C.M.; Guarnieri, C. H9c2 cardiac myoblasts undergo apoptosis in a model of ischemia consisting of serum deprivation and hypoxia: Inhibition by PMA. FEBS Lett. 2003, 536, 85–91. [Google Scholar] [CrossRef]
- Vial, C.; Owen, P.; Opie, L.; Posel, D. Significance of release of adenosine triphosphate and adenosine induced by hypoxia or adrenaline in perfused rat heart. J. Mol. Cell. Cardiol. 1987, 19, 187–197. [Google Scholar] [CrossRef]
- Zieseniss, A.; Hesse, A.R.; Jatho, A.; Krull, S.; Hölscher, M.; Vogel, S.; Katschinski, D.M. Cardiomyocyte-specific transgenic expression of prolyl-4-hydroxylase domain 3 impairs the myocardial response to ischemia. Cell. Physiol. Biochem. 2015, 36, 843–851. [Google Scholar] [CrossRef]
- Jing, L.; Li, Q.; He, L.; Sun, W.; Jia, Z.; Ma, H. Protective effect of tempol against hypoxia-induced oxidative stress and apoptosis in H9c2 cells. Med. Sci. Monit. Basic. Res. 2017, 23, 159. [Google Scholar] [CrossRef]
- Sato, T.; Takeda, N. The roles of HIF-1α signaling in cardiovascular diseases. J. Cardiol. 2023, 81, 202–208. [Google Scholar] [CrossRef]
- Datta Chaudhuri, R.; Banik, A.; Mandal, B.; Sarkar, S. Cardiac-specific overexpression of HIF-1α during acute myocardial infarction ameliorates cardiomyocyte apoptosis via differential regulation of hypoxia-inducible pro-apoptotic and anti-oxidative genes. Biochem. Biophys. Res. Commun. 2021, 537, 100–108. [Google Scholar] [CrossRef]
- Ikeda, M.; Ide, T.; Tadokoro, T.; Miyamoto, H.D.; Ikeda, S.; Okabe, K.; Ishikita, A.; Sato, M.; Abe, K.; Furusawa, S. Excessive hypoxia-inducible factor-1α expression induces cardiac rupture via p53-dependent apoptosis after myocardial infarction. JAHA 2021, 10, e020895. [Google Scholar] [CrossRef]
- Feng, C.-C.; Lin, C.-C.; Lai, Y.-P.; Chen, T.-S.; Marthandam Asokan, S.; Lin, J.-Y.; Lin, K.-H.; Viswanadha, V.P.; Kuo, W.-W.; Huang, C.-Y. Hypoxia suppresses myocardial survival pathway through HIF-1α-IGFBP-3-dependent signaling and enhances cardiomyocyte autophagic and apoptotic effects mainly via FoxO3a-induced BNIP3 expression. Growth Factors 2016, 34, 73–86. [Google Scholar] [CrossRef]
- Lee, S.H.; Wolf, P.L.; Escudero, R.; Deutsch, R.; Jamieson, S.W.; Thistlethwaite, P.A. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N. Engl. J. Med. 2000, 342, 626–633. [Google Scholar] [CrossRef]
- Adinolfi, E.; Callegari, M.G.; Ferrari, D.; Bolognesi, C.; Minelli, M.; Wieckowski, M.R.; Pinton, P.; Rizzuto, R.; Di Virgilio, F. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol. Biol. Cell 2005, 16, 3260–3272. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 receptor in infection and inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Schmalzing, G.; Markwardt, F. The elusive P2X7 macropore. Trends Cell Biol. 2018, 28, 392–404. [Google Scholar] [CrossRef]
- Zhang, S.; Bi, Y.; Xiang, K.; Tang, Y. P2X7 Receptor Facilitates Cardiomyocyte Autophagy After Myocardial Infarction via Nox4/PERK/ATF4 Signaling Pathway. Cell Biochem. Funct. 2025, 43, e70078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Brewer, A.C.; Schröder, K.; Santos, C.X.; Grieve, D.J.; Wang, M.; Anilkumar, N.; Yu, B.; Dong, X.; Walker, S.J. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 18121–18126. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, R.; Wang, W.; Liu, J.; Jin, X.; Li, Y.; Li, L.; Lei, B. P2X7 Receptor Antagonist Attenuates Retinal Inflammation and Neovascularization Induced by Oxidized Low-Density Lipoprotein. Oxid. Med. Cell. Longev. 2021, 2021, 5520644. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yu, J.; Liu, P.; Zeng, T.; Zeng, X. Astragaloside IV protects cardiomyocytes against hypoxia injury via HIF-1α and the JAK2/STAT3 pathway. Ann. Transl. Med. 2021, 9, 1435. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin Dayel, A.F.; Alhejji, R.M.; Alonazi, A.S.; Alrasheed, N.M. Role of P2X7 Receptor on Hypoxia-Induced Vascular Endothelial Growth Factor Gene Expression in H9c2 Rat Cardiomyocytes. J. Cardiovasc. Dev. Dis. 2025, 12, 438. https://doi.org/10.3390/jcdd12110438
Bin Dayel AF, Alhejji RM, Alonazi AS, Alrasheed NM. Role of P2X7 Receptor on Hypoxia-Induced Vascular Endothelial Growth Factor Gene Expression in H9c2 Rat Cardiomyocytes. Journal of Cardiovascular Development and Disease. 2025; 12(11):438. https://doi.org/10.3390/jcdd12110438
Chicago/Turabian StyleBin Dayel, Anfal F., Reem M. Alhejji, Asma S. Alonazi, and Nouf M. Alrasheed. 2025. "Role of P2X7 Receptor on Hypoxia-Induced Vascular Endothelial Growth Factor Gene Expression in H9c2 Rat Cardiomyocytes" Journal of Cardiovascular Development and Disease 12, no. 11: 438. https://doi.org/10.3390/jcdd12110438
APA StyleBin Dayel, A. F., Alhejji, R. M., Alonazi, A. S., & Alrasheed, N. M. (2025). Role of P2X7 Receptor on Hypoxia-Induced Vascular Endothelial Growth Factor Gene Expression in H9c2 Rat Cardiomyocytes. Journal of Cardiovascular Development and Disease, 12(11), 438. https://doi.org/10.3390/jcdd12110438





