Early Ventricular Arrhythmias Correlate with Adverse Outcome in Takotsubo Syndrome: Analysis of a Large Single-Center Database
Abstract
1. Introduction
2. Methods
Statistics
3. Results
3.1. Baseline Characteristics
3.2. In-Hospital Arrhythmias
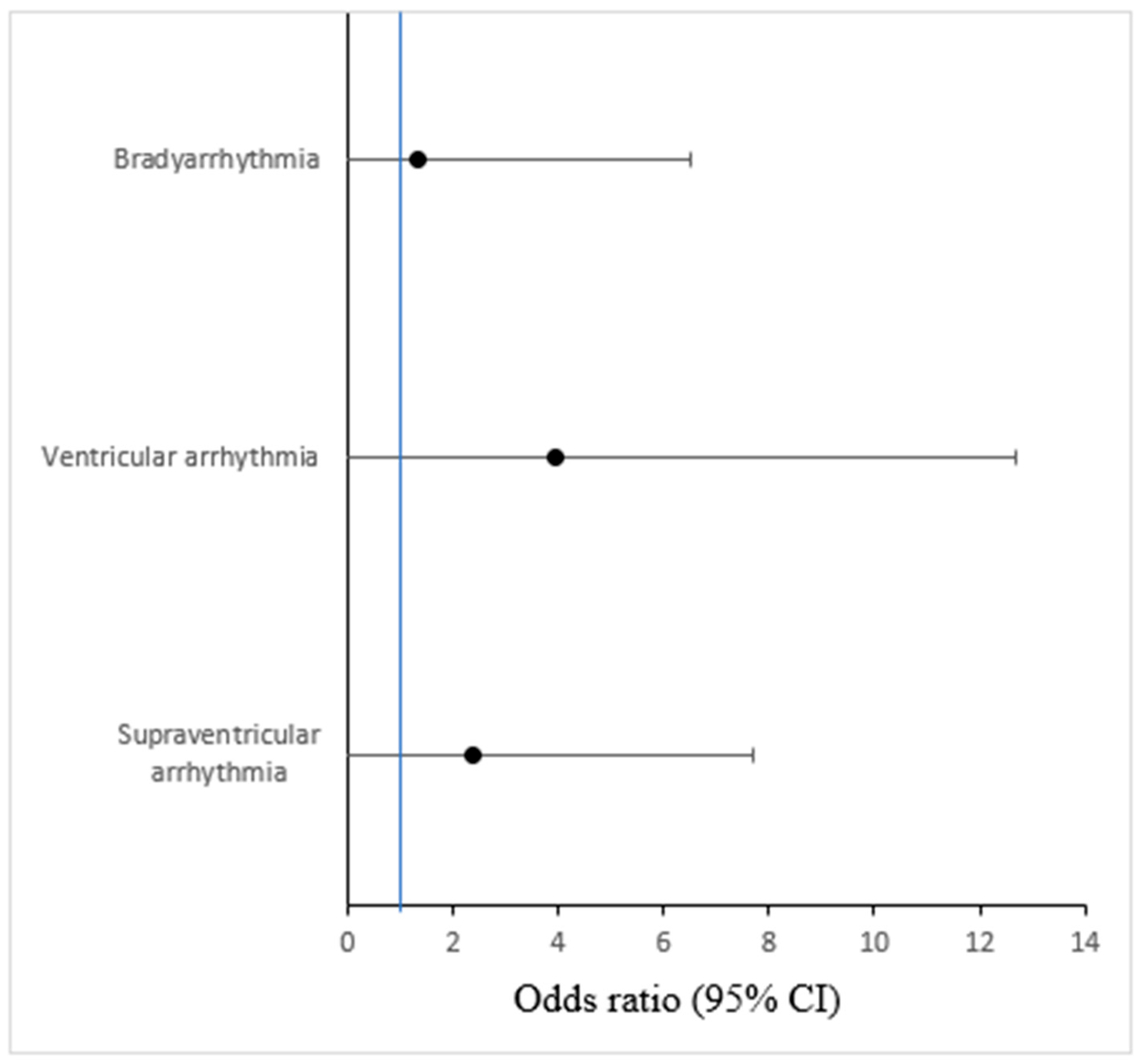
3.3. Arrhythmias and Outcome
3.4. Electrocardiographic Parameters and Cardiovascular Risk Factors
3.5. Subgroup Analysis of TTS Patients Triggered by Life-Threatening Medical Conditions
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurisu, S.; Sato, H.; Kawagoe, T.; Ishihara, M.; Shimatani, Y.; Nishioka, K.; Kono, Y.; Umemura, T.; Nakamura, S. Tako-tsubo-like left ventricular dysfunction with ST-segment elevation: A novel cardiac syndrome mimicking acute myocardial infarction. Am. Heart J. 2002, 143, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Akashi, Y.J.; Goldstein, D.S.; Barbaro, G.; Ueyama, T. Takotsubo Cardiomyopathy. Circulation 2008, 118, 2754–2762. [Google Scholar] [CrossRef]
- Templin, C.; Ghadri, J.R.; Diekmann, J.; Napp, L.C.; Bataiosu, D.R.; Jaguszewski, M.; Cammann, V.L.; Sarcon, A.; Geyer, V.; Neumann, C.A.; et al. Clinical Features and Outcomes of Takotsubo (Stress) Cardiomyopathy. N. Engl. J. Med. 2015, 373, 929–938. [Google Scholar] [CrossRef]
- Brandspiegel, H.Z.; Marinchak, R.A.; Rials, S.J.; Kowey, P.R. A Broken Heart. Circulation 1998, 98, 1349. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Sarcon, A.; Diekmann, J.; Bataiosu, D.R.; Cammann, V.L.; Jurisic, S.; Napp, L.C.; Jaguszewski, M.; Scherff, F.; Brugger, P.; et al. Happy heart syndrome: Role of positive emotional stress in takotsubo syndrome. Eur. Heart J. 2016, 37, 2823–2829. [Google Scholar] [CrossRef]
- Prasad, A.; Lerman, A.; Rihal, C.S. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am. Heart J. 2008, 155, 408–417. [Google Scholar] [CrossRef]
- Previtali, M.; Repetto, A.; Camporotondo, R.; Citro, R.; Faggiano, P.; Bovelli, D.; Baldini, E.; Pasquetto, G.; Ascione, L.; Vignali, L.; et al. Clinical characteristics and outcome of left ventricular ballooning syndrome in a European population. Am. J. Cardiol. 2011, 107, 120–125. [Google Scholar] [CrossRef]
- Lyon, A.R.; Citro, R.; Schneider, B.; Morel, O.; Ghadri, J.R.; Templin, C.; Omerovic, E. Pathophysiology of Takotsubo Syndrome. J. Am. Coll. Cardiol. 2021, 77, 902–921. [Google Scholar] [CrossRef] [PubMed]
- Wittstein, I.S.; Thiemann, D.R.; Lima, J.A.C.; Baughman, K.L.; Schulman, S.P.; Gerstenblith, G.; Wu, K.C.; Rade, J.J.; Bivalacqua, T.J.; Champion, H.C. Neurohumoral features of myocardial stunning due to sudden emotional stress. N. Engl. J. Med. 2005, 352, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Hiestand, T.; Ghadri, J.-R.; Templin, C.; Jäncke, L.; Hänggi, J. Takotsubo Syndrome—Predictable from brain imaging data. Sci. Rep. 2017, 7, 5434. [Google Scholar] [CrossRef]
- Suzuki, H.; Matsumoto, Y.; Kaneta, T.; Sugimura, K.; Takahashi, J.; Fukumoto, Y.; Takahashi, S.; Shimokawa, H. Evidence for brain activation in patients with takotsubo cardiomyopathy. Circ. J. 2014, 78, 256–258. [Google Scholar] [CrossRef]
- Wittstein, I.S. Stress cardiomyopathy: A syndrome of catecholamine-mediated myocardial stunning? Cell. Mol. Neurobiol. 2012, 32, 847–857. [Google Scholar] [CrossRef]
- Ieva, R.; Santoro, F.; Ferraretti, A.; Spennati, G.; de Gennaro, L.; Di Biase, M.; Brunetti, N.D. Hyper-acute precipitating mechanism of Tako-Tsubo cardiomyopathy: In the beginning was basal hyperkinesis? Int. J. Cardiol. 2013, 167, e55-7. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, S.W.; Pink, V.R.; Lesser, J.R.; Garberich, R.F.; Maron, M.S.; Maron, B.J. Clinical Profile of Patients with High-Risk Tako-Tsubo Cardiomyopathy. Am. J. Cardiol. 2015, 116, 765–772. [Google Scholar] [CrossRef]
- Koh, Y.; Voskoboinik, A.; Neil, C. Arrhythmias and Their Electrophysiological Mechanisms in Takotsubo Syndrome: A Narrative Review. Heart Lung Circ. 2022, 31, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Stiermaier, T.; Eitel, C.; Denef, S.; Desch, S.; Schuler, G.; Thiele, H.; Eitel, I. Prevalence and Clinical Significance of Life-Threatening Arrhythmias in Takotsubo Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 2148–2150. [Google Scholar] [CrossRef]
- Pant, S.; Deshmukh, A.; Mehta, K.; Badheka, A.O.; Tuliani, T.; Patel, N.J.; Dabhadkar, K.; Prasad, A.; Paydak, H. Burden of arrhythmias in patients with Takotsubo Cardiomyopathy (Apical Ballooning Syndrome). Int. J. Cardiol. 2013, 170, 64–68. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Lang, S.; Ansari, U.; Tülümen, E.; Schramm, K.; Fastner, C.; Zhou, X.; Hoffmann, U.; Borggrefe, M.; Akin, I. Prevalence of malignant arrhythmia and sudden cardiac death in takotsubo syndrome and its management. Europace 2018, 20, 843–850. [Google Scholar] [CrossRef]
- Auzel, O.; Mustafic, H.; Pillière, R.; El Mahmoud, R.; Dubourg, O.; Mansencal, N. Incidence, Characteristics, Risk Factors, and Outcomes of Takotsubo Cardiomyopathy with and Without Ventricular Arrhythmia. Am. J. Cardiol. 2016, 117, 1242–1247. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Santoro, F.; Stiermaier, T.; Möller, C.; Guastafierro, F.; Novo, G.; Novo, S.; Santangelo, A.; Mariano, E.; Romeo, F.; et al. Prevalence, management, and outcome of adverse rhythm disorders in takotsubo syndrome: Insights from the international multicenter GEIST registry. Heart Fail. Rev. 2020, 25, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Gili, S.; Cammann, V.L.; Schlossbauer, S.A.; Kato, K.; D’Ascenzo, F.; Di Vece, D.; Jurisic, S.; Micek, J.; Obeid, S.; Bacchi, B.; et al. Cardiac arrest in takotsubo syndrome: Results from the InterTAK Registry. Eur. Heart J. 2019, 40, 2142–2151. [Google Scholar] [CrossRef]
- Madhavan, M.; Prasad, A. Proposed Mayo Clinic criteria for the diagnosis of Tako-Tsubo cardiomyopathy and long-term prognosis. Herz 2010, 35, 240–244. [Google Scholar] [CrossRef]
- Stiermaier, T.; Rommel, K.-P.; Eitel, C.; Möller, C.; Graf, T.; Desch, S.; Thiele, H.; Eitel, I. Management of arrhythmias in patients with Takotsubo cardiomyopathy: Is the implantation of permanent devices necessary? Heart Rhythm. 2016, 13, 1979–1986. [Google Scholar] [CrossRef]
- El-Battrawy, I.; Erath, J.W.; Lang, S.; Ansari, U.; Behnes, M.; Gietzen, T.; Zhou, X.; Borggrefe, M.; Akin, I. Takotsubo syndrome and cardiac implantable electronic device therapy. Sci. Rep. 2019, 9, 16559. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Damonte, J.I.; Moroni, F.; Ravindra, K.; Westman, P.; Chiabrando, J.G.; Bressi, E.; Li, P.; Kapoor, K.; Mao, Y.; et al. QT Prolongation and In-Hospital Ventricular Arrhythmic Complications in Patients with Apical Ballooning Takotsubo Syndrome. JACC Clin. Electrophysiol. 2022, 8, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Imran, T.F.; Rahman, I.; Dikdan, S.; Shah, R.; Niazi, O.T.; Thirunahari, N.; Alhaj, E.; Klapholz, M.; Gaziano, J.M.; Djousse, L. QT Prolongation and Clinical Outcomes in Patients with Takotsubo Cardiomyopathy. Pacing Clin. Electrophysiol. 2016, 39, 607–611. [Google Scholar] [CrossRef] [PubMed]
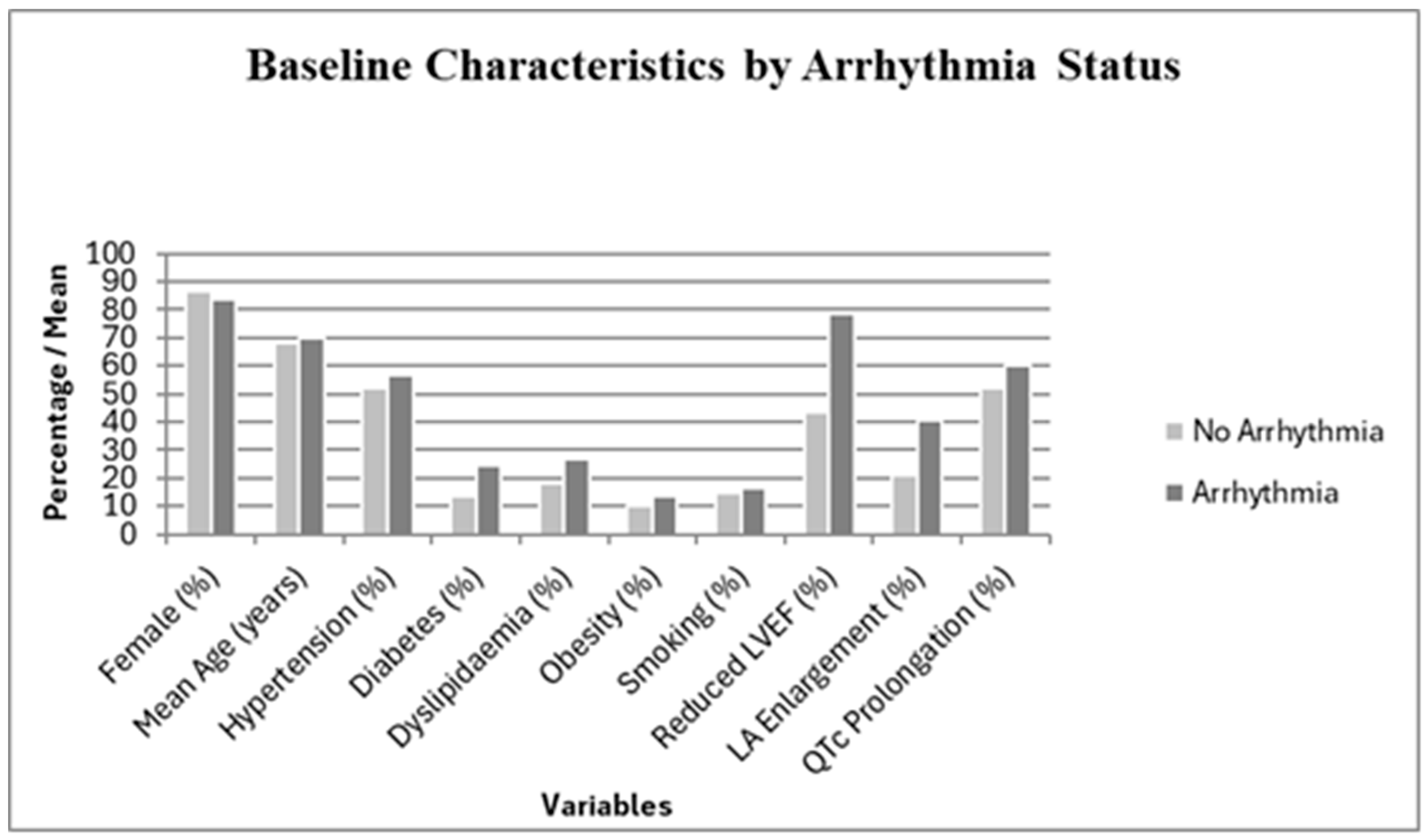
| Variable | Arrhythmias (n = 37) | No Arrhythmias (n = 67) | p-Value |
|---|---|---|---|
| Female sex (%) Age at diagnosis (years) | 31 (83.8%) 69.7 ± 10.2 | 58 (86.6%) 67.9 ± 11.8 | 0.724 0.361 |
| QRS duration (ms) | 96.5 ± 12.4 | 92.1 ± 10.3 | 0.082 |
| QTc interval (ms) | 468.2 ± 32.5 | 451.7 ± 28.9 | 0.018 * |
| LV dysfunction at diagnosis (%) | 29 (78.4%) | 29 (43.3%) | 0.003 * |
| Hypertension (%) | 21 (56.8%) | 35 (52.2%) | 0.642 |
| Diabetes mellitus (%) | 9 (24.3%) | 9 (13.4%) | 0.192 |
| Dyslipidemia (%) | 10 (27.0%) | 12 (17.9%) | 0.177 |
| Smoking (%) | 6 (16.2%) | 10 (14.9%) | 0.698 |
| Obesity (%) | 5 (13.5%) | 7 (10.4%) | 0.650 |
| Variable | Non-Medical Trigger (n = 93) | Medical Trigger (n = 11) | p-Value |
|---|---|---|---|
| Age at diagnosis (years) | 66.4 ± 14.2 | 64.7 ± 12.0 | 0.64 |
| Female sex, n (%) | 80 (86.0%) | 8 (72.7%) | 0.28 |
| LV dysfunction at diagnosis, n (%) | 71 (76.3%) | 10 (90.9%) | 0.34 |
| Composite adverse outcome, n (%) | 44 (47.3%) | 2 (18.2%) | 0.066 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Güler-Eren, S.; Güner, F.; Wanjek, C.-T.; Könemann, H.; Alhourani, N.; Kreimer, F.; Wolfes, J.; Rath, B.; Ellermann, C.; Köbe, J.; et al. Early Ventricular Arrhythmias Correlate with Adverse Outcome in Takotsubo Syndrome: Analysis of a Large Single-Center Database. J. Cardiovasc. Dev. Dis. 2025, 12, 437. https://doi.org/10.3390/jcdd12110437
Güler-Eren S, Güner F, Wanjek C-T, Könemann H, Alhourani N, Kreimer F, Wolfes J, Rath B, Ellermann C, Köbe J, et al. Early Ventricular Arrhythmias Correlate with Adverse Outcome in Takotsubo Syndrome: Analysis of a Large Single-Center Database. Journal of Cardiovascular Development and Disease. 2025; 12(11):437. https://doi.org/10.3390/jcdd12110437
Chicago/Turabian StyleGüler-Eren, Sati, Fatih Güner, Charleen-Therese Wanjek, Hilke Könemann, Nawar Alhourani, Fabienne Kreimer, Julian Wolfes, Benjamin Rath, Christian Ellermann, Julia Köbe, and et al. 2025. "Early Ventricular Arrhythmias Correlate with Adverse Outcome in Takotsubo Syndrome: Analysis of a Large Single-Center Database" Journal of Cardiovascular Development and Disease 12, no. 11: 437. https://doi.org/10.3390/jcdd12110437
APA StyleGüler-Eren, S., Güner, F., Wanjek, C.-T., Könemann, H., Alhourani, N., Kreimer, F., Wolfes, J., Rath, B., Ellermann, C., Köbe, J., Reinke, F., Frommeyer, G., Wegner, F., & Eckardt, L. (2025). Early Ventricular Arrhythmias Correlate with Adverse Outcome in Takotsubo Syndrome: Analysis of a Large Single-Center Database. Journal of Cardiovascular Development and Disease, 12(11), 437. https://doi.org/10.3390/jcdd12110437








